Jones D.S.J., Pujado P.R. Handbook of Petroleum Processing
Подождите немного. Документ загружается.


THE REFINERY ENVIRONMENTAL ISSUES 663
123
10
4
10
3
10
2
4681 2
1.5
2.0
3.0
10.0
5.0
34 681 2 34 6 81
NH
3
in Solution (ppm by wt)
NH
3
Partial Pressure psia
10.0
8
6
4
3
2
1.0
8
6
4
3
2
0.1
8
6
4
3
2
0.1
Molar Ratio
of NH
3
/H
2
S
in Solution.
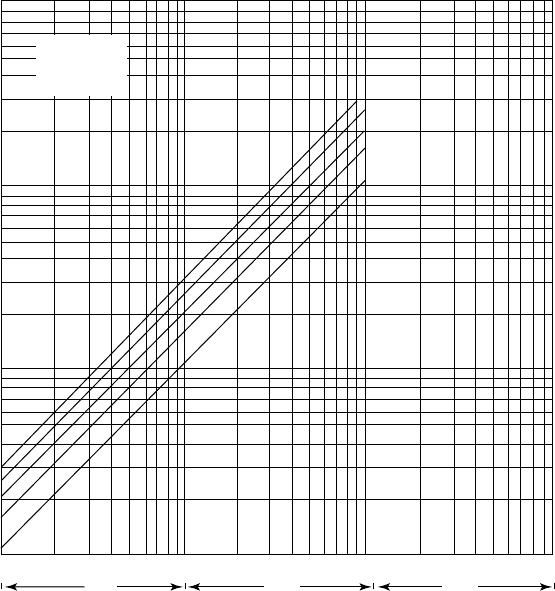
Figure 14.A.7. Partial pressure of NH
3
over aqueous solutions of H
2
S and NH
3
at 210
◦
F.
664 CHAPTER 14
1
0.1
2
3
4
6
8
0.1
2
3
4
6
8
1.0
2
3
4
6
8
10.0
234
10
2
10
3
NH
3
in Solution (ppm by wt)
NH
3
Partial Pressure psia
10
4
681 2 34 681 2 34 6 81
Molar Ratio
of NH
3
/H
2
S
in Solution.
2.0
1.5
3.0
5.0
10.0
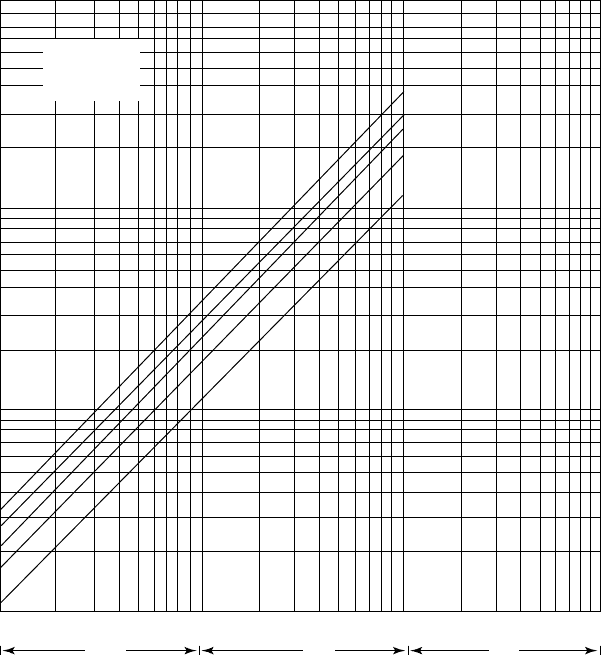
Figure 14.A.8. Partial pressure of NH
3
over aqueous solutions of H
2
S and NH
3
at 220
◦
F.
THE REFINERY ENVIRONMENTAL ISSUES 665
1
0.1
2
3
4
6
8
0.1
2
3
4
6
8
1.0
2
3
4
6
8
10.0
234681 234681 23
10
4
10
3
10
2
4681
NH
3
Partial Pressure psia
NH
3
in Solution (ppm by wt)
Molar Ratio
of NH
3
/H
2
S
in Solution.
10.0
5.0
3.0
2.0
1.5
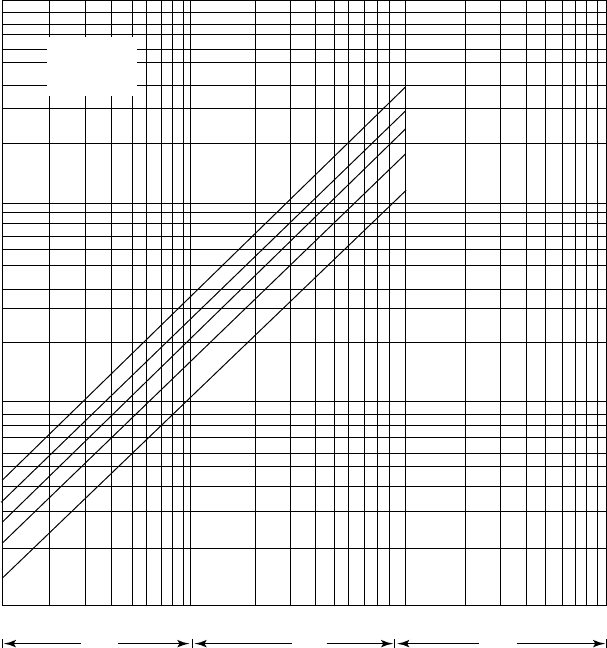
Figure 14.A.9. Partial pressure of NH
3
over aqueous solutions of H
2
S and NH
3
at 225
◦
F.
666 CHAPTER 14
1
0.1
2
3
4
6
8
0.1
1.5
2.0
3.0
5.0
10.0
2
3
4
6
8
1.0
2
3
4
6
8
10.0
234681 2
NH
3
in Solution (ppm by wt)
34 1 2 3
10
4
10
3
10
2
468186
NH
3
Partial Pressure psia
Molar Ratio
of NH
3
/H
2
S
in Solution.
Figure 14.A.10. Partial pressure of NH
3
over aqueous solutions of H
2
S and NH
3
at 230
◦
F.

THE REFINERY ENVIRONMENTAL ISSUES 667
Appendix 14.2: Example of the design of a sour water stripper with no reflux
Specification
Feed: The tower is to be designed to handle 200 gpm (at 100
◦
F) of sour water con-
taining 10,000 ppm of H
2
S and 7,500 ppm of NH
3
(by weight).
Unit: The unit shall be a trayed column using sieve trays (efficiency of 0.5) with no
reflux.
Steam rate: Refinery 50 psig saturated steam shall be used at a rate of 1.3 lbs/gpm of
feed.
Tower pressure and temperature: The vapors leaving the tower top shall have sufficient
pressure to enter a ‘Rat Tail’burner in a nearby heater. The tower top pressure shall
be 20 psia. The feed entering the top tray shall be preheated to a temperature of 200
◦
F.
Total tower pressure drop shall be 2 psi.
Stripped water specification: The tower shall be designed and operated to remove
99.0% of the H
2
S in the feed and 95% of the NH
3
.
The design
Assume the stripping will be accomplished using four theoretical trays. Then at an
efficiency of 0.5 the number of actual trays will be 8. The pressure drop per tray will
be 0.25 (that is 2 psi/8 trays).
Calculate feed mass per hour:
Water @ 100
◦
F has a specific volume of 0.1207 gals/lb.
Then 200 gpm of water =
200×60
0.1207
= 99,420 lbs/hr.
Feed will be:
lbs/hr moles/hr
Water 99,420 5,523.3
NH
3
745.65 43.86 (7,500 ppm by wt)
H
2
S 994.2 29.24 (10,000 ppm by wt)
Calculate stripping steam:
Tower bottom pressure will be 20 psia + (8 × 0.25) = 22 psia.
From steam tables tower bottom temperature will be water at 22 psia = 233
◦
F
668 CHAPTER 14
Feed temperature = 200
◦
F
Then steam used for heating = 99,420(33/924) = 3,550 lbs/hr.
And steam used for stripping = 15,550 − 3,550 = 12,000 lbs/hr.
Calculate stripped water quantity and composition:
Stripped water shall contain feed water plus condensate. 99,420 + 3,550 = 102,970
lbs/hr
NH
3
in stripped water shall be 5% of total = 37.28 lbs/hr = 2.19 moles/hr = 361
ppm by wt
H
2
S in stripped water shall be 1% of total = 9.94 lbs/hr = 0.29 moles/hr = 100 ppm
by wt
Calculate the overhead vapor partial pressures:
Overhead vapor leaving the tower V
o
will be as follows:
moles/hr lbs/hr PP psia
NH
3
41.67 708 1.13
H
2
S 28.95 984 0.78
Steam 667 12,000 18.09
Total 737.62 13,692 20.00
Temperature of top theoretical tray is steam at a saturation pressure of 18.09 psia
= 223
◦
F.
Top tray calculation:
Vo
F
Theo Tray 1
L
1 V2
Assume a ratio of NH
3
/H
2
S as 3.8 moles that is (17/34) × 3.8 = 1.9bywt.
From Figure A5.9 NH
3
at a partial pressure of 1.13 will be 4,600 ppm by wt H
2
S ppm
by wt will be 4,600/1.9 = 2,421 ppm
From Figure A5.4 partial pressure of H
2
S = 0.75 which is acceptably close to 0.78,
which was established.
Note: Should the Partial Pressure of H
2
S be substantially different to that for V
o
then
a different ratio of the two components would have to be chosen and the calculation
repeated.
Calculate liquid from top tray L
1
:
ppm lbs/hr moles/hr
NH
3
4,600 457 26.9
H
2
S 2,421 241 7.08
THE REFINERY ENVIRONMENTAL ISSUES 669
Calculate vapor from Theo tray 2 V
2
Moles vapor for NH
3
and H
2
S will be those moles in V
o
+ L
1
− F
Pressure on tray Theo tray 2 will be (1/0.5) = 2 actual trays @ 0.25 psi pressure drop =
20 psia + 0.5 = 20.5 psia.
moles/hr PP psia
NH
3
24.71 0.73
H
2
S 6.79 0.2
Steam 667 19.57
Total 698.5 20.5 Tray temp (from Steam tables = 226
◦
F)
Calculate balance over theoretical tray 2:
V3 L2
V2 L1
Tray 2
Assume NH
3
/H
2
S ratio is 5.0 molar and 2.5 ppm by weight.
NH
3
@ a PP of 0.73 and 5.0 molar ratio = 2,400 ppm by wt (from Figure 14.A.10).
H
2
S ppm is 2,400/2.5 = 960.
From Figure A5.5 H
2
S ppm = 0.19, which is a satisfactory match.
Liquid from tray 2. L
2
:
ppm lbs/hr moles/hr
NH
3
2,400 278.4 16.37
H
2
S 960 95.5 2.81
Vapor from tray 3 V
3
:
Total tray Pressure = 21 psia and temperature is 231
◦
F
Vapor from tray 3 = V
2
+ L
2
− L
1
moles/hr PP psia
NH
3
14.18 0.44
H
2
S 2.52 0.08
Steam 667 20.52
Total 683.7 21.00

670 CHAPTER 14
Calculate balance over theoretical tray 3:
V3 L2
V4 L3 Tray 3
Assume NH
3
/H
2
S ratio is 6.5 Molar and 3.25 by weight.
NH
3
ppm from Figure 14.A.10 = 1,600 ppm by weight.
H
2
S is 493 ppm from Figure 14.A.6 PP of H
2
S is 0.085 which is a satisfactory match.
Liquid from theoretical tray 3, L
3
:
ppm lbs/hr moles/hr
NH
3
1,600 159.1 9.35
H
2
S 493 49 1.3
Vapor from theoretical tray 4, V
4
:
Tray pressure 21.5 psia Temperature 232
◦
F
V
4
= V
3
+ L
3
− L
2
Moles/hr PP psia
NH
3
7.16 0.23
H
2
S 1.01 0.032
Steam 667 21.24
Total 675.17 21.5
Calculate balance over theoretical tray 4:
V4 L3
Vb
L4 Tray 4.
Assume NH
3
/H
2
S ratio is 6.6 molar and 3.3 by weight
THE REFINERY ENVIRONMENTAL ISSUES 671
NH
3
ppm from Figure 14.A.10 is 610. H
2
S ppm is 185 PP psia of H
2
S from Fig-
ure 14.A.6 is 0.032 which is a satisfactory match.
Liquid from theoretical tray 4, L
4
ppm lbs/hr moles/hr
NH
3
610 60.6 3.57
H
2
S 184.8 18.4 0.54
Vapor from tower bottom V
b
Pressure 22 psia Temperature 233
◦
F
V
b
= V
4
+ L
4
− L
3
moles/hr PP psia
NH
3
1.38 0.045
H
2
S 0.25 0.008
Steam 667 21.947
Total 668.83 22.0
Calculate the bottom of the tower:
Vb L4 Steam
Stripped Water
Assume NH
3
/H
2
S ratio is 7.0 molar and 3.5 by weight
NH
3
ppm from fig A5.10 is 180
H
2
S ppm is 51.4 PP psia of H
2
S from Figure A5.6 is 0.008 (extrapolated) which is a
satisfactory match.
Contaminants in Stripped Liquid Product

672 CHAPTER 14
ppm lbs/hr
NH
3
180 17.9
H
2
S 51 5.1
Conclusion
The tower will handle 200 gpm of sour water and remove 97.6 % weight of NH
3
and
99.49% weight of H
2
S using 8 actual trays and 1.3 lbs/hr per gpm of feed. Feed will
be preheated to 200
◦
F by heat exchange with tower bottoms before entering on the
top tray of the tower.
Appendix 14.3: Example design of an API separator
Specification
It is required to design an oil/water separator to handle the normal quoted rainfall and
process waste from a 4,500 BPSD hydro-skimming refinery. The quantity of in flow
to the separator is estimated to be 600 gpm. The normal rundown temperature for
this stream is taken as 100
◦
F. The design of the separator shall be in accordance with
the appropriate section of the API Manual—sixth edition. The following data shall
be used in the design:
Specific gravity of water 0.995
Specific gravity of the oil 0.890
Viscosity of water 0.7 Centipoise.
Diameter of the oil globules 0.006 ins
The oil shall be removed from the separator by means of oil skimming pipes and an
oil sump designed to meet the oil influent content of 400 ppm by volume.
The design
The rising rate of the oil is calculated from the equation:
V
r
= 6.69 × 10
4
×
d
2
× S
µ
where
V
r
= rising rate of oil in ft/min.
d = diameter of oil globule in ins = 0.006
S = difference in the SGs of oil and water phases = 0.105
µ = Viscosity of the water phase in centipoise = 0.7
