Jan Lindhe. Clinical Periodontology
Подождите немного. Документ загружается.

PERIODONTITIS AS A RISK FOR SYSTEMIC DISEASE • 373
Hujoel and co-workers evaluated the same popula-
tion cohort as DeStefano and colleagues but for a
longer 21-year follow-up. The Hujoel et al. study ex-
tensively adjusted for possible confounding factors,
and this may account for the lack of any detected
significant relationship after adjustment. It is also
likely that significant misclassification occurred in the
periodontal status over time, with those classified as
having no periodontal disease at baseline developing
periodontal disease over the 21 years of study. Lastly,
misclassification of those having periodontal disease
at baseline may have occurred over time as a result of
treatment and extractions (Hujoel et al. 2000).
Table 16-3 summarizes the strength and consis-
tency of associations for oral conditions as risk factors
for coronary heart disease. It is interesting to note the
consistency of the associations across studies that
used different study designs and a variety of measures
for both the exposure and outcome.
Specificity of the associations between
periodontitis and coronary heart disease
The specificity of the associations between oral condi-
tions and heart disease are summarized in Table 16-4.
The associations in these studies have been adjusted
for established risk factors for atherosclerosis and
coronary heart disease, indicating that the associa-
tions found are not confounded by those factors. The
student should remember that the criterion of speci-
ficity suggests that the condition being studied such
as periodontitis is more likely to be a risk factor if it is
specifically related to a single disease and not multiple
diseases. Data relating periodontitis as a risk for pre-
term low birthweight infants, diabetes and other sys-
temic conditions suggest that periodontitis does not
fully meet the criterion of specificity. However, it is the
combined weight of the evidence that is most impor-
tant. It is of course possible for an exposure to be a risk
factor for multiple conditions. Cigarette smoking is
one such example (Beck et al. 1998).
Correct time sequence
Table 16-5 presents data which examine the time se-
quence for an association between oral conditions and
atherosclerosis/coronary heart disease. Because the
reviewed case control studies conducted by Matilla
and co-workers (1989, 1993) selected subjects on the
basis of diagnosed coronary heart disease, they lack
information on whether periodontitis preceded the
cardiovascular diagnosis. The Matilla et al. (1995)
study is a follow-up of patients who were hospitalized
with myocardial infarction; therefore the disease was
present prior to oral status measurement. The DeSte-
fano et al. study is a cohort study so it is assumed that
any NHANES I subjects who had heart disease at
baseline were not included in the analysis. The Beck
and co-workers study enrolled only participants who
were systemically healthy, so oral disease present at
baseline did not precede the development of the car-
diovascular outcome. The outcome variable in the
Genco et al. study was new cardiovascular disease.
The outcome variable in the Joshipura et al. study was
new coronary heart disease. Thus there are at least a
few studies that meet the time sequence criterion for
establishing periodontitis as a risk for coronary heart
disease (Beck et al. 1998, Garcia et al. 2001).
Degree of exposure
The degree of exposure of the suspected risk factor is
considered important in establishing causation be-
cause increasing dose levels of the purported risk
factor or exposure should lead to increasing prob-
abilities for the disease.
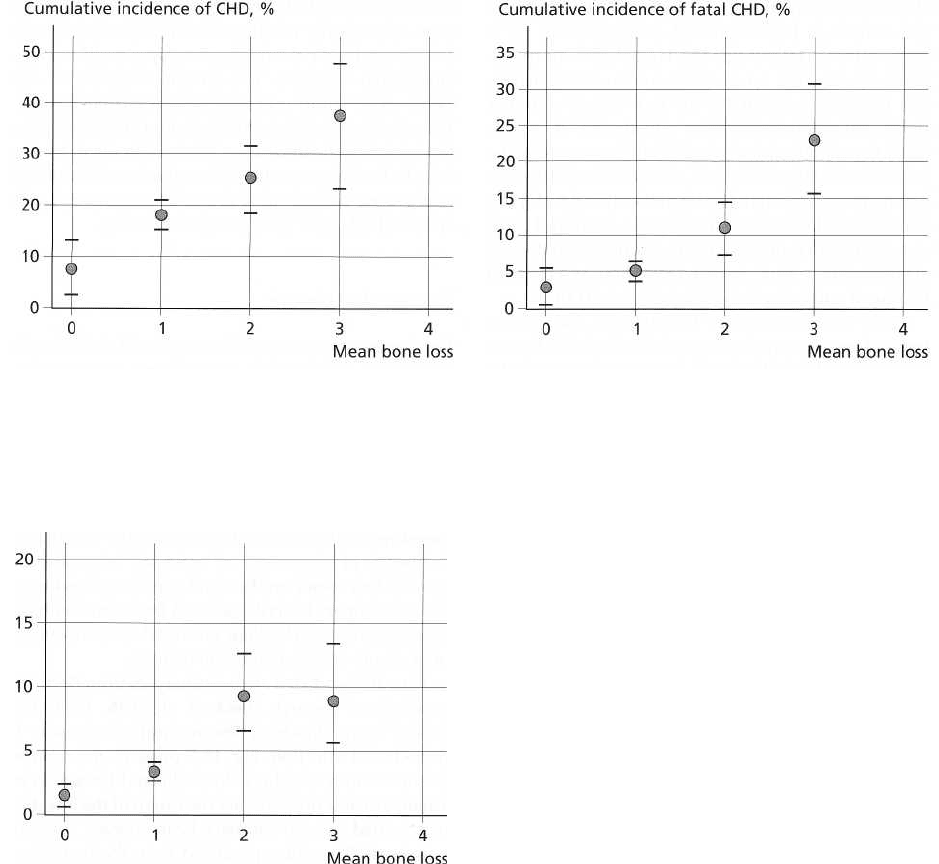
Figs. 16-4, 16-5 and 16-6 are taken from Beck and
co-workers' reports (Beck et al. 1996, 1998). One can
note that with increasing periodontitis severity as
measured with bone loss there is a higher cumulative
frequency of occurrence of coronary heart disease.
Levels of alveolar bone loss and cumulative incidence
of total coronary heart disease and fatal coronary heart
disease indicated a biologic gradient between severity
of exposure and occurrence of disease.
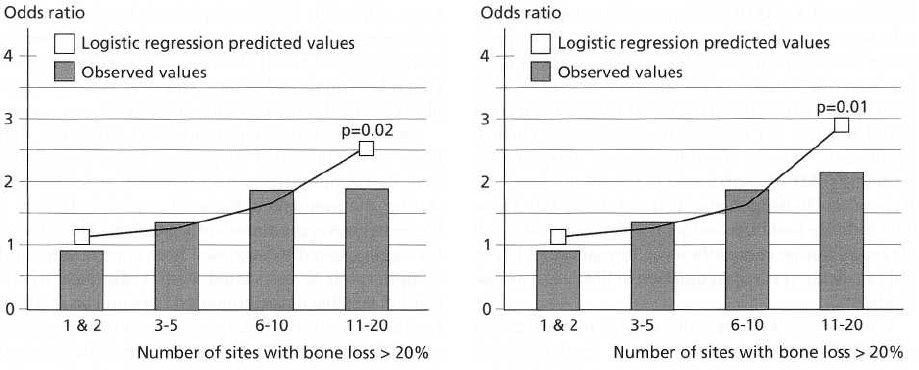
Figs. 16-7 and 16-8 are new analyses from Beck and
co-workers' research (Beck et al. 1996, 1998) that
looked more closely at the amount of exposure to
periodontal infection. Fig. 16-7 presents the number
of sites with > 20% bone loss adjusted for age in an
attempt to describe whether the extent of the infection
was related to total coronary heart disease. The line
represents the values predicted from the logistic re-
gression model, assuming a linear dose-response re-
lationship between increasing numbers of sites with
greater than 20% hone loss and total coronary heart
disease, adjusting for age. The model is significant
with a P value of 0.02 and shows that the predicted
odds ratios run from 1.04 for 1 or 2 sites up to 2.5 for
11 to 20 sites with bone loss greater than 20%.
Fig. 16-8 presents the same information, except that
the relationship between the number of sites of bone
loss and total coronary heart disease was adjusted for
all relevant risk factors. Both figures provide support
for a dose-response gradient between extent of peri-
odontal bone loss and coronary heart disease inde-
pendent of other known risk factors.
Arbes et al. (1999) recently evaluated the associa-
tion between periodontal disease and coronary heart
disease in the third National Health and Nutrition
Examination Survey (NHANES III) and found that the
odds for a history of heart attack increased with the
severity of periodontal disease. With the highest se-
verity of periodontal disease, the odds ratio was 3.8
(
95% confidence interval; 1.5-9.7) as compared to no
periodontal disease after adjustment for age, gender,
race, poverty, smoking, diabetes, high blood pressure,
374 • CHAPTER
16
None
20% 40% 60% 80%
N=74 N=812 N=199 N=31
N=2
Fig. 16-4. Age-adjusted level of bone loss and cumula
-
tive incidence of coronary heart disease.
Cumulative incidence of stroke, %
None
20% 40% 60% 80%
N=74 N=812 N=199 N=31
N=2
Fig. 16-6. Age-adjusted level of bone loss and cumula
-
tive incidence of stroke.
body mass index and serum cholesterol. This cross-
sectional study adds additional evidence for an asso-
ciation seen in the Beck et al. (1996) study, and shows
a dose response with high levels of periodontal dis-
ease associated with higher prevalence of reported
heart attack.
Biological plausibility
It has been particularly helpful in the ongoing study
of risk to try to understand the "biologic plausibility"
or scientific logic that would explain a link between
periodontitis and atherosclerosis/coronary heart dis-
ease. As noted earlier, for many years infection has
been suspected as a risk factor for atherosclerosis and
coronary heart disease (Thorn et al. 1992, Loesche
1994, Danesh et al. 1997). Other suspected etiologic
None
20% 40% 60% 80%
N=74 N=812 N=199 N=31
N=2
Fig. 16-5. Age-adjusted level of bone loss and cumula
tive incidence of fatal coronary heart disease.
infectious agents in atherogenesis include cytomega-
lovirus, herpes virus,
H. pylori
and C.
pneumonice.
The
inflammatory response to these infections, and the
large body of evidence on inflammatory pathways in
atherogenesis, certainly provide reasons to believe
that
the inflammation resulting from periodontal in-
fection and periodontitis may contribute to coronary
heart disease (Beck et al. 1998, Ross 1999).
Several investigators have suggested that there is
marked variability in individual host responses to
bacterial challenge with periodontitis (Offenbacher
1996, Beck et al. 1998, Page 1998a,b). Such variability
has been attributed to individual differences in T cell
and monocyte functions, with such differences in part
having a genetic basis. Some individuals may respond
to a microbial or lipopolysaccharide (LPS) challenge
with an abnormally high inflammatory response, as
reflected in the release of high levels of pro-inflamma-
tory mediators such as PGE
2
, IL-1(3, and TNF-u. In
laboratory tests, peripheral blood monocytes from
these hyperinflammatory monocyte phenotype pa-
tients secrete three-fold to ten-fold greater amounts of
these mediators in response to LPS than those from
normal monocyte phenotype individuals. Such obser-
vations have led to the hypothesis that the variation
in inflammatory responses may be a direct conse-
quence of at least two factors: those genes that regulate
the T cell-monocyte response, and the host-microbial
environment, which can trigger and modulate the
response (Offenbacher 1996, Beck et al. 1998, Page
1998a,b).
Beck, Offenbacher and colleagues propose that the
natural histories of both periodontitis and coronary
heart disease/atherosclerosis may in fact be related to
a common hyper-reactive inflammatory phenotype or
response. They further list several pathogenic charac-
teristics shared by the two diseases (Beck & Offen-
bacher 1998, Beck et al. 1998, Offenbacher et al. 1999):
PERIODONTITIS AS A RISK FOR SYSTEMIC DISEASE • 375
Fig. 16-7. Odds ratios and 95% confidence intervals for
number of sites with > 20% bone loss and total coro-
nary heart disease adjusted for age.
1.
Cells of the monocytic lineage and the attendant
cytokines play a critical role in initiating and propa
-
gating both atheroma formation and periodontitis.
2.
The hyper-inflammatory phenotype appears to be
under both genetic and environmental influence.
Monocytic hyper-responsiveness to LPS has been
genetically mapped tentatively to the area of HLA-
DR3/3 or -DQ which is the region where increased
susceptibility to Type 1 diabetes mellitus has been
proposed to reside. Furthermore, dietary-induced
elevation of serum low-density lipoprotein has
been shown to upregulate monocytic responses to
LPS, thereby providing a behavorial or environ-
mental influence on the macrophage phenotype.
Known risk factors for coronary heart disease such
as dietary fat intake may therefore enhance mono
-
cyte secretion of inflammatory and tissue destruc-
tive cytokines, and via this common mechanism,
may contribute to the severity of the expression of
coronary heart disease and periodontal disease.
3.
Periodontal infections may directly contribute to
the pathogenesis of atherosclerosis and throm-
boembolic events by providing repeated systemic
challenges with LPS and inflammatory cytokines.
There is also evidence that some of the bacteria found
in dental plaque may have a direct effect on
atherosclerosis and thromboembolic events. Herzberg
and co-workers have reported that the oral Gram-
positive bacterium S.
sanguis
and the Gram-negative
periodontal pathogen
P.
gingivalis
have been shown to
induce platelet aggregation and activation through
the
expression of collagen-like platelet aggregation-
associated proteins. The aggregated platelets may
then play a role in atheroma formation and throm-
boembolic events (Herzberg et al. 1983, Herzberg &
Meyer 1996, 1998).
A recent study by Haraszthy et al. identified peri-
odontal pathogens in human carotid atheromas
Fig. 16-8. Odds ratios and 95% confidence intervals for
number of sites with > 20% bone loss and total coro
-
nary heart disease adjusted for age and other relevant
risk factors.
(Haraszthy et al. 2000). Fifty carotid atheromas ob-
tained at endarterectomy were analyzed for the pres-
ence of bacterial 16-S rDNA by PCR using synthetic
oligonucleotide probes specific for the periodontal
pathogens A.a.,
B. forsythus, P. gingivalis
and
P.
interme-
dia.
Thirty per cent of the specimens were positive for
B. forsythus,
26% positive for
P.
gingival is,
18% positive
for A.
a.
and 14% positive for
P.
intermedia. Chlamydia
pneumoniae
DNA was detected in 18% of the athero-
mas. These and other studies suggest that periodontal
pathogens may be present in atherosclerotic plaques,
where, like other infectious organisms, they may play
a
role in atherogenesis (Genco et al. 1999).
Experimental evidence
There is ongoing research to see if well-controlled
prospective studies in animal models and in human
subjects can confirm or extend the evidence for a role
for periodontitis as a risk for systemic disease. Beck
and co-workers (1998) have studied the effects of P.
gingivalis
infection and high-fat diets in atheroma for-
mation in mice. Using a knock-out mouse model ge-
netically susceptible to cardiovascular disease (het-
erozygotic APOE), animals were given a high-fat or
low-fat diet. Small steel coil chambers were implanted
subcutaneously and the rejection of the chambers after
inoculation with
P.
gingivalis
HG405 was examined.
The animals were first immunized with a dose of
heat-killed
P.
gingivalis
and 21 days later challenged
with live
P.
gingivalis.
The sloughing of the chamber
would represent an intense inflammatory reaction to
the infectious agent. Initial findings suggest that a
high-fat diet in the susceptible mouse strain was asso
-
ciated with a tendency to exhibit increased risk of
serious inflammation. This initial study at least links
dietary fat intake in a susceptible mouse to an inflam
-
matory response to a periodontal pathogen,
P.
gingi-
376 • CHAPTER 16
valis.
Geva et al. (2000) in this group have reported that
infection of mice with a more virulent P.
gingivalis
strain leads to increased atheroma size and calcifica-
tion with the amount of calcification increasing with
the length of exposure. In no instance was calcification
found in mice not exposed to
P.
gingivalis.
Further,
significantly greater amounts of bone morphogenic
protein-2 (BMP-2) were found in the atheromas of the
P.
gingivalis
challenged mice (Chung et al. 2002). These
data indicate that infection with a virulent strain of P.
gingivalis
can promote atheroma formation and arte-
rial calcification via up-regulation of BMPs in a mouse
model.
When one looks at the collective evidence gathered
so far, it seems clear that, at least from historical
epidemiological data, there is a compelling link be-
tween periodontitis and coronary heart disease. There
are also emerging data which strongly suggest that
period ontitis may be a risk for pregnancy complica-
tions such as preterm low birthweight infants.
PERIODONTITIS AS A RISK FOR
PREGNANCY COMPLICATIONS
Beginning around 1996, with a landmark report by
Offenbacher and colleagues (Offenbacher et al. 1996),
there has been an increasing interest and much re-
search into whether periodontitis may be a possible
risk factor for preterm low birthweight infants.
Preterm infants who are born with low birth-
weights represent a major social and economic public
health problem, even in industrialized nations. Al-
though there has been an overall decline in infant
mortality in the US over the past 40 years, preterm low
birthweight remains a significant cause of perinatal
mortality and morbidity. Accordingly, a 47% decrease
in the infant mortality rate to a level of 13.1 per 1000
live births occurred between 1965 and 1980, but the
mortality rate and the incidence of preterm delivery
have not significantly changed since the early 1990s
(
Offenbacher et al. 1996, 1998, Champagne et al. 2000).
Preterm low birthweight deliveries represent ap-
proximately 10% of annual births in industrialized
nations and account for two-thirds of overall infant
mortality. Approximately one-third of these births are
elective while two-thirds are spontaneous preterm
births. About a half of the spontaneous preterm births
are due to premature rupture of membranes and the
other half are due to preterm labor. For the spontane-
ous preterm births, 10-15% occur before 32 weeks
gestation, result in very low birthweight (< 1500 g) and
often cause long-term disability, such as chronic respi
-
ratory diseases and cerebral palsy (Offenbacher et al.
1996, 1998, Champagne et al. 2000).
Among the known risk factors for preterm low
birthweight deliveries are young maternal age (< 18
years) drug, alcohol and tobacco use, maternal stress,
genetic background, and genitourinary tract infec
tions. Although 25-50% of preterm low birthweight
deliveries occur without any known etiology, there is
increasing evidence that infection may play a signifi-
cant role in preterm delivery (Hill 1998, Goldenberg et
al. 2000, Sobel 2000, Williams et al. 2000).
One of the more important acute exposures that
have been implicated in preterm birth is an acute
maternal genitourinary tract infection at some point
during the pregnancy. Bacterial vaginosis (BV) is a
Gram-negative, predominantly anaerobic infection of
the vagina, usually diagnosed from clinical signs and
symptoms. It is associated with a decrease in the
normal lactobacillus-dominated flora and an increase
in anaerobes and facultative species including
Gard-
nerella vaginalis, Mobiluncus curtsii, Prevotella bivia
and
Bacteroides ureolyticus.
Bacterial vaginosis is a rela-
tively common condition that occurs in about 10% of
all pregnancies. It may ascend from the vagina to the
cervix and even result in inflammation of the mater-
nal-fetal membranes (chorioamnionitis). Extending
beyond the membranes, the organisms may appear in
the amniotic fluid compartment that is shared with the
fetal lungs and/or may involve placental tissues and
result in exposure to the fetus via the bloodstream.
Despite the observed epidemiological linkage of bac-
terial vaginosis with preterm birth, the results from
randomized clinical trials to determine the effects of
treating bacterial vaginosis with systemic antibiotics
on incident preterm birth are equivocal (Goldenberg
et al. 2000). Still, there are compelling data linking
maternal infection and the subsequent inflammation
to preterm birth. It appears that inflammation of the
uterus and membranes represents a common effector
mechanism that results in preterm birth, and thus,
either clinical infection or subclinical infection is a
likely stimulus for increased inflammation.
In the early 1990s, Offenbacher and his group hy-
pothesized that oral infections, such as periodontitis,
could represent a significant source of both infection
and inflammation during pregnancy. Offenbacher
noted that periodontal disease is a Gram-negative
anaerobic infection with the potential to cause Gram
-
negative bacteremias in persons with periodontal dis
-
ease. He hypothesized that periodontal infections,
which serve as reservoirs for Gram-negative anaero-
bic organisms, lipopolysaccharide (LPS, endotoxin)
and inflammatory mediators including PGE
2
and
TNF-a, may be a potential threat to the fetal-placental
unit (Collins et al. 1994a,b).
As a first step in testing this hypothesis, Offen-
bacher and colleagues conducted a series of experi-
ments in the pregnant hamster animal model. It had
been noted earlier by Lanning et al. (1983) that preg-
nant hamsters challenged with E.
coli
LPS had malfor
-
mation of fetuses, spontaneous abortions and low
fetal weight. The work by Lanning and co-workers
clearly demonstrated that infections in pregnant ani-
mals could elicit many pregnancy complications in-
cluding spontaneous abortion, preterm labor, low
birthweight, fetal growth restriction and skeletal ab-
PERIODONTITIS AS A RISK FOR SYSTEMIC DISEASE • 377
normalities. It was not clear however if these findings
from E. coif endotoxin would be similar if endotoxin
from oral anaerobes was studied. First of all, LPS from
Gram-negative enteric organisms differs in structure
and biological activity from oral LPS. Thus, Offen-
bacher needed to demonstrate that LPS from oral
organisms had similar effects on fetal outcomes when
administered to pregnant animals. Secondly, the oral
cavity represents a distant site of infection. Although
pneumonia has been a recognized example of a dis-
tant site of infection triggering maternal obstetric
complications, it was important to demonstrate that
distant, non-disseminating infections with oral patho
-
gens could elicit pregnancy complications in animal
models. Thirdly, oral infections are chronic in nature.
Increased obstetric risk is generally associated with
acute infections that occur during pregnancy. Thus, in
concept, maternal adaptation to a chronic infectious
challenge was assumed to afford protection to the
fetus, even during acute flare-ups that may occur
during pregnancy.
Offenbacher's landmark hamster studies (Collins
et
al. 1994a,b) demonstrated that chronic exposure to
oral pathogens like
P. gingivalis
in a chamber model
(
Genco & Arko 1994) does not in fact afford protection,
but actually enhances the feto-placental toxicity of
exposure during pregnancy. Thus during pregnancy
the mother does not become "tolerant" of infectious
challenge from oral organisms. Offenbacher also
wanted to demonstrate that the low-grade infections
with low numbers of oral pathogens were not of suf-
ficient magnitude to induce maternal malaise or fever.
He noted however a measurable local increase of PG
E
2
and TNF-a in chamber fluid with
P. gingivalis
infection
as well as a 15-18% decrease in fetal weight. Further,
the magnitude of the PGE
2
and TNF-a response was
inversely related to the weight of the fetuses, mimick-
ing the intra-amniotic changes seen in humans with
preterm low birthweight. LPS dosing experiments
demonstrated that higher levels of LPS could induce
fever and weight loss in pregnant animals and re-
sulted in more severe pregnancy outcomes including
spontaneous abortions and malformations. These
more noteworthy outcomes were not seen in the low
challenge-oral infection models, but rather resulted in
a consistent decrease in fetal weight. Previous sensiti-
zation or exposures to these pathogens prior to preg-
nancy enhanced the severity of the fetal growth re-
striction when a secondary exposure occurred during
pregnancy.
Offenbacher and colleagues then went on to study
infection and pregnancy in the hamster by experimen
-
tally inducing periodontal disease in the animal
model (Collins et al. 1994b). Four groups of animals
were fed either control chow or plaque-promoting
chow for an 8-week period to induce experimental
periodontitis prior to mating. Two additional groups
of animals (i.e. one control chow and one plaque-pro-
moting chow) received exogenous
P. gingivalis
via oral
gavage. Animals fed the plaque-promoting diet begin-
ning 8 weeks prior to mating developed periodontitis.
These animals also had litters with a mean fetal weight
of 1.25 ± 0.07 g that was 81% of the weight of the
control groups. Animals receiving both plaque-pro-
moting diet and
P. gingivalis
gavage also had signifi-
cantly smaller fetuses. The mean fetal weight for this
group was 1.20 ± 0.19 g which represented a signifi-
cant 22.5% reduction in fetal weight compared to
controls. Exogenous
P. gingivalis
challenge by gastric
gavage did not appear to promote either more severe
periodontal disease or more severe fetal growth re-
striction. This experiment indicated that experimen-
tally induced periodontitis in the hamster could also
alter fetal weight in the hamster.
Other factors appear also to be involved in the
findings of low fetal weight in the hamster study.
There was a statistically significant elevation of intra-
amniotic fluid levels of both PGE
2
and TNF-a. This
finding suggests that periodontal infection can result
in a change in the fetal environment. It is possible that
both PGE
2
and TNF-a are produced by the periodon-
tium and appear in the systemic circulation to even-
tually cross the chorioamniotic barrier and finally
appear in the fluid. It is more likely, however, that
blood-borne bacterial products such as endotoxin tar
-
get the chonoamniotic plexus to trigger local PG
E
2
and
TNF-a synthesis. These animal studies by Offen-
bacher and colleagues provided important proof-of-
concept experiments and raised the possibility that
distant, low grade oral infections might also trigger
inflammation of the human maternal-fetal unit in a
manner analogous to that seen with reproductive tract
infections (Collins et al. 1994a,b).
In a subsequent landmark study, Offenbacher and
colleagues conducted a case-control study on 124
pregnant or postpartum women (Offenbacher et al.
1996). Preterm low birthweight cases were defined as
a mother whose infant had a birthweight of less than
2500 g and also had one or more of the following:
gestational age < 37 weeks, preterm labor or preterm
premature rupture of membranes. Controls were all
mothers whose infant had a normal birthweight. As-
sessments included a broad range of known obstetric
risk factors such as tobacco usage, drug use and alco
-
hol consumption, level of prenatal care, parity, geni-
tourinary tract infections and weight gain during
pregnancy. Each subject received a full-mouth peri-
odontal examination to determine clinical attachment
levels. Mothers of preterm low birthweight cases and
first birth PLBW cases had significantly more ad-
vanced periodontal disease as measured with attach-
ment loss than the respective mothers of normal birth
-
weight controls. Multivariate logistic regression mod-
els, controlling for other known risk factors and co-
variates, demonstrated that periodontitis was a statis
-
tically significant risk factor for preterm low birth-
weight, with adjusted odds ratios of 7.9 and 7.5 for all
PLBW cases and primiparous PLBW cases respec-
tively. This research by Offenbacher and colleagues
was the first to demonstrate an association between
378 • CHAPTER
16
periodontal infection and adverse pregnancy out-
comes in humans (Offenbacher et al. 1996).
Jeffcoat and Hauth have recently confirmed this
association in a larger case-control study. Gathering
data on 1313 mothers, Jeffcoat and Hauth reported
that maternal periodontitis was an independent risk
factor for preterm birth. With increasing severity of
periodontal disease as an exposure, there was an in-
creased risk for preterm birth with odds ratios ranging
from 4.45 to 7.07 for moderate to severe periodontitis,
adjusting for age, race, smoking and parity (Jeffcoat et
al. 2001a,b).
Offenbacher's group at the University of North
Carolina has been conducting a large prospective mo-
lecular epidemiological study designed to examine
the
role of maternal periodontal infections on abnor
mal
pregnancy outcomes. The principal goal of the
study
is to determine whether the presence of mater
nal
periodontal disease represents a significant inde-
pendent risk factor for preterm birth and low birth-
weight in the context of other established risk factors.
Maternal periodontal disease was assessed at baseline
(prior to 26 weeks gestation) and at postpartum to
examine for periodontal disease progression during
pregnancy. This group measured periodontal disease
as an exposure in three ways: (1) clinical signs — by
assessment of disease extent and severity (Carlos et al.
1986), (2) inflammatory response — by concentrations
of inflammatory mediators (PGE
2
and IL-1(3) within
the gingival crevicular fluid (GCF), and (3) microbial
burden — by determining levels of periodontal patho-
gens. Serum antibody responses to specific oral organ
-
isms were also measured. Extensive maternal obstet-
ric data were collected including medical, social and
clinical OB histories. The presence of bacterial vagi-
nosis as a potential covariate or confounder was as-
sessed by clinical exam, history and quantitative ex-
amination of the composition of the vaginal flora by
wet mount, Gram stain and whole chromasomal DNA
macroarray for specific indicator vaginal and cervical
organisms. Neonatal exposures and outcomes were
determined including fetal cord blood measures of
antibody to oral organisms and levels of inflammatory
mediators. Data have to date been collected in over
1200 mothers.
The findings from this large prospective cohort
study very nicely confirm and extend the case-control
observations that Offenbacher reported earlier. At
baseline, periodontal disease independently enhances
the risk of preterm birth, e.g. for moderate-severe
disease adjusted odds ratio = 3.0 for GA< 37 weeks
and odds ratio of 7.9 adjusting for race, age, socio-eco
-
nomic status, smoking, parity, bacterial vaginosis and
chorioamnionitis (Lieff et al. 2000). In addition, peri-
odontal disease increased the relative risk for fetal
growth restriction (decreased weight for gestational
age, adjusting for parity, race and baby sex) as well as
pregnancy-induced hypertension, preeclampsia and
neonatal death.
Of particular interest in this ongoing study is the
finding that not only does the presence of periodontal
disease early in pregnancy appear to confer risk, but
also if periodontal disease becomes more severe dur-
ing pregnancy, this periodontitis worsening inde-
pendently enhances the risk of preterm birth. When
periodontal disease is present both at baseline and
also progressing during pregnancy the odds ratio for
preterm birth is 10.9 adjusting for age, race, previous
preterm births, parity, smoking and social status (Lieff
et al. 2000). Clinical diagnosis of vaginosis or subclini
-
cal vaginosis chorioamnionitis did not confound the
relationship between periodontal disease and preterm
birth. Complementing these recent epidemiologic
findings, Madianos et al. (2002) have presented initial
findings of fetal immunoglobulin (IgM) specific for
periodontal pathogens. Although they detected IgM
specific to
P.
giiigivalis
and
B. fobs/thus,
among others,
in
fetal cord blood samples from both low and normal
birthweight infants, these preliminary data at least
confirm that the fetus can be challenged by maternal
periodontal bacteria and can mount an independent
host response.
PERIODONTITIS AS A RISK FOR
DIABETIC COMPLICATIONS
Similar to cardiovascular disease, diabetes mellitus is
a
common, multifactorial disease process involving
genetic, environmental and behavioral risk factors.
Affecting up to 5% of the general population and over
124 million persons worldwide (King et al. 1998), this
chronic condition is marked by defects in glucose
metabolism that produce hyperglycemia in patients.
Diabetes mellitus is broadly classified under two ma-
jor types (American Diabetes Association Expert
Committee on the Diagnosis and Classification of Dia
-
betes Mellitus 1997). In patients with Type 1 diabetes,
(formerly called insulin-dependent diabetes mellitus),
the defect occurs at the level of the pancreatic beta cells
that are destroyed. Consequently Type 1 diabetics
produce insufficient levels of the hormone insulin for
homeostasis. In contrast, patients with Type 2 diabetes
(formerly called non-insulin-dependent diabetes mel-
litus), exhibit the defect at the level of the insulin
molecule or receptor. Cells in Type 2 diabetics cannot
respond or are resistant to insulin stimulation. Diabe
-
tes mellitus is usually diagnosed via laboratory fast-
ing blood glucose levels that are greater than 126
mg/dL. Additionally, casual or non-fasting blood glu-
cose values are elevated above 200 mg/dL. Thirdly,
diabetic patients exhibit abnormal glucose tolerance
tests (i.e. blood glucose levels greater than 140 mg/dL
at 2 hours following a 100 g glucose load). Elevated
glycated hemoglobin levels (HbAI and HbA1c) com-
prise a fourth laboratory parameter and one that pro-
vides a 30 to 90-day record of the patient's glycemic
status. Classic signs and symptoms of diabetes in-
clude polyuria, polydipsia, polyphagia, pruritis,
PERIODONTITIS AS A RISK FOR SYSTEMIC DISEASE •
379
weakness and fatigue. End-stage diabetes mellitus is
characterized by problems with several organ systems
including micro and macro-vascular disease (athero-
sclerosis), retinopathy, nephropathy, neuropathy and
indeed periodontal disease.
Although environmental exposures, viral infection,
autoimmunity and insulin resistance are currently
considered to play principal roles in the etiology of
diabetes mellitus (Yoon 1990, Atkinson & Maclaren
1990), pathogenesis of the disease and end-organ
damage relies heavily on the formation and accumu-
lation of advanced glycation end products (AGES)
(
Brownlee 1994). Accordingly, the chronic hyperglyce-
mia in diabetes results in the non-enzymatic and irre
-
versible glycation of body proteins. These AGES, in
turn, bind to specific receptors for advanced glycation
end products (RAGEs) on monocytes, macrophages
and endothelial cells, and alter intracellular signaling
(
transduction) pathways (Esposito et al. 1992, Kirstein
et al. 1992). With AGE-RAGE binding, monocytes and
macrophages are stimulated to proliferate, up-regu-
late pro-inflammatory cytokines and produce oxygen
free radicals (Vlassara et al. 1988, Yan et al. 1994, Yui
et al. 1994). While oxygen free radicals directly dam-
age host tissues, pro-inflammatory cytokines like IL-1,
IL-6 and TNF-a exacerbate this damage via a cascade
of catabolic events and the recruitment of other im-
mune cells (T and B lymphocytes). Patients with dia-
betes exhibit elevated levels of AGES in tissues includ
-
ing those of the periodontium (Brownlee 1994,
Schmidt et al. 1998). Diabetics also present with ele-
vated serum and gingival crevicular fluid levels of
pro-
inflammatory cytokines (Nishimura et al. 1998, Salvi
et al. 1998). Furthermore, monocytes isolated
from
diabetics and stimulated with LPS secrete higher
concentrations of pro-inflammatory cytokines and
Prostaglandins (Salvi et al. 1998). Chronic hyperglyce
-
mia, the accumulation of AGEs and the hyper-inflam
-
matory response may promote vascular injury and
altered wound healing via increased collagen cross-
linking and friability, thickening of basement mem-
branes and altered tissue turnover rates (Weringer &
Arquilla 1981, Lien et al. 1984, Salmela et al. 1989,
Cagliero et al. 1991). Lastly, diabetic patients exhibit
impairments in neutrophil chemotaxis, adherence
and phagocytosis, (Bagdade et al. 1978, Manoucherhr
-
Pour et al. 1981, Kjersem et al. 1988) and thus are at
high risk for infections like periodontitis.
Numerous epidemiologic surveys demonstrate an
increased prevalence of periodontitis among patients
with uncontrolled or poorly controlled diabetes mel-
litus. For example, Cianciola et al. (1982) reported that
13.6% and 39% of Type 1 diabetics, 13-18 and 19-32
years of age respectively, had periodontal disease. In
contrast, none of the non-diabetic sibling controls and
2.5% of the non-diabetic, unrelated controls exhibited
clinical evidence of periodontitis. In a classic study,
Thorstensson and Hugoson (1993) examined the se-
verity of periodontitis in patients with diabetes melli-
tus and compared severity of periodontitis with the
duration a patient had been diagnosed with diabetes.
In looking at three age cohorts, 40-49 years, 50-59 years
and 60-69 years, the 40-49 years age group diabetics
had more periodontal pockets >_ 6 mm and more ex-
tensive alveolar bone loss than non-diabetics. In this
same age group, there were also more subjects with
severe periodontal disease experience among the dia-
betics than among the non-diabetics. In noting that the
younger age diabetics had more periodontitis than the
older age diabetics, these authors reported that early
onset of diabetes is a much greater risk factor for
periodontal bone loss than mere disease duration.
Safkan-Seppala & Ainamo (1992) conducted a cross-
sectional study of 71 Type 1 diabetics diagnosed with
the condition for an average of 16.5 years. Diabetics
identified with poor glycemic control demonstrated
significantly more clinical attachment loss and radio-
graphic alveolar bone resorption as compared to well-
controlled diabetics with the same level of plaque
control. Two longitudinal cohort studies monitoring
Type 1 diabetics for 5 and 2 years respectively docu-
mented significantly more periodontitis progression
among diabetics overall and among those poorly con-
trolled (Sappala et al. 1993, Firath 1997). Investigators
from the State University of New York at Buffalo have
published a number of landmark papers document-
ing the periodontal status of Pima Indians, a popula-
tion with a high prevalence of Type 2 diabetes melli-
tus. Shlossman et al. (1990) first documented the peri
-
odontal status of 3219 subjects from this unique popu
lation. Diagnosing Type 2 diabetes with glucose toler-
ance tests, the investigators found a higher prevalence
of clinical and radiographic periodontitis for diabetics
versus non-diabetics independent of age. This inves-
tigative group next focused on a cross-sectional analy
-
sis of 1342 dental subjects (Emrich et al. 1991). A
logistic regression analysis indicated that Type 2 dia-
betics were 2.8 times more likely to exhibit clinical
attachment loss and 3.4 times more likely to exhibit
radiographic alveolar bone loss indicative of perio-
dontitis relative to non-diabetic controls. In a larger
study of 2273 Pima subjects, 60% of Type 2 diabetics
were affected with periodontitis versus 36% of non-
diabetic controls (Nelson et al. 1990). When a cohort
of 701 subjects with little or no evidence of periodon-
titis at baseline were followed for approximately 3
years, diabetics were 2.6 times more likely to present
with incident alveolar bone resorption as compared to
non-diabetics. Taylor et al. (1998a,b) similarly re-
ported higher odds ratios of 4.2 and 11.4 for the risk of
progressive periodontitis among diabetic Pima Indi-
ans in general and poorly controlled diabetics (i.e.
with glycated hemoglobin levels > 9%) respectively.
The studies cited above reiterate diabetes as a modi
fier or risk factor for periodontitis. Recently, new data
have emerged indicating that the presence of perio-
dontitis or periodontal infection can increase the risk
for diabetic complications, principally poor glycemic
control. Taylor et al. (1996) first tested this hypothesis
using longitudinal data on 88 Pima subjects. Severe
38o •
CHAPTER
16
periodontitis at baseline as defined clinically or ra-
diographically was significantly associated with poor
glycemic control (glycated hemoglobin > 9%). Other
significant covariates in the regression modeling in-
cluded subject age, smoking, and baseline severity
and duration of Type 2 diabetes. Given these findings
the next logical question is whether periodontal treat-
ment can improve glycemic control. Several investiga-
tors have sought to answer this question using peri-
odontal mechanical treatment as the intervention
(
Seppala & Ainamo 1994, Aldridge et al. 1995, Smith
et al. 1996, Christgau et al. 1998, Stewart et al. 2001).
These studies in general have failed to detect an im-
provement in glycated hemoglobin level with scaling
and root planing alone. Grossi et al. (1997) reported
more compelling findings from an intervention trial
featuring 113 Pima Indians with Type 2 diabetes and
periodontitis who received both mechanical and an-
timicrobial treatment. At baseline, participants were
treated with scaling and root planing plus one of five
antimicrobial regimens: (1) water (placebo) rinse and
peroral doxycycline (100 mg q.d. for 2 weeks), (2)
0.
12`% chlorhexidine rinse and peroral doxycycline, (3)
povidone-iodine rinse and peroral doxycycline, (4)
0.
12% chlorhexidine rinse and peroral placebo, or (5)
povidone-iodine rinse and peroral placebo. Subjects
were evaluated using clinical, microbiological and
laboratory parameters prior to therapy and at 3 and 6
months. All treatment groups on average demon-
strated clinical and microbiological improvements;
however, those groups treated with adjunctive peroral
tetracycline exhibited significant and greater reduc-
tions in pocket depth and subgingival detection rates
for
P.
Ring=ivalis
as compared to the groups receiving
peroral placebo. Most strikingly, diabetic subjects re-
ceiving mechanical therapy plus peroral tetracycline
demonstrated significant, 10% reductions in their gly
-
cated hemoglobin levels. Two small, uncontrolled co-
hort studies with nine Type 1 diabetic-periodontitis
patients each similarly reported improvements in gly-
cemic control with combination mechanical-antimi-
crobial therapy (Williams & Mahan 1960, Miller et al.
1992). These limited lines of evidence suggest that
untreated periodontal infections may increase a dia-
betic patient's risk for poorer glycemic control and
subsequent systemic complications.
PERIODONTITIS AS A RISK FOR
RESPIRATORY INFECTIONS
There is lastly emerging evidence that in certain at risk
populations, periodontitis and poor oral health may
be associated with several respiratory conditions. Res
-
piratory diseases contribute considerably to morbid-
ity and mortality in human populations. Lower respi-
ratory infections were ranked as the third most com-
mon cause of death worldwide in 1990, and chronic
obstructive pulmonary disease was ranked sixth
(
Scannapieco 1999).
Bacterial pneumonia is either community-acquired
or hospital-acquired (nosocomial). Community-ac-
quired pneumonia is usually caused by bacteria that
reside on the oropharyngeal mucosa, such as
Strepto-
coccus
puculuouia
and
Haernophilus influenza.
Alterna-
tively, hospital-acquired pneumonia is often caused
by bacteria within the hospital or health care environ
-
ment, such as Gram-negative bacilli,
Pseudomonas
aeruginosa
and
Staphylococcus aureus
(Scannapieco
1999). As many as 250 000 to 300 000 hospital-acquired
respiratory infections occur in the US each year with
an estimated mortality rate of about 30%. Pneumonia
also contributes to a significant number of other
deaths by acting as a complicating or secondary factor
with other diseases or conditions.
Chronic obstructive pulmonary disease (COPD) is
another common severe respiratory disease charac-
terized by chronic obstruction to airflow with excess
production of sputum resulting from chronic bronchi
-
tis and/or emphysema. Chronic bronchitis is the re-
sult of irritation to the bronchial airway causing an
expansion of the propagation of mucus-secreting cells
within the airway epithelium. These cells secrete ex-
cessive tracheobronchial mucus sufficient to cause
cough with expectoration for at least 3 months of the
year over two consecutive years. Emphysema is the
distention of the air spaces distal to the terminal bron
-
chiole with destruction of the alveolar septa (Scan-
napieco 1999).
Beginning in 1992 with a report by Scannapieco's
group at SUNY-Buffalo (Scannapieco et al. 1992), sev
-
eral investigators have hypothesized that oral and/or
periodontal infection may increase the risk for bacte-
rial pneumonia or COPD.
It seems quite plausible from all the evidence re-
viewed in this chapter that the oral cavity may also
have a critical role in respiratory infections. For exam
-
ple, oral bacteria from the periodontal pocket can be
aspirated into the lung to cause aspiration pneumo-
nia. The teeth may also serve as a reservoir for respi-
ratory pathogen colonization and subsequent noso-
comial pneumonia. Typical respiratory pathogens
have been shown to colonize the dental plaque of
hospitalized intensive care and nursing home pa-
tients. Once established in the mouth, these pathogens
may be aspirated into the hung to cause infection. Also,
periodontal disease-associated enzymes in saliva may
modify mucosal surfaces to promote adhesion and
colonization by respiratory pathogens, which are then
aspirated into the lungs. These same enzymes may
also destroy salivary pellicles on pathogenic bacteria
to hinder their clearance from the mucosal surface.
Lastly, cytokines originating from periodontal tissues
may alter respiratory epithelium to promote infection
by respiratory pathogens (Scannapieco 1999).
At present, cross-sectional epidemiologic studies
from Frank Scannapieco's group in Buffalo and Cath-
erine Hayes's group in Boston are pointing to a sig-
PERIODONTITIS AS A RISK FOR SYSTEMIC DISEASE • 381
Fig. 16-9. Mean probing depth measurements of 4-5
mm have been customarily used in epidemiology stud
ies to designate a subject as being healthy or having
pe
riodontitis.
nificant association between poor oral health such as
periodontitis and pulmonary conditions. In a recent
report examining data from the third National Health
and Nutrition Examination Survey (NHANES III),
Scannapieco and Ho reported that patients with a
history of chronic obstructive pulmonary disease had
significantly more periodontal attachment loss (1.48 ±
1.35 mm) than subjects without COPD (1.17 ± 1.09
mm) p = 0.0001 (Scannapieco & Ho 2001).
SUMMARY
This chapter has examined the evidence, gathered by
many investigators since the early 1990s, which sug
-
gests that periodontitis may be a risk for certain sys-
temic conditions such as cardiovascular disease, pre
-
term low-birthweight infants, diabetes mellitus and
pulmonary disease. Collectively, the findings gath-
ered for the most part from large epidemiological data
sets, are very compelling. It would certainly appear
that at the least, in a diverse group of study subjects,
periodontitis is strongly associated with systemic con
-
ditions. But there is much yet to be done to elucidate
and understand the exact nature of the relationship of
periodontitis to a person's overall risk for systemic
disease.
The student of dentistry will note that in this chap
-
ter the measures of periodontal disease used in the
epidemiological data sets were "all over the board".
Most of the studies tried to relate some type of clinical
sign of periodontitis such as pocket depth, attachment
loss or bone loss on radiographs to a systemic condi-
tion (Fig. 16-9). Two studies used self-reported disease
and asked the subject if he/she had periodontitis with
a yes/no answer. And as was noted in this chapter,
while one study might distinguish between health
and periodontitis if pocket depths were < 4mm
(
health) or >_ 4 mm (periodontitis), another study
might use 5 mm to distinguish between health and
periodontitis. Thus in one study subjects having
pocket depths of >_ 4 mm would be diagnosed with
periodontitis while in another study that subject
would have been called healthy. Further, "self-re-
ported" periodontal disease with a yes/no answer is
likely to have considerable error such that those data
may be of limited use. Still, collectively, the epidemiol
-
ogical data point to a strong and noteworthy relation
-
ship between periodontitis and systemic conditions.
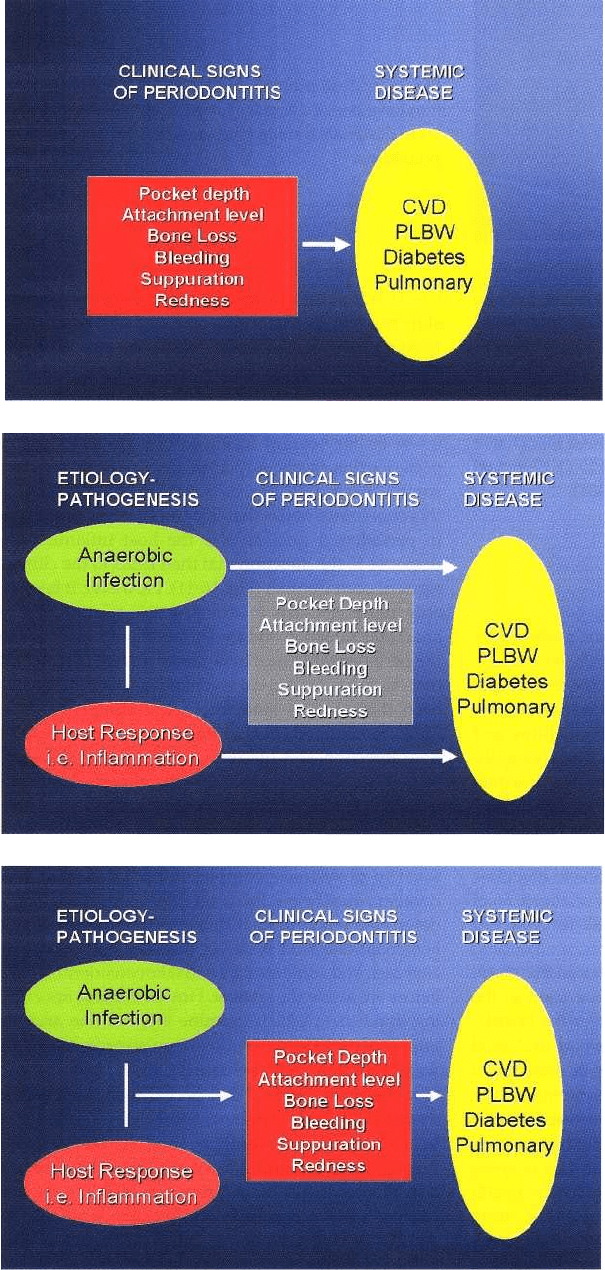
In April 2001, an AAP-NIDCR Conference was held in
Bethesda, MD, which focused on "The periodontitis-
systemic connection". One of the major points made
at this conference focused on the need for future stud
-
ies to go far beyond trying to link clinical signs of
periodontitis (such as pocket depth) with systemic
complications (Fig. 16-10). Scientists and clinicians at
this conference noted that it is the anaerobic infection
in periodontal pockets and the host inflammatory
response to the infection that then leads to the clinical
signs of periodontitis (Fig. 16-11). However, it is prob
-
ably more relevant to examine the relationship of
anaerobic infection and the inflammatory response in
the periodontal tissues to systemic disease (Fig. 16-12)
than to focus on clinical signs of periodontitis as has
been done in the epidemiological studies to date.
Thus, research workers in the future need to explore
how infection and inflammation in the periodontal
pocket may better explain the role of periodontitis as
a
risk for systemic disease.
Lastly, students of dentistry will ask, "if you treat
periodontitis, do you prevent the onset or reduce the
severity of systemic complications?" It is clear that
dentistry must now focus on intervention studies to
determine whether treating periodontitis will have a
beneficial effect on systemic diseases. This is not an
easy task and some studies will take considerable time
before we know the answer. However, there are excit
-
ing initial data which examine intervention and the
impact of periodontal treatment on preterm low birth
-
weight infants. In a preliminary study, Lamster and
his colleagues reported that periodontal intervention
decreased the risk of preterm low birthweight infants.
In a study of 195 young women at the School of
Pregnant and Parenting Teens in Central Harlem, the
overall prevalence of preterm low birthweight was
14.
6%. PLBW occurred in 18.8% of the women who
did
not receive periodontal intervention but in only
6.7%
of the women who received periodontal therapy
(
Mitchell-Lewis et al. 2000). Lopez and co-workers, in
a
noteworthy abstract, have recently reported that
periodontal therapy significantly reduces the risk of
preterm low birthweight in a study of 351 pregnant
women. The total incidence of PLBW in this cohort of
subjects was 6.26%. In women treated for periodonti
-
tis, the incidence of PLBW was only 1.84% while this
382 • CHAPTER
16
Fig. 16-10. Most studies to date
have used a clinical sign of perio-
dontitis such as probing depth to
relate periodontitis to systemic
complications.
Fig. 16-11. In addition to clinical
measures of disease severity, the
anaerobic bacterial burden and
the inflammatory response must
be taken into account in linking
periodontitis to systemic disease.
Fig. 16-12. Studying the anaerobic
bacterial burden and the inflam-
matory response may be more
critical than measures of pocket
depth in finding a relationship be-
tween periodontitis and systemic
complications.
incidence was 10.11% in untreated women. Lopez
concluded that periodontitis was an independent risk
factor for PLBW and that periodontal treatment sig-
nificantly reduced the risk of PLBW. These two studies
are compelling findings indeed and suggest that at
least
for preterm low birthweight babies, reducing
periodontal infection and disease can be very benefi
cial.
There are also studies being designed to look at
the
effect of intervention on cardiovascular disease.
Our
group, along with colleagues at Boston Univer
sity,
SUNY-Buffalo, University of Maryland and Ore
gon
Health Science University, have initiated plans for
