Jones D.S.J., Pujado P.R. Handbook of Petroleum Processing
Подождите немного. Документ загружается.

PETROLEUM PRODUCTS AND A REFINERY CONFIGURATION 69
Table 2.12. Oxygenates commonly used in gasoline (subject to phase-out in the U.S.A.)
Oxygen Water
Name Formula RON RVP psig %wt solubility %
∗
Methyl tertiary butyl
ether (MTBE)
(CH
3
)
3
COCH
3
110–112 8 18 4.3
Ethyl tertiary butyl
ether (ETBE)
(CH
3
)
3
COC
2
H
5
110–112 4 16 1.2
Tertiary amyl methyl
ether (TAME)
(CH
3
)
2
(C
2
H
5
)COCH
3
103–105 4 16 1.2
Ethanol C
2
H
5
OH 112–115 18 35 100
∗
Wt % soluble in water.
See also Part 3 of this chapter.
Using the oxygenates. Oxygenates were used originally simply as a additive to im-
prove octane number. However, because of their oxygen content they are now added
also ton reduce the carbon monoxide and hydrocarbon in the emission gases. There
are a number of oxygenates now used in gasoline manufacture some of the more
common are given in Table 2.12.
The EPA have established limits for the use of each oxygenates in gasoline blends.
For example, MTBE may be blended up to 15 %vol subject to an overall limit of
2.7 %wt oxygen content. The role of MTBE and other oxygenates in the U.S. was
discontinued in 2002 after it was discovered that underground storage tanks had not
been upgraded to retain reformulated gasoline. As a consequence tanks were leaking
gasoline that contained MTBE into the ground and drinking water systems.
Diesel fuel
The policies in place to control, or at least to endeavor to control, emissions from
motive fuels has moved from gasoline engines to diesel engines. Similar to the gasoline
fuels diesel has also changed in composition to meet these environmental constraints
while still maintaining a required performance standard. These changes have imposed
a major impact on the refining industry at a time when the demand for diesel is
increasing. Again as in the case of the gasoline fuel reformulated diesel fuel becomes
part of the modern diesel specification and composition. These are described and
discussed in the following sections.
The diesel engine
Before dealing with the diesel fuel it may be advantageous to review briefly the
principle of the diesel engine itself, diesel engines have historically been preferred in
70 CHAPTER 2
many applications because of their simplicity of design, power, durability, and higher
fuel efficiency. Diesel engines fit into three categories and these are:
r
Low speed engines
r
Medium speed engines
r
High speed engines
Low speed engines. These operate at less than 300 revs per min (rpm), and are used
for applications that require sustained heavy loads at constant engine speed. Examples
are the main propulsion engines in marine vessels and engines used in electric power
generation.
Medium speed engines. These operate at speeds between 300 and 1,000 rpm, and are
used for applications with fairly high loads, and relatively constant speeds. Engines
used in auxiliary power plants on marine vessels, and in smaller power generation
plants are examples of this type of diesel engine.
High speed engines. Operate at speeds above 1000 rpm, and are designed for frequent
and wide variations in load and speed. These are the type of engines used for road
transport and Diesel locomotives.
Almost all diesel engines use a standard four stroke design which are, intake, com-
pression, power, and exhaust. During the intakes stroke, air alone enters the cylinder,
during the compression stroke the air is compressed by the upward movement of the
piston. The final temperature and pressure reached in the cylinder is a function of
the compression ratio, engine speed, and engine design. Pressures of 450 psig, and
temperatures of 500
◦
F are typical. Shortly before the end of the compression stroke,
one or more jets of fuel are injected into the cylinder. The fuel pump introducing these
jets is usually cam driven type and operates at pressures between 1,800 and 30,000
psig. The fuel does not ignite immediately. There is a delay period when the fuel
droplets vaporize and reach their ignition temperature. The extent of this time delay is
affected by the design of the engine, the temperatures of the inlet air and fuel, and the
degree to which the injected fuel is atomized on entering the cylinder. This ignition
delay must be kept as short as possible to avoid accumulation of fuel in the cylinder
before ignition. Such a situation causes diesel knock when this large quantity of fuel
detonates.
After ignition, fuel injection continues for a portion of the power stroke. This fuel
burns almost instantaneously with the remaining air and combustion products in the
cylinder. This more controlled burning period is referred to as diffusion combustion.
Fuel injection is stopped part way through the power stroke. Finally the exhaust stroke
purges the combustion products from the cylinder, and the cycle begins again.
PETROLEUM PRODUCTS AND A REFINERY CONFIGURATION 71
Design improvements to the basic diesel engine
Fuel injection improvements. Most diesel engines built in the 1980s were equipped
with indirect fuel injection system (IDI). Modern engines are equipped with the
direct fuel injection (DI) which is designed for the geometry of the combustion
chamber, and is significantly more efficient. (Note: This can well be compared with
the direct injection concept in gasoline engines which was introduced around this time
also).
Four valve cylinder heads. This promotes better movement of both combustion air
and exhaust gases. This causes high turbulence of the fuel and air in the combustion
chamber resulting in good distribution of the fuel and increased flame speed which
reduces the ignition delay time.
Electronically controlled fuel injection timing. This allows for the injection of a small
quantity of fuel before the main charge into combustion chamber. The result is a
quieter combustion minimizing the amount of premixed combustion and maximizing
the more controlled diffusion combustion.
Increasing the amount of air introduced into the cylinder. Using a Turbocharger,
which is an air compressor driven by a turbine using exhaust gas energy, the mass of
air introduced into the cylinder is increased. This enables the engine to burn more
fuel thus increasing its power output. Using this concept the power output for a diesel
engine can be increased by as much as 50% for the same engine displacement.
The parameters of diesel fuel
The following are the major parameters in meeting the diesel fuel specification with
respect to engine performance or emission or both:
r
The cetane number
r
Aromatic content
r
Density
r
Sulfur content
r
Distillation
r
Viscosity
r
Cloud and pour points
r
Flash point
Most of these parameters have been defined elsewhere in this book. This section then
deals with the effect these parameters have on the performance of the diesel engine or
the emission of undesirable components from the engine or both. Where the definition
72 CHAPTER 2
of the parameter is not dealt with in detail elsewhere in the book, then definition is
included here.
Cetane number. This is the result of an engine test that compares the ignition delay
for a fuel. For this test two reference fuels are chosen. The first is normal cetane
(C
16
) and the second is an isomer of cetane which is heptamethylnonane. The normal
cetane is arbitrarily given the cetane number of 100, while the isomer as the second
reference fuel is assigned a cetane number of 15. The fuel being tested is run in a
standard test engine. The cetane number is derived by comparing the ignition delay
of the test diesel with a blend of the two reference fuels. The cetane number is then
calculated using the equation:
Cetane Number = % normal cetane + 0.15 ×% heptamethylnonane.
Higher cetane numbers indicates that the fuel has a shorter ignition delay. The higher
the cetane number also results in less CO and unburnt hydrocarbons in the engine
emission gases. This has a greater effect in the older diesel engine. Modern engines
are equipped with retarded ignition timing and increasing the cetane number has a
smaller effect on these more modern engines.
Aromatics. The aromatic content of diesel fuel can be measured for single ring aro-
matics, multi-ring or poly-aromatic hydrocarbons (PAH). Some studies show that
reducing the aromatics results in the reduction of all regulated emissions, but other
studies have indicated that the reduction of emissions of unburned hydrocarbons,
NO
x
, and particulates can only be achieved by reducing multi-ring aromatics.
Density. As density is a measure of the mass per unit volume, diesel fuels of low
density require a longer injection time to deliver the same mass of fuel into the
cylinder. The longer the injection time the lower is the peak temperatures which, in
turn, results in lower No
x
formation. At high loads and engine speeds, the longer
injection interval causes some incomplete combustion, resulting in a high emission
of unburned hydrocarbons and CO. When the load is being increased however the
lower density fuel results in less over-fueling, which actually decreases the emission
of particulates, hydrocarbons, and CO.
Sulfur. The sulfur in diesel fuel is burned to SO
2
, a portion of which is further oxidized
to sulfates. This binds with water to form a portion of the particulate matter. Because
only a small percent of the total sulfur in fuel is oxidized to sulfates the contribution
of sulfates to the total particulates is quite small. However if an oxidation catalyst
is used to reduce emission of hydrocarbons, CO, and particulate matter a significant
amount of the SO
2
is converted to sulfates and consequently making a significant
contribution to the particulates in the emission gases.
PETROLEUM PRODUCTS AND A REFINERY CONFIGURATION 73
Distillation. The distillation range of diesel fuel has a significant influence on engine
performance. This is especially so in medium and high speed engines. If the fuel is too
volatile the engine loses power and efficiency because of vapor lock in the fuel system
or poor droplet penetration into the cylinder. On the other hand if the volatility of the
fuel is too low, the engine will lose power and efficiency as a result of poor atomization
of the fuel. Both the front end and the back end of the distillation are important. If the
10 %vol point is too high, the engine will have difficulty starting. A low 50% point
reduces particulate emissions and odor. Because heavier molecules are more difficult
to burn, both soot and the soluble organic fraction (SOF) of the particulate emissions
are increased if the 90% point is too high, the emission of unburned hydrocarbons
will also increase.
Viscosity. Fuel viscosity has an important effect on the fuel pump and injector system.
The shape of the fuel spray is affected by viscosity. If this is too high, the fuel will
not be properly atomized into the cylinder, which will result in poor combustion, loss
of power, and efficiency, with an increase in CO and hydrocarbon emission. Another
effect of poor atomizing of the fuel will be to allow the fuel to impinge on the cylinder
walls, and remove the lubricating film. This results in excessive wear and increase of
hydrocarbon emissions.
If the viscosity of the fuel is too low, the injection spray is too soft and will not
penetrate far enough into the cylinder. A loss of power and efficiency will occur due
to this. Where the fuel system is also lubricated by the fuel, as is the case in some
engine designs, increased wear to the system will result through low fuel viscosity.
Cloud and pour point. Cold weather performance of diesel fuel is a key considera-
tion for users. Actual specifications for cold flow properties are based on expected
temperature extremes and different test methods are used in different parts of the
world. In the United States cloud point is used as an indicator of the cold flow
properties of the fuel. Cloud point is the temperature at which wax begins to pre-
cipitate out of the fuel. The longer paraffin molecules in the fuel precipitate as a wax
when the temperature falls below the cloud point. This wax clogs unheated fuel lines
and filters. The more paraffinic a fuel the higher will be its cloud point. In some
parts of the world, pour point is used as an indication of the lowest temperature at
which a fuel can be pumped. Pour points are generally 4–5
◦
C lower than the cloud
point.
Flash point. The flash point of a fuel is the temperature that the fuel must be heated
to produce an ignitable mixture of vapor and air above the surface of the liquid. This
property is only important for safe handling and storage of the fuel. It has no effect
on the performance of the fuel or its emission properties. If the flash point is too low,
fire or explosion could occur when it is handled.
74 CHAPTER 2
Meeting the diesel fuel parameters
There are various solutions for meeting the parameters of diesel fuel in the design
of the fuel. These solutions range from fractionation, adding improvers, and more
complex hydro-processing. Using modern developments in hydro-processing it is
possible to convert low-grade blend stocks, such as FCCU or thermal cracker prod-
uct streams to good diesel precursors. Among the more important parameters to be
met both from the performance and the emission aspects of the fuel are discussed
below.
Increasing cetane value. The simplest way to improve cetane number is to use an
appropriate ignition improvement additive. These are mainly alkyl nitrates. The ef-
fectiveness of cetane improvement additives tend to be linear with addition rate, how-
ever the improvement can vary with different diesel blend stock. Paraffins which
already have high cetane value respond best to the additives. Aromatics on the
other hand, which have a low cetane number have a poorer response. About 500
wppm of standard alkyl nitrate will increase the cetane value by 2–5 numbers.
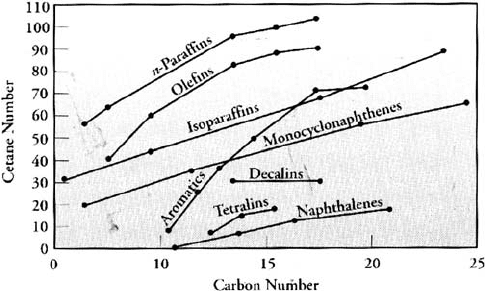
Another way to increase cetane number is to modify the hydrocarbon type in the
diesel blend. Figure 2.2 shows the relationship between molecular types and cetane
number.
Straight chain paraffin molecules have the highest cetane numbers while multiple
ring aromatics have the lowest cetane number improvement also occurs during the
hydro-treating of the gas oil fractions used as blend components in the diesel prod-
uct. During the hydro-treating to remove the organic sulfur and nitrogen species
found in these untreated gas oil fractions, the molecules containing these items are
opened and saturated to form paraffins and naphthenes. At the same time some of the
Figure 2.2. Cetane number of hydrocarbon types.
PETROLEUM PRODUCTS AND A REFINERY CONFIGURATION 75
aromatic molecules contained in the gas oils are also opened and saturated increas-
ing the paraffin/naphthene content of the hydro-treated product. As a result of this
ring opening and saturation a cetane number improvement of between 3 and 5 num-
bers can be expected. Hydro-cracking of heavy gas oil fractions, and hydro-treating
of thermal cracked gas oils and FCC gas oils significantly increase product cetane
numbers.
Meeting sulfur content. Sulfur content of 0.05 %wt is now quite a common require-
ment for diesel fuel. In most cases this level of sulfur can only be met by extensive
hydro-treating or hydro-cracking to remove the sulfur from the middle distillates that
will be used to blend the diesel product. Some refineries select their crude slate with
low sulfur crudes to reduce the hydro-treating demand. The impact on refiners to meet
this parameter extends beyond the severity of hydro-treating that may exist. It impacts
on the availability of hydrogen in the front end to the capacity for handling the sour
gases (H
2
S in particular) in the back end of the process. It is likely that the legislative
sulfur content in diesel may be reduced still further in the near future. This makes the
selection between crude slate demand for low sulfur crude and a modified refinery
configuration and operation a critical one.
Reducing aromatic content. With the increasing demand for diesel fuel, particularly
in North America and Europe, refiners will need to use streams normally routed to
heating oils of fuel as components for the diesel product blend. These streams are
of lower quality and in particular contain higher quantity of aromatics that will be
acceptable as diesel blend stocks without severe treating to improve their blending
characteristics. While high severity hydro-treating to remove sulfur and nitrogen helps
in reducing aromatics in acceptable diesel blend stocks, it will not be sufficient to
improve the quality of the heating oil and fuel oil blend stocks to meet the diesel pool
requirement. Hydro treating using nickel molybdenum catalyst (de-aromatization
process) is being considered for the purpose of upgrading these poorer blend stocks.
This de-aromatization process consists of two stages. The first is processing over a
conventional Co–Mo hydro-treating catalyst to remove sulfur and then to process over
the Ni–Mo catalyst. The nickel catalyst is poisoned by sulfur, thus the two stages. The
economics of using the de-aromatization process must be carefully evaluated because
the metal content of the nickel catalyst represents a significant investment. The process
however operates at a higher space velocity and lower pressure than the conventional
Co–Mo hydro-treating process.
Improvement of cold flow properties. Problems with meeting the cold flow properties
of diesel fuel are associated with the presence of straight chain paraffins. Although
these have higher cetane numbers they pose cold flow limitations because they tend to
precipitate as a wax at low temperatures. The simplest and probably the best solution
to this cold flow property of these paraffins is the use of additives. These additives are
however costly ranging from below $50 per thousand bbls for pour point reduction
76 CHAPTER 2
to as high as $250 per thousand bbls for cloud point reduction (these are year 2000
dollars). Another alternative is to reduce the diesel FBP and add kerosene. This
action removes the heavier paraffinic molecules, which tend to precipitate at higher
temperature. Reducing the cut point from 700
◦
Fto640
◦
F reduces the yield of diesel
by 6 %vol on crude. Solvent dewaxing is another option. In this process the wax
forming components are selectively removed from the diesel product. This is only
considered however for stocks from high paraffin crudes. Selective hydrocracking of
the straight chain paraffins to smaller paraffin molecules is an option. This however
is accompanied by a reduction in yield and cetane number. Finally isomerization of
the straight chain paraffins to branched paraffins minimizes cetane and yield loss is
a viable option. However these isomerization catalysts contain noble metals and are
therefore quite expensive and require the two stage process, desulfurization followed
by the isomerization stage.
2.4 A refinery process configuration development
There are many steps necessary to complete a refining company’s marketing strategy
and production objectives. These steps may culminate in building new processing
facilities or revamping existing ones. The course of action that will ultimately be
adopted will, almost invariably, be decided on an economic basis reflecting the com-
pany’s future profitability picture. Among the most important of these steps however
will be the development of several refining scenarios and processing configurations.
A process configuration and its accompanying Block Flow Diagram is a blue print
and a basis for economic study and decision making. The final and accepted configu-
ration will also be the foundation for the design of new facilities or the revamping of
existing ones. The block flow diagram shows the calculated results of process plant
design capacity, the quantity of process streams to and from each process plant, the
sequence of the plants to one another in the refining scheme, and finally the blending
recipes (streams and their quantities that make up the finished product blends). Such
a development requires the in-depth knowledge of the refining industry and relies,
in no small measure, on the expertise of those companies that license processes and
their technology.
The configuration example described here would be one of many that would be
examined meeting the production and economic objectives of a particular refinery
operation. In this example a refining company wishes to examine a new ‘Grass Roots’
configuration that will produce the following products from a medium Middle East
crude, whose assay TBP curve is given as Figure 2.1.
The product specifications are given in the respective text, and the production limits
are as follows:
PETROLEUM PRODUCTS AND A REFINERY CONFIGURATION 77
Crude feed 50,000 BPCD
LPG 3,289 BPCD (Max)
Gasoline 22,170 BPCD (Max)
20,390 BPCD (Min)
Kerosene No target
Auto diesel 8,830 BPCD (Max)
3,000 BPCD (Min)
Gas oil 8,553 BPCD (Max)
2,630 BPCD (Min)
Marine diesel 3,000 BPCD (Max)
2,300 BPCD (Min)
Heavy fuel 8,500 BPCD (Max)
6,500 BPCD (Min)
Bunker fuel No restrictions
A solution
For this configuration the following processes will be examined:
r
Atmos crude dist unit (CDU)
r
Vacuum dist unit (VDU)
r
Light ends dist units
r
Naphtha hydrotreater (Nap Hds)
r
Catalytic reformer
r
Lt gas oil hydrotreater (LGO Hds)
r
Hy gas oil hydrotreater (HGO Hds)
r
Fluid catalytic cracker (FCCU)
r
Isom/alkylation plant
r
Thermal cracker (TC)
r
Gas treating and sulfur recovery processes
The stream quantities to each of these units will be developed as follows:
The atmospheric crude distillation unit. The crude is split to satisfy the quantities and
distillation characteristics of the finished products required. For this example these
are as follows:
Gas to C
5
1.71 %vol on crude
C
5
to 210
◦
F 10.44 %vol on crude Light naphtha (LSR)
210 to 380
◦
F 14.20 %vol on crude Heavy naphtha (HSR)
380 to 520
◦
F 9.09 %vol on crude Kerosene
520 to 650
◦
F 13.83 %vol on crude Diesel
650
◦
F+ 50.73 %vol on crude Residue (feed to crude vacuum unit)

78 CHAPTER 2
Table 2.13. ASTM and TBP of the crude distillate cuts
C
5
to 210
◦
F 210 to 380
◦
F 380 to 520
◦
F 520 to 650
◦
F
ASTM
◦
F TBP
◦
F ASTM
◦
F TBP
◦
F ASTM
◦
F TBP
◦
F ASTM
◦
F TBP
◦
F
IBP 90 30 198 132 363 310 451 392
10 %vol 115 78 235 196 387 357 507 480
30 135 116 260 242 407 395 533 527
50 148 140 280 276 424 425 551 558
70 162 163 301 308 440 451 567 583
90 182 191 332 349 462 481 593 618
FBP 225 238 390 412 493 515 622 650
Then from the cut points corresponding to the stream’s volume % on crude the fol-
lowing ASTM and TBP of the distillate streams leaving the unit are calculated. The
ASTM (Starting at the C
5
+ level) are developed using the 50% point of each cut and
the 70% point converted to ASTM (using the Edmister correlation) and a straight line
drawn through them to give the ASTM distillation. These are then converted to TBP
(again using the Edmister correlation) and are shown in Table 2.13.
These curves are given in Figure 2.3.
From these curves the table below gives a component break down of the distillate
streams (Table 2.14).
The properties of these distillate cuts are determined using the component mid vol% on
crude multiplied by its volume on the respective cut or by use of indices as previously
described.
The following table gives the component properties from the assay data (Table 2.15).
Using the methods described earlier in this chapter the products from the atmospheric
crude unit including their salient properties are as follows (Table 2.16).
The vacuum crude unit, VDU. The feed to the vacuum distillation unit will be the
atmospheric residue from the CDU. This stream will be treated in the same manner
as the distillate streams shown above for the CDU. Thus the distillate streams and the
vacuum residue stream including their salient properties are shown in Table 2.17.
The product Streams from this unit are:
LVGO (Light Vacuum Gas Oil)—650 to 750
◦
F (49.27–60.12 %vol on crude)
HVGO (Heavy Vacuum Gas oil)—750 to 930
◦
F (60.12–78.67 %vol on crude)
Vacuum Residue—+930
◦
F (21.33 %vol on crude)
