Jan Lindhe. Clinical Periodontology
Подождите немного. Документ загружается.

THE TRANSMUCOSAL ATTACHMENT •
837
pared the implant (ITI O Dental Implant System) sites
in such a way that at the probing experiment some
regions were healthy, a few sites exhibited signs of
mucositis and some sites exhibited more advanced
peri-implantitis. Probes with different geometry were
inserted into the pockets using a standardized prob-
ing procedure and a small force of only 0.2 N. The
probes were anchored and block biopsies were har-
vested. The probe locations were studied in histologic
ground sections. The authors reported that the mean
"
histologic" probing depth at healthy sites was 1.75
mm, i.e. similar to the depth (about 2 mm) recorded
by Ericsson & Lindhe (1993). The corresponding
depth at sites with mucositis and peri-implantitis was
1.62 mm and 3.8 mm respectively. Lang et al. (1994)
further stated that at healthy and mucositis sites, the
probe tip identified "the connective tissue adhesion
level" (i.e. the base of the barrier epithelium) while at
peri-implantitis sites, the probe exceeded the base of
the ulcerated pocket epithelium by a mean distance of
0.5 mm. At such peri-implantitis sites the probe
reached the base of the inflammatory cell infiltrate.
Schou et al. (2002) compared probing measure-
ments at implants and teeth in eight cynomolgus mon
-
keys. Ground sections were produced from tooth and
implant sites that were (1) clinically healthy, (2)
slightly inflamed (mucositis/gingivitis), and (3) se-
verely inflamed (peri-implantitis/peridodontitis) and
in which probes had been inserted. An electronic
probe (Peri-Probe
®
) with a tip diameter of 0.5 mm and
a standardized probing force of 0.3-0.4 N was used. It
was demonstrated that the probe tip was located at a
similar distance from the bone in healthy tooth sites
and implant sites. On the other hand, at implants
exhibiting mucositis and peri-implantitis, the probe
tip was consistently identified at a more apical posi-
tion than at corresponding sites at teeth (gingivitis and
periodontitis). The authors concluded that (1) probing
depth measurements at implant and teeth yielded
different information, and (2) small alterations in
probing depth at implants may reflect changes in soft
tissue inflammation rather than loss of supporting
tissues.
By comparing the findings from the studies re-
ported above, it becomes apparent that meaningful —
in comparison to tooth sites — probing depth and
probing attachment level measurements at implant
sites can be obtained only if the force used during
probing is light, i.e. about 0.2—0.3 N. If a greater force
is utilized, the attachment between the mucosa and
the implant surface may be mechanically compro-
mised; the mucosa becomes dislocated in lateral, "api
-
cal", direction and the probe tip is allowed to end close
to the bone level. In this context it should be realized
that the probing force used by different professionals
varies between 0.5 and 1.3 N (Freed et al. 1983). Fur-
ther, in the presence of inflammation in the peri-im-
plant mucosa, the probe penetrates to a more "apical"
position than at inflamed sites at teeth.
REFERENCES
Abrahamsson, l., Berglundh, T., Glantz, P.O. & Lindhe, J. (1998).
The mucosal attachment at different abutments. An experi-
mental study in dogs.
Journal of Clinical Periodontology 25,
721-727.
Abrahamsson, I., Berglundh, T., Wennstrom, J. & Lindhe, J.
(
1996). The peri-implant hard and soft tissues at different
implant systems. A comparative study in the dog.
Clinical Oral
Implants Research
7, 212-219.
Abrahamsson, I., Zitzmann, N.U., Berglundh, T., Linder, E.,
Wennerberg, A. & Lindhe, J. (2002) The mucosal attachment
to titanium implants with different surface characteristics. An
experimental study in dogs.
Journal of Clinical Periodontology
(
in press).
Abrahamsson, I., Zitzmann, N.U., Berglundh, T., Wennerberg,
A.
& Lindhe, J. (2001). Bone and soft tissue integration to
titanium implants with different surface topography. An ex-
perimental study in the dog.
Journal of Maxillofacial Implants
16, 323-332.
Berglundh, T. (1999). Soft tissue interface and response to micro-
bial challenge. In: Lang, N.P., Lindhe, J. & Karring, T., eds.
Implant dentistry. Proceedings from 3rd European Workshop on
Periodontology.
Berlin: Quintessence, pp. 153-174.
Berglundh, T. & Lindhe, J. (1996). Dimensions of the peri-impl an
t
mucosa. Biological width revisited.
Journal of Clinical Perio-
dontology
23,
971-973.
Berglundh, T., Lindhe, J., Ericsson, I, Marinello, C.P., Liljenberg,
B.
& Thomsen, P. (1991). The soft tissue barrier at implants
and teeth.
Clinical Oral Implants Research 2,
81-90.
Berglundh, T., Lindhe, J., Jonsson, K. & Ericsson, 1. (1994). The
topography of the vascular systems in the periodontal and
peri-implant tissues dog.
Journal of Clinical Periodontology
21,
189-193.
Buser, D., Weber, H.P., Donath, K., Fiorellini, J.P., Paquette, D.W.
& Williams, R.C. (1992). Soft tissue reactions to non-sub-
merged unloaded titanium implants in beagle dogs.
Journal
of
Periodontology
63,
226-236.
Ericsson, I. & Lindhe, J. (1993). Probing depth at implants and
teeth.
Journal of Clinical Periodontology
20, 623-627.
Freed, H.K, Capper, R.L, & Kalkwarf, K.L. (1983). Evaluation of
periodontal probing forces.
Journal of Periodontology
54, 488-
492.
Gould, T.R.L., Westbury, L. & Brunette, D.M. (1984). Ultrastruc-
tural study of the attachment of human gingiva to titanium
in
vivo.
Journal of Prosthetic Dentistry 52,
418-420.
Lang, N.P, Wetzel, A.C., Stich, H. & Caffesse, R.G. (1994). His-
tologic probe penetration in healthy and inflamed peri-im-
plant tissues.
Clinical Oral Implants Research 5,
191-201.
Moon, LS, Berglundh, T., Abrahamsson, I., Linder, E. & Lindhe,
J.
(1999). The barrier between the keratinized mucosa and the
dental implant. An experimental study in the dog.
Journal of
Clinical Periodontology 26,
658-663.
Schou, S., Holmstrup, P., Stolze, K., Hjorting-Hansen, E., Fien,
N.
E. & Skovgaard, L.T. (2002). Probing around implants and
teeth with healthy or inflamed marginal tissues. A histologic
comparison in cynomolgus monkeys
(Macaca fascicularis).
Clinical Oral Implants Research
13, 113-126.
CHAPTER 36
Radiographic Examination
HANS-GORAN GRONDAHL
Basic radiologic principles
Special requirements in the periodontally
compromised patient
Radiographic techniques for primary
preoperative evaluations
Radiographic techniques for secondary
preoperative evaluations
Postoperative radiography
Digital intraoral radiography
BASIC RADIOLOGIC PRINCIPLES
Whenever radiographic methods are used to acquire
information in a clinical context it is of great impor-
tance that one makes sure that the benefit of using
them exceeds the costs involved. When radiographic
methods are employed, the costs are not only of a
monetary nature. Not least important are those which
comprise radiation risks. With the growing number of
subjects for whom implant treatment is considered,
there is a risk that the population dose will increase.
This is because both preoperatively and postopera-
tively, more radiographs are often required when im-
plant treatment is performed than when conventional
prosthetic treatment is done. In addition, radiographic
methods which deliver higher radiation doses to the
patient than do conventional methods are sometimes
used.
It must thus be remembered that radiography
should be based on a comprehensive clinical exami-
nation from which is determined the clinically indis-
pensable information that can only be obtained
through radiographic methods. Furthermore, the re-
quired information should be obtained with tech-
niques yielding the smallest possible radiation dose.
SPECIAL REQUIREMENTS IN THE
PERIODONTALLY COMPROMISED
PATIENT
The placement of implants in the partially dentate
patient and, particularly, in the periodontally compro
-
mised patient, requires careful attention to both the
remaining teeth and the potential implant sites. The
state of the remaining teeth in a patient in whom
implant treatment is contemplated must be thor-
oughly evaluated to enable necessary treatment to be
performed and a long-term prognosis to be made
before the implant treatment is initiated. Failures to
diagnose and treat pathologic conditions in and
around remaining teeth can seriously compromise the
results of implant therapy in both the short-term and
the long-term perspective. Hence, the partially den-
tate patient must be subjected to a detailed clinical and
radiographic examination of the teeth and surround-
ing alveolar hone. The examination should include the
potential implant sites to determine the presence of
pathologic conditions, root remnants, foreign bodies
and other factors which may require surgical interven
-
tion and a subsequent healing period before a decision
to insert implants can be made.
RADIOGRAPHIC TECHNIQUES
FOR
PRIMARY PREOPERATIVE
EVALUATIONS
Intraoral and panoramic radiography
The radiographic technique of choice is the intraoral
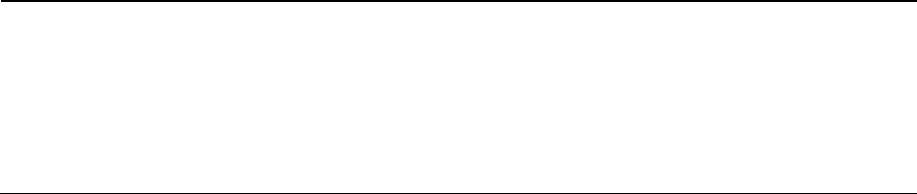
paralleling technique (Fig. 36-1) with projections per-
pendicular to the tangent of the dental arch in the areas
of interest. The bisecting-angle technique should be
avoided because this distorts vertical dimensions. To
decrease doses as much as possible, fast (E or F speed)
films should be used in combination with narrowly
collimated X-ray beams. When applied also to the
edentate regions the intraoral technique provides
RADIOGRAPHIC EXAMINATION •
8
39
Fig. 36-1. The best radiographic
depiction of remaining teeth and
edentate regions is provided by
the
intraoral paralleling technique.
Fig. 36-2. In intraoral radiographs the mesiodistal dimension of the intended implant site can be evaluated. The
number of implants that can be inserted can thus be assessed. Intraoral radiographs also provide a preliminary esti
-
mate of the vertical bone dimension.
valuable information concerning the mesiodistal di-
about the potentially available bone height relative to,
mension of the region in which implants are consid- for example, the mandibular canal and the maxillary
ered and, thus, about the number of implants that can
sinus (Fig. 36-2). From the intraoral radiographs it can
be inserted. The radiographs also provide information therefore be determined in which cases implant treat-
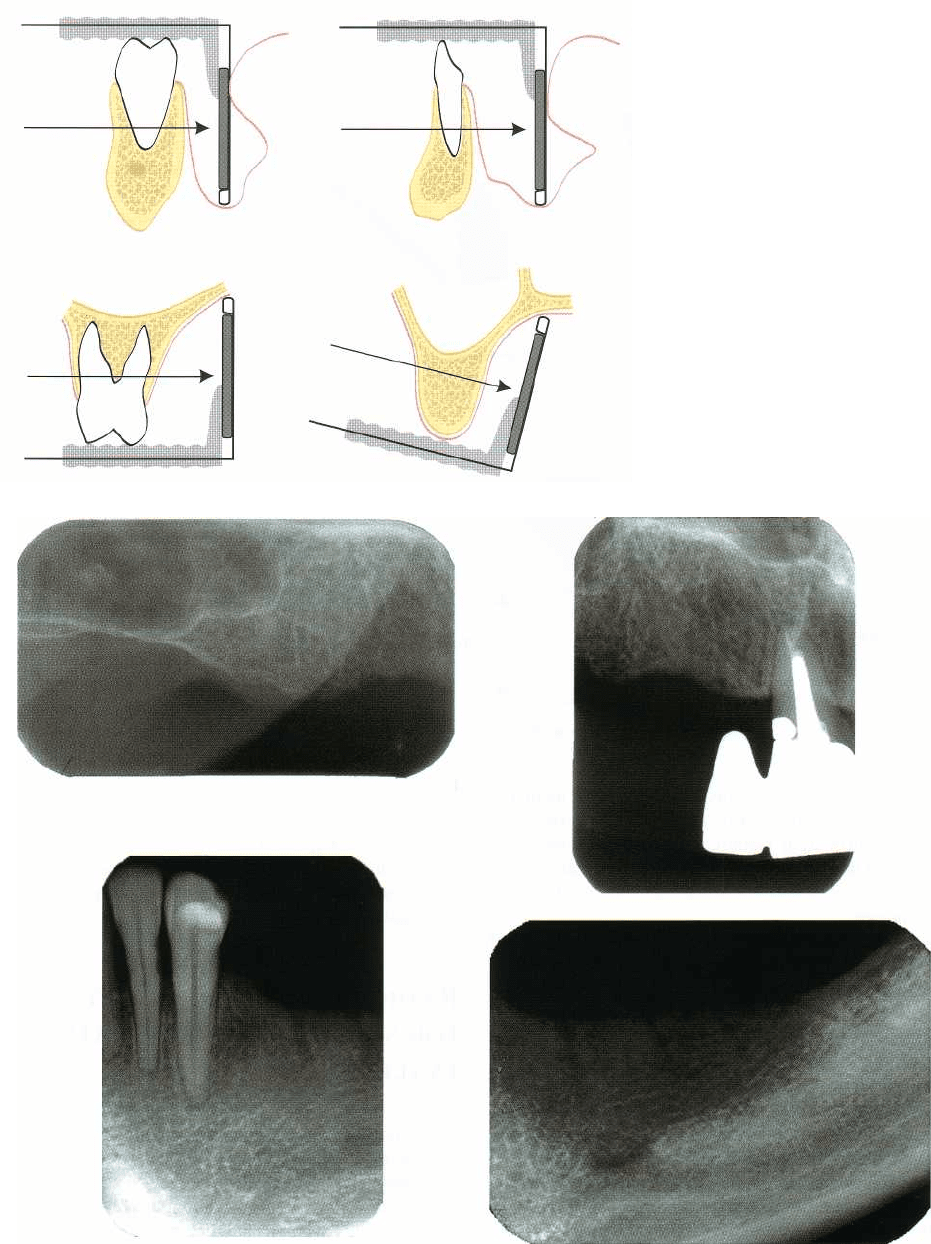
840 • CHAPTER 36
Fig. 36-3. To be able to correctly es
-
timate horizontal distances in the
region of interest, radiographs
must be taken with the incident
beam perpendicular to the tan-
gent of the alveolar process (a).
An
incorrect beam angulation can
make the mesiodistal dimension
appear smaller than it is (b).
Fig. 36-4. Ideally the implant
should be surrounded by bone in
its entire length. The bone height
available for implant insertion
therefore corresponds to the dis-
tance from a bone level, where the
bone width is sufficient, to a limit-
ing anatomic structure. Bone
width is most accurately meas-
ured in tomograms. A measure of
the total bone height can be used
as a reference during surgery.
ment cannot be performed due to lack of available
bone volume unless bone augmentation procedures
are performed. It cannot, however, be determined
whether implant treatment can be made because of the
lack of information about the buccolingual bone di-
mensions.
While panoramic radiography can provide some of
the information that is necessary to determine
whether implant treatment may be contemplated, its
lack of detail often prohibits a sufficiently accurate
diagnosis of tooth-related diseases. Furthermore, in
panoramic radiographs distortions are frequently
found, above all concerning horizontal dimensions
(
Tronje 1982). This makes panoramic images less well
suited for accurate estimates of the amount of bone
available in the mesiodistal direction, particularly in
the anterior parts of the jaws. However, when the
anatomic conditions make it impossible to place in-
traoral films parallel to the vertical axis of the alveolar
process, a better estimate of the bone height can be
made in panoramic radiographs. It is important that
due account is taken of the magnification in pano-
ramic radiographs, as this can vary between pano-
ramic units.
When implants are to be inserted between teeth,
between a tooth and the mental foramen, or between
a
tooth and the anterior border of the maxillary sinus,
supplementary intraoral radiographs should always
be obtained. They should be taken with a direction of
the X-ray beam perpendicular to the tangent of the
alveolar arch (Fig. 36-3). Inaccurate horizontal angu-
lation of the X-ray beam can easily make the distances
of interest appear too small or, less frequently, too large
(Grondahl et al. 1996).
RADIOGRAPHIC TECHNIQUES FOR
SECONDARY PREOPERATIVE
EVALUATIONS
An important objective of the preoperative radio-
graphic evaluation of the implant patient is to deter-
mine the height and width of the bone available for
implant insertion. Ideally, the bone width should al-
low complete coverage of all implant threads on both
the buccal and the lingual sides. The available bone
height must therefore be estimated from that part of
the alveolar bone in which a sufficient bone width is
found to a site specific anatomic border in the vertical
direction, e.g. the lower border of the nasal cavity, the
lower border of the maxillary sinus or the upper bor-
der of the mandibular canal. Sufficiently accurate es-
timations of bone width and height cannot be ob-
tained without cross-sectional tomography. To obtain

RADIOGRAPHIC EXAMINATION • 841
Fig. 36-5. Cross-sectional tomography of the mandible
provides information about the position of the
mandibular canal relative to the marginal bone crest
and about the width of the mandible.
a reference measurement that can be used during
surgery in order to determine to what depth the drill-
ing can be performed, measures should also be taken
from the marginal border of the alveolar crest to the
anatomic structure that limits the depth to which the
preparation can be made (Fig. 36-4).
To achieve ideal conditions for a successful integra
-
tion of the implant with the surrounding bone it is
important that good stability of the implant can be
obtained during the healing period (Sennerby et al.
1992, Ivanoff et al. 1996). The most important factor in
this respect is the presence of a sufficient amount of
compact bone in which the implant can be anchored.
The compact bone at the marginal bone crest can
provide stability of the marginal part of the implant.
Stability of its "apical" part can, in the anterior part of
the mandible, be obtained by anchoring the implant
in a layer of cortical bone at the base of the mandible.
In the maxilla the lower border of the nasal cavity or
the maxillary sinus can provide the necessary "apical"
stability. If neither of these possibilities are at hand,
stability of the "apical" part can sometimes be
achieved by placing it in a layer of buccal or, more
often, lingual bone cortex. When an apically located
cortical layer cannot be used for anchoring the im-
plant, a relatively narrow width of the jaw bone in
combination with a thick, cortical marginal border
may provide proper conditions for immediate im
plant
stability. On the other hand, a wide alveolar bone
with
a thin layer of compact bone at the alveolar crest
often
provides less than optimal conditions for im
plant
treatment. However, the presence of thick trabeculae
in the spongious bone can provide the neces
sary
conditions for good primary stability. Adequate
information about bone width and bone content con-
sequently is of importance in the planning of where
and how to place implants. While bone width can be
determined by tomography, the number and size of
bone trabeculae can be difficult to evaluate. The best
information is provided by the intraoral radiographs
(
Lindh et al. 1996a) but it must be remembered that
the trabecular pattern seen in these images primarily
reflects the conditions in the junctional area between
compact and trabecular bone (van der Stelt 1979,
Lindh et al. 1996b). Thus, the presence in the radio-
graph of a trabecular pattern is no guarantee that bone
trabeculae will be found in the interior parts of the jaw
bone. On the other hand, the absence of such a pattern
strongly indicates a definite absence of bone trabecu-
lae. In such cases, nutrient canals are frequently seen.
Absence of bone trabeculae and presence of nutrient
canals are also a frequent finding in alveolar processes
of a narrow buccolingual dimension.
An accurate estimate of the distance between the
marginal bone crest and the lower border of the nasal
cavity or the maxillary sinus is necessary in order to
select implants of appropriate lengths for placement
in the maxilla. Rather than choosing an implant that
does not reach the border, an implant should be used
that just penetrates the cortical border to obtain the
necessary anchorage.
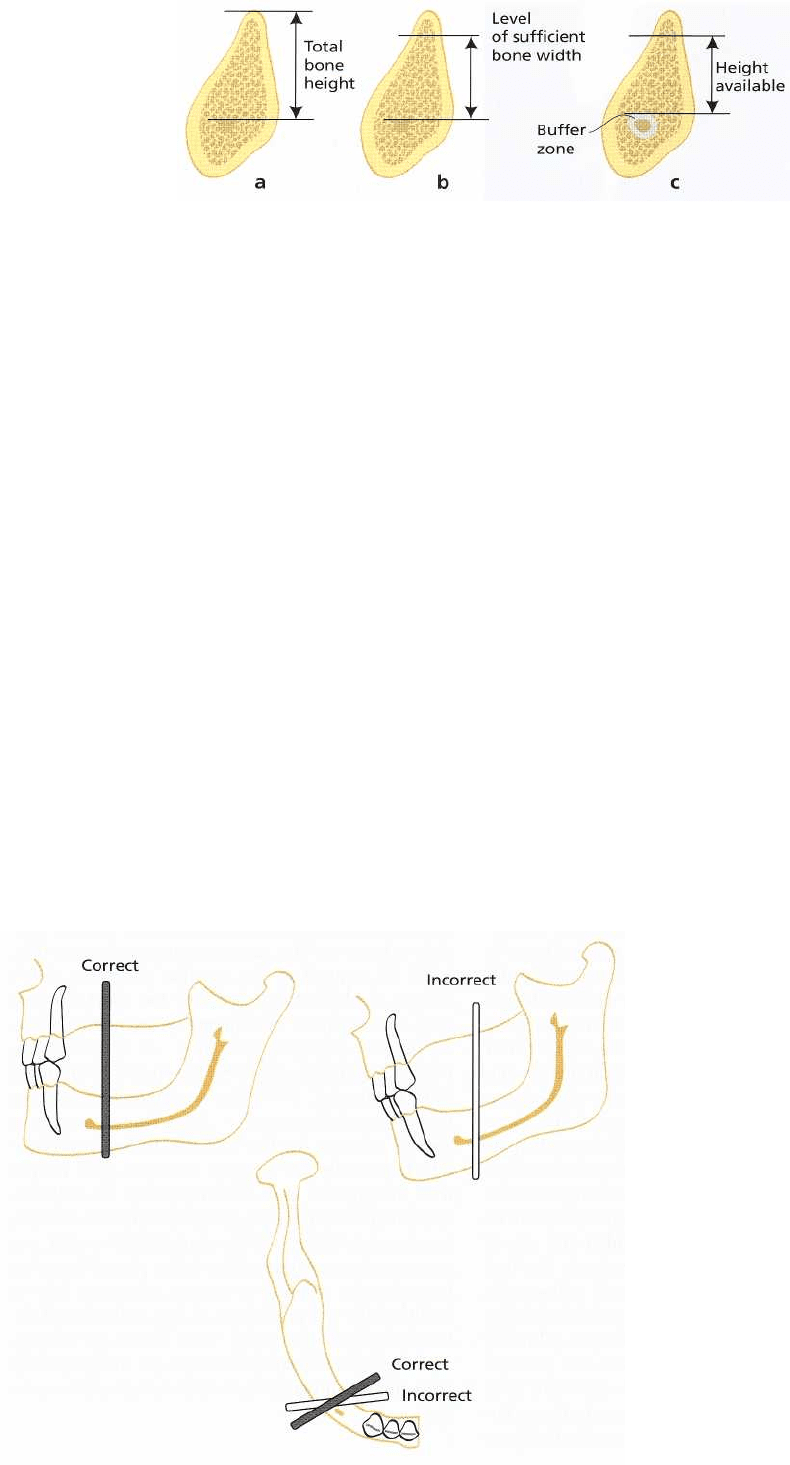
In the mandible, the distance between the marginal
bone crest and the upper border of the mandibular
canal must be determined with great accuracy so that
the insertion of implants, or the preparation preceding
the insertion of an implant, does not interfere with the
infra-alveolar neurovascular bundle. If it does, perma
-
nent paresthesia may follow. Since this is a serious
complication, the risk for it must be minimized. Only
cross-sectional tomography can provide a good
enough depiction of the mandibular canal and pro-
vide a basis for the necessary measurements (Fig.
36-
5). In contrast to the maxilla, one must rather choose
an implant that is a little too short than too
long.
Radiographic measurements are neither so ac-curate
nor so precise that they can be completely
trusted (
Grondahl et al. 1991, Ekestubbe & Grondahl
1993,
Lindh et al. 1995). One must therefore decrease
the
calculated distances by 1-2 mm. Due account must
also be taken of the fact that the drilling procedure
which precedes the implant insertion goes deeper
than the implant itself. One must also take into ac-
count that the upper part of the implant cannot always
be placed at the level of the marginal bone crest, e.g.
in cases when an implant has to be placed buccal or
lingual to the upper bone margin or when a narrow
width of the marginal bone makes reduction of the
bone height necessary (Fig. 36-6). Hence, the need for
a reference measurement between the marginal bone
crest and the upper border of the mandibular canal is
obvious.
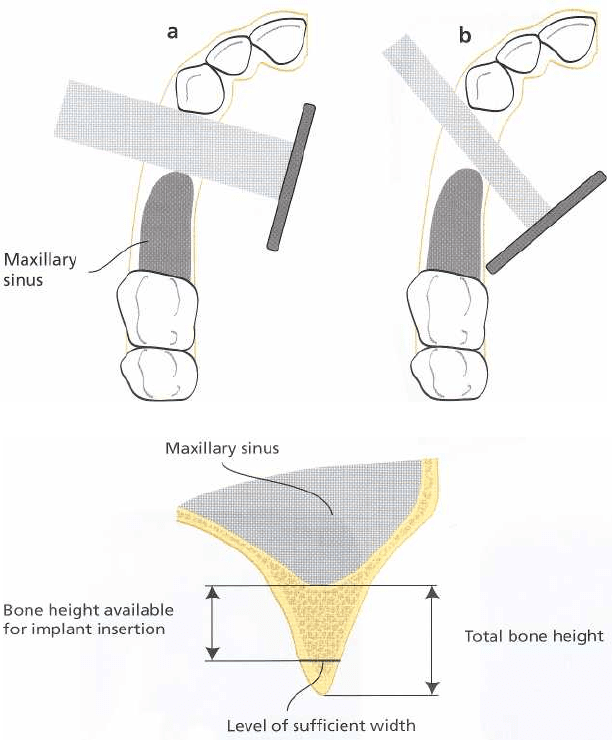
842 • CHAPTER 36
Fig. 36-6. The total height between the upper border of the mandibular canal and the marginal bone crest can be
used as a reference during surgery (a). The total bone height may have to be reduced due to too narrow a
marginal
bone width (b). To decrease the risk for nerve damage, the bone height measurements should be further
reduced (c).
Conclusion
A comprehensive clinical examination must precede
the radiographic examination. The latter should be
done with techniques yielding the lowest possible
doses, yet all clinically necessary information.
Failure to diagnose and treat pathologic conditions
in and around remaining teeth can seriously compro-
mise the results of implant therapy.
The intra-oral paralleling technique is recom-
mended for an estimate of horizontal dimensions of
the
intended implant site, and for a preliminary esti
mate
of its vertical dimensions.
When panoramic techniques are used, supplemen-
tary intraoral radiographs should always be obtained
when horizontal distances are critical.
For the best estimate of height and width of the
implant site, cross-sectional tomography should be
carried out.
To avoid damages of the infra-alveolar neurovascu-
lar bundle, a safety margin should be applied to the
calculated distances between the marginal bone crest
and the upper border of the mandibular canal.
Requirements for cross-sectional tomography
In cross-sectional tomography with conventional to-
mographic techniques (motion tomography) one
must
take into account the curvature of the jaws. For
each
intended implant site, the placement of the to-
mographic layers must be individualized. This is pos-
sible with some computer-operated tomographic
units
because of special software. Other units require
that
the patient is moved and his/her head rotated in
the
horizontal plane to achieve the appropriate posi
tion. A
correct position of the tomographic layer im
plies that it
is perpendicular to the tangent of the jaw
curvature
and to a horizontal reference plane (Fig.
36-7). In the
maxilla the reference plane is the hard
palate, in the
mandible the mandibular canal, which
in the premolar
to first molar region often runs paral
lell to the base of
the mandible. To obtain correct
positions of the
tomographic layer relative to one of
these horizontal
reference planes the patient's head
may have to be
slightly tilted forwards or backwards.
The latter is the
case for a tomographic examination
in the mandibular
premolar and molar regions. Incor
rect angulation of
the tomographic layer decreases the visibility of the
cortical bone plates and the mandibu-
Fig. 36-7. In cross-sectional to-
mography a proper placement of
the tomographic layers is essential
for correct estimates of bone
height and width.

RADIOGRAPHIC EXAMINATION • 843
lar canal and can lead to incorrect estimates of both
bone height and width (Grondahl et al. 1996).
Implants in the premolar and molar regions
In the maxillary premolar and molar regions, the ex-
tension of the maxillary sinus limits the amount of
bone available for implant placement both in the hori-
zontal and the vertical direction. In cases where teeth
remain in the anterior part of the jaw, but are missing
posteriorly, one must be able to determine the number
of implants that can be inserted between the most
posteriorly positioned tooth and the anterior border
of
the maxillary antrum. An estimate of the horizontal
dimension of the potential implant site can be made
from panoramic or intraoral radiographs. If the im-
plant site is within a curved part of the jaw, measure-
ments from panoramic radiographs can be inaccurate.
Orthoradially obtained radiographs are therefore to be
preferred. These can also be used for a preliminary
estimate of the available bone height between the
marginal bone crest and the lower border of the max-
illary sinus. When the film cannot be placed parallel
to
the vertical axis of the alveolar process, the vertical
measurements are best made in the panoramic image.
The bone height actually available for implant in-
sertion also depends upon the width of the alveolar
process. For example, when the bone width is too
narrow in the marginal part of the alveolar process,
the
bone height has to be surgically reduced until a
level
is reached where the bone width is sufficient for
implant placement. To determine the height of the
bone in areas with proper width, supplementary to-
mography is often needed. This can also establish
whether bone of sufficient width is present lingual to
the maxillary sinus (Fig. 36-8), which neither intraoral
nor panoramic radiographs can reveal (Grondahl et al.
1996). To be able to give as accurate information as
possible about the height and width of the jaw bone,
the tomographic layers must be perpendicular to the
hard palate and to the tangent of the jaw curvature.
Because of the shape of the dental arch, each side and
region must be examined with individual adjustments
of the direction of the X-ray beam (Eckerdal & Kvint
Fig. 36-8. Tomography can reveal whether bone of suffi
-
cient width for implant placement is present on the lin
-
gual side of the maxillary sinus.
1986). To obtain the best possible image quality the
amount of scatter radiation should be small. This is
achieved by narrowly collimated X-ray beams that
also
reduce the radiation dose to the patient.
In the premolar and molar regions of the mandible,
the position of the mental foramen and the mandibu-
lar canal must be identified. If there is a certain mini-
mum distance between a tooth and the anterior border
of the mental foramen, it may be possible to insert an
implant between the tooth and the foramen. Because
the mental foramen on the one hand and the root of
an anteriorly positioned tooth on the other are at
different distances from the X-ray source, the horizon-
tal distance between the tooth and the foramen can be
misinterpreted. In images taken from a mesio-oblique
direction the distance can appear too long, while it can
appear too short in images taken from a disto-oblique
direction. Therefore, the X-ray beam must be perpen-
dicular to the tangent of the dental arch in the area
between the foramen and the anteriorly positioned
tooth (Fig. 36-9).
The mandibular canal often makes a more or less
Fig. 36-9. To enable a correct estimate of the distance between the mental foramen and an anteriorly positioned
tooth, a correct horizontal angulation of the X-ray beam (a) is essential. Incorrect angulations can make the
distance
appear too large (b) or too small (c).
8
44 • CHAPTER 36
Fig. 36-10. Cross-sectional tomography of a mandibu
-
lar molar region revealing a deep lingual concavity
anteriorly convex loop before it reaches the mental
foramen. To avoid damaging the nerve, the distance
between the anterior border of the mental foramen and
the tooth must allow for some safety margin
between
the foramen and an implant.
The insertion of implants above the mandibular
canal should be preceded by a radiographic evalu
ation
which provides information not only on the
width of
the jaw bone and the distance between the
upper
border of the mandibular canal and the mar
ginal
bone crest, but also of the cross-sectional shape
of the
jaw. Not infrequently, the width of the jaw is
limited by
lingual concavities, most notably the sub-
mandibular
gland fossa within which a branch from the facial
artery can be found. Failure to take account
of this
cavity can lead to a lingual perforation of the
mandible
during surgery and damage to the artery.
The
subsequent bleeding can be life threatening
(
Bruggenkate et al. 1993). Failure to observe the some-
times pronounced lingual inclination of the posterior
part of the alveolar process can also lead to uninten-
tional perforation of the lingual border of the mandi-
ble. The necessary information about lingual concavi-
ties or a marked lingual inclination of the posterior
part of the mandible can only be obtained through
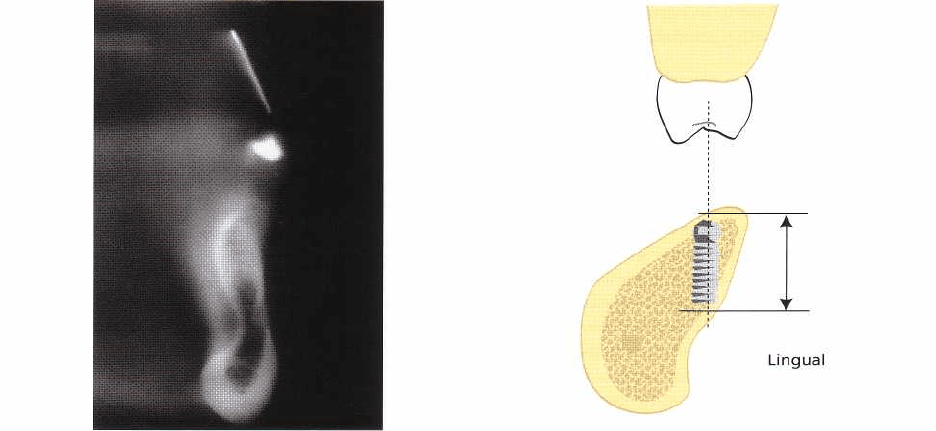
cross-sectional tomography (Fig. 36-10). This not only
prevents serious complications, it also provides infor-
mation about where sufficient amount of bone can be
found in which the apical part of the implant can be
anchored (Fig. 36-11). Tomographic images which in-
clude the crowns of the teeth in the maxilla also pro-
vide guidance for an appropriate buccolingual place-
ment and inclination of the mandibular implant.
Regardless of technique used, the tomographic lay-
Fig. 36-11. Cross-sectional tomography of the posterior
parts of the mandible can reveal a lingual inclination of
the alveolar process and the presence of a lingual fossa.
It can also be used to determine proper implant place-
ment for good primary stability as well as optimal
placement and inclination relative to teeth in the max-
illa.
ers should be placed as perpendicular as possible to
the mandibular canal and to the tangent of the jaw
curvature. This provides the most distinct images of
the canal and the borders of the mandible while offer-
ing the best possibilities for reliable measurements.
Although it is true that the mandibular canal occa-
sionally can be difficult to perceive even in high quality
tomograms (Lindh et al. 1992) the combination of
intraoral, panoramic and tomographic images usually
provides a solid radiographic foundation for sub-
sequent treatment decisions.
Conclusion
The horizontal dimension of an intended implant site
can be determined from intraoral or panoramic radio-
graphs. In curved parts of the dental arch, measure-
ments in panoramic radiographs can be inaccurate
due to distortions.
Preliminary estimates of the bone height can be
made in intraoral radiographs, provided that a paral-
leling technique has been used, or in panoramic radio-
graphs.
Determination of the bone height actually available
is best made in cross-sectional tomograms in which
the width of the jaw bone also can be determined.
Cross-sectional tomography should be done per-
pendicular to the tangent of the dental arch and per-
pendicular to a horizontal reference plane, the hard
palate for maxillary examinations and the base of the
mandible for mandibular examinations.
To determine the distance between the mental fora-
men and an anteriorly positioned tooth, intraoral ra-
diographs should be obtained with an X-ray beam
RADIOGRAPHIC EXAMINATION •
8
45
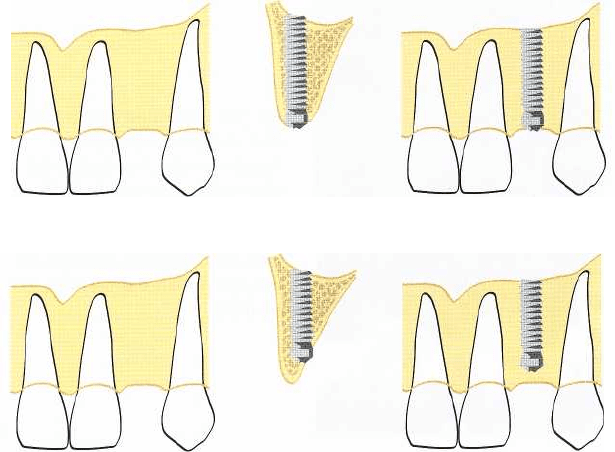
Fig. 36-12. The eventual position
of the marginal part of an implant
depends on the width of the mar-
ginal bone. Tomography can re-
veal where sufficient bone width
is located and, thus, where the
marginal part of the fixture will
become placed once the necessary
bone reduction has been made.
direction perpendicular to the tangent of the dental
arch.
Cross-sectional tomography in the mandible is
needed to determine the distance between the mar-
ginal bone crest and the upper border of the mandibu
-
lar canal as well as the presence of lingual concavities
and the inclination of the alveolar process.
Conventional versus computed tomography
Conventional tomography is to be preferred for im-
plant treatment in the partially dentate patient not
least because computed tomography delivers consid-
erably larger radiation doses (Clark et al. 1990, Ekes-
tubbe et al. 1993, Fredriksen et al. 1995). For conven
-
tional tomography, equipment with which multidirec-
tional tomography – hypocycloidal or spiral – can be
performed provides the best image quality due to less
disturbing ghost shadows from surrounding struc-
tures (Curry et al. 1990). When computed tomography
has to be done, because of lack of conventional to-
mographic equipment, lower doses can be achieved
through a lowering of the X-ray tube current (Ekes-
tubbe et al. 1996). If possible, so-called direct com-
puted tomography should be done instead of axial
tomography, which requires subsequent reformatting
to obtain cross-sectional images. Direct computed to-
mography, in which the scan planes are positioned as
in conventional tomography, requires a careful, indi-
vidual positioning of the patient relative to each re-
gion of interest, to obtain high quality images (Lindh
et al. 1995, Grondahl et al. 1996). In many patients this
can be difficult to achieve. In addition, the presence of
metal components within the scan plane can cause
disturbing artifacts.
Conclusion
Conventional tomography delivers lower doses than
computed tomography and is therefore to be pre-
ferred.
Multidirectional tomography provides the best im-
age quality due to a smaller amount of ghost shadows
from surrounding structures.
Lower doses in computed tomography can be
achieved by a lowering of the X-ray tube current.
In computed tomography, direct techniques pro-
vide better image quality than techniques requiring
image reformatting. However, direct techniques can
be difficult to use clinically.
The single implant case
When an implant is to be inserted between neighbor-
ing teeth, the distance between the opposing root
surfaces must be determined to make sure that the
implant does not become placed too close to either
tooth. It has been shown that marginal bone loss can
occur at the approximal bone surfaces facing the im-
plant and that this bone loss becomes more pro-
nounced, the closer to the tooth the implant is placed
(
Esposito et al. 1993, Andersson et al. 1995). Another
factor which can affect the marginal bone at adjacent
teeth is the vertical position at which the most mar-
ginal part of the implant is positioned relative to the
neighboring teeth. A long vertical distance between
the level of the marginal part of the implant and the
bone level at adjacent root surfaces is unfavorable and
the more so, the smaller the horizontal distance be-
tween the implant and the root surface. In a region
with a narrow marginal width of the alveolar bone,
and therefore a need to reduce its height to reach a
bone level of sufficient width, the eventual vertical
position of the marginal part of the implant can be best
predicted through a preceding tomographic examina-
tion (Fig. 36-12).
The most accurate estimation of the distance be-

846 • CHAPTER 36
Fig. 36-13. The most correct infor-
mation about the distance be-
tween opposing root surfaces is
obtained from an orthoradially ob-
tained radiograph relative to the
region of implant placement (a). A
mesio-oblique beam direction can
make the distance appear too
small
(b).
Fig. 36-14. If the horizontal distance between a tooth
and the incisive canal appears too small for implant
placement, tomography may reveal sufficient bone
width anterior to the incisive canal.
tween neighboring root surfaces is made from or-
thoradially obtained intraoral radiographs (Fig. 36-
13).
Panoramic radiographs can easily depict such a
distance
incorrectly and make it either too large or too small. This
is especially the case in the anterior regions
of the jaws
and depends on incorrect patient position
ing in the
panoramic machine (Grondahl et al. 1996).
In the
anterior part of the maxilla, one must also take
the
proximity of the incisive canal into account. In
cases
where the mesiodistal distance between the ca-
nal and
the intended implant site appears too small in
an
intraoral radiograph, tomography can be used to
determine whether sufficient amount of bone is pre-
sent
buccal to the canal (Figs. 36-14, 36-15).
Tomography within a small region surrounded by
teeth can lead to disturbing ghost shadows from the
Fig. 36-15. Intraoral radiographs (a) in which the distance between the lateral incisor and the incisive canal appears
too small for implant placement. A tomographic examination (b) reveals sufficient bone width anterior to the canal.
