Jan Lindhe. Clinical Periodontology
Подождите немного. Документ загружается.

DENTAL PLAQUE AND CALCULUS • 93
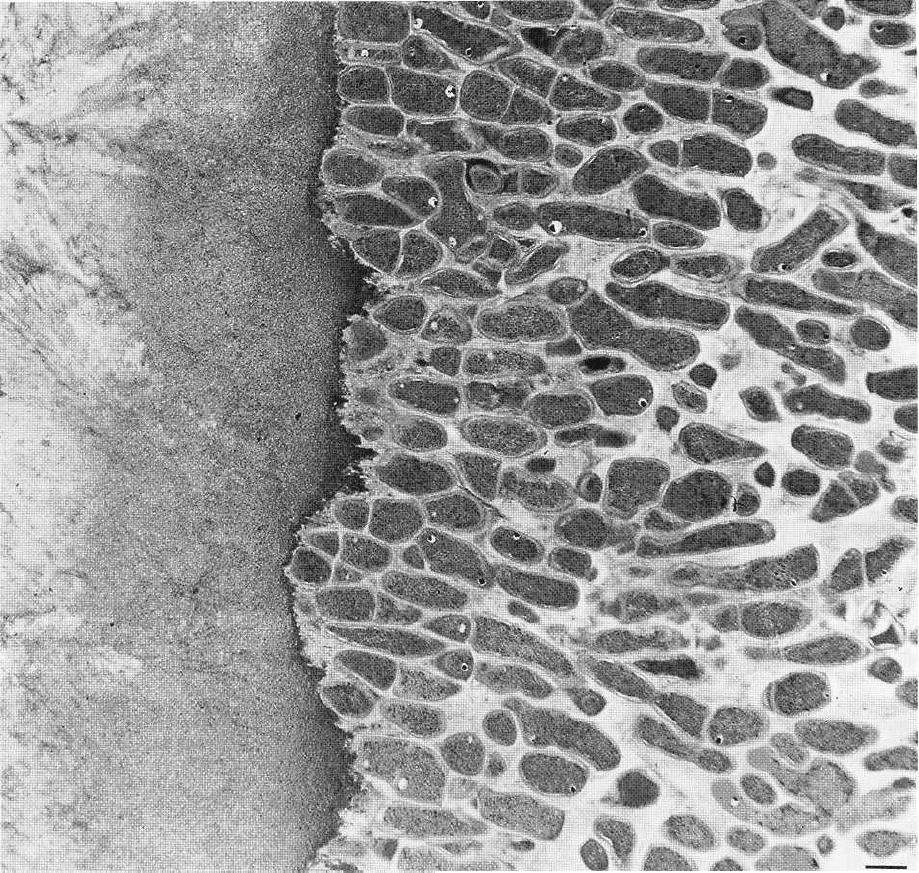
Fig. 3-27. Thin section of supragingival plaque on a root surface (to the left). The Gram-positive bacteria are ori-
ented in a palisading arrangement. Magnification x 6400. Bar: 1µm. From Listgarten (1976).
trix between them. This outer part of the microbial
accumulation in the periodontal pocket adheres
loosely to the soft-tissue pocket wall (Listgarten 1976)
.
In cases of juvenile periodontitis (Listgarten 1976,
Westergaard et al. 1978) the bacterial deposits in deep
pockets are much thinner than those found in adult
forms of periodontal disease. Areas of the tooth sur-
face in the periodontal pocket may sometimes even be
devoid of adherent microbial deposits. The cuticular
material has an uneven thickness (Figs. 3-30, 3-31).
The adherent layer of microorganisms varies consid-
erably in thickness and shows considerable variation
in arrangement. It may exhibit a palisaded organiza-
tion of the bacteria (Fig. 3-32). The microorganisms in
this layer are mainly cocci, rods or filamentous bacte-
ria, primarily of the Gram-negative type (Fig. 3-33). A
surface layer with some Gram-positive cocci, fre-
quently associated with filamentous organisms in the
typical corncob configuration, may also be found.
Subgingivally located bacteria appear to have the
capacity to invade dentinal tubules, the openings of
which have become exposed as a consequence of in-
flammatory driven resorptions of the cementum (
Adriaens et al. 1988). Such a habitat might serve as
the source for bacterial recolonization of the subgingi-
val space following treatment of periodontal disease.
The mechanisms involved in such reversed invasion
of the subgingival space are unknown.
The sequential events taking place during the de-
velopment of subgingival plaque have not been stud-
ied in man. However, in dogs, subgingival plaque
may develop in the gingival sulcus within a few days,
if oral hygiene is discontinued (Matsson & Attstrom
1979, Ten Napel et al. 1983). From these studies it has
been established that early dental plaque in the dog
has many structural similarities with that occurring in
man. This applies to the supragingival plaque (Fig. 3-
21a) as well as to the subgingival accumulation (Fig.
3-21b). The deposits may either appear as an apical
continuation of the supragingival plaque, or as dis-
94 • CHAPTER 3
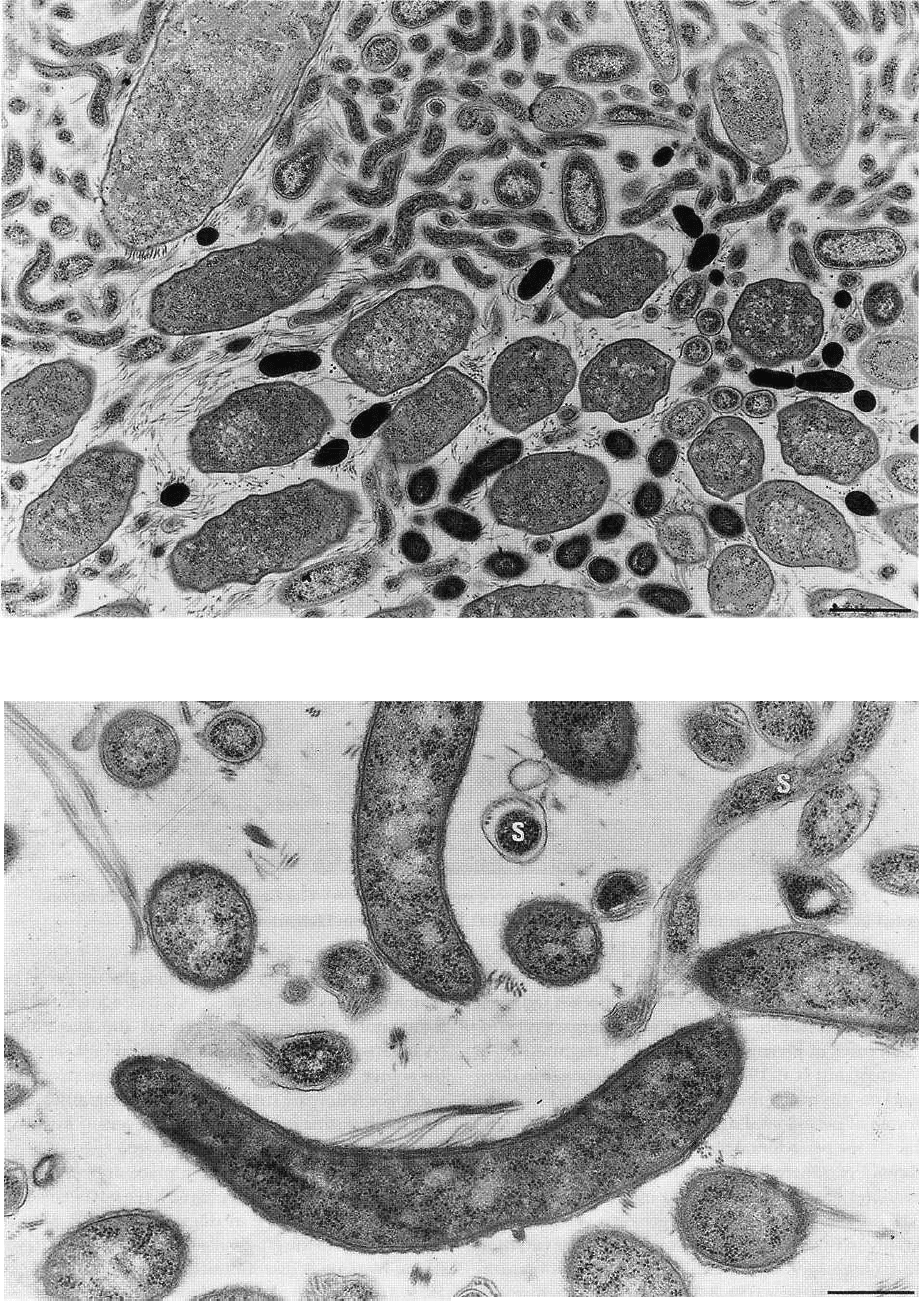
Fig. 3-28. Thin section of subgingival plaque from a deep periodontal pocket. Small microorganisms predominate,
many of which are spirochetes. Magnification x 13 000. Bar: 1 pm. From Listgarten (1976).
Fig. 3-29. Thin section of subgingival plaque from a deep periodontal pocket with many spirochetes (S), which are
recognized by their axial filaments. In the lower part of the figure is a curved organism with flagella at its concave
surface. Magnification x 25 000. Bar: 0.5 gm. From Listgarten (1976).

DENTAL PLAQUE AND CALCULUS • 95
Fig. 3-30. Thin section of deposit in deep pocket of pa-
tient with juvenile periodontitis. The cementum (C) is
covered with cuticular material and cellular remnants.
Magnification x 5500. Bar: 1 pm. From Westergaard et
al. (1978).
Fig. 3-31. Thin section of deposit in deep pocket of pa-
tient with juvenile periodontitis. A cuticle of uneven
thickness is seen to the right on the cementum. A small
colony of degenerating bacteria adheres to the cuticle
in the upper part of the illustration, and below a single
rod-shaped microorganism is partly embedded in the
cuticle. Magnification x 5500. Bar: 1 pm. From Wester-
gaard et al. (1978).
Fig. 3-32. Thin section of plaque in deep pocket of pa-
tient with juvenile periodontitis. Densely packed Gram-
positive rods grow perpendicular to the cementum to
the right in the illustration. Magnification x 23 000. Bar:
0.5 µm. From Westergaard et al. (1978).
crete aggregates at some distance from the supragingi-
val deposit. Old established subgingival plaque shows
considerable variation in bacterial composition between
dogs: in some, a subgingival microbiota dominated by
spirochetes is seen; in others, colonies of Gram-
negative cocci and rods are found in the
gingival crevice, whereas spirochetes are virtually ab-
sent (Soames & Davies 1975, Theilade & Attstrom
1985). A characteristic feature of subgingival plaque is
the presence of leukocytes interposed between the
surfaces of the bacterial deposit and the gingival sul-
cular epithelium (Fig. 3-34). Some bacteria may be
96 • CHAPTER 3
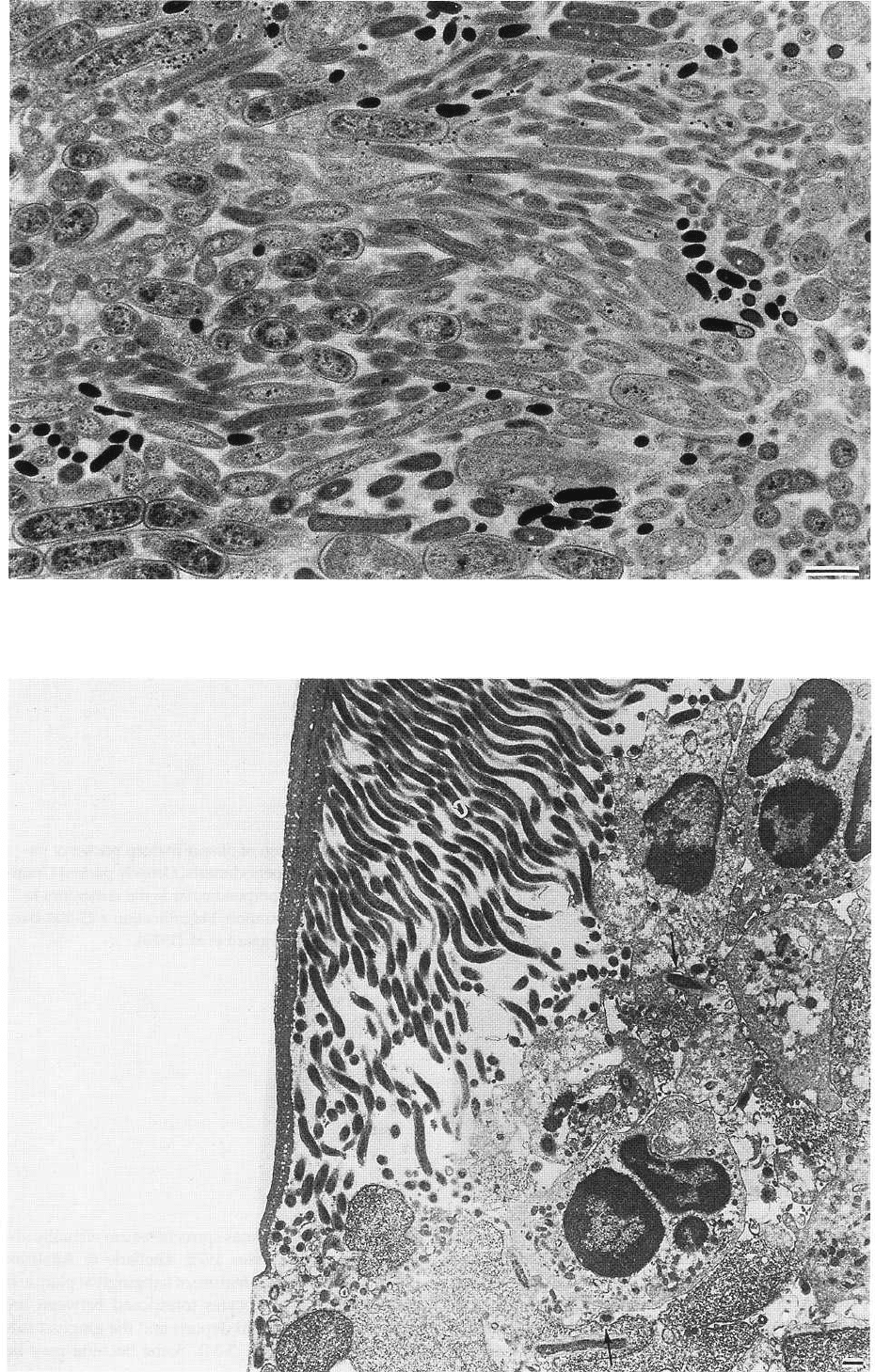
Fig. 3-33. Thin section of plaque in deep pocket of patient with juvenile periodontitis. The bacterial flora is charac-
terized by cocci, rods or filamentous organisms, primarily of the Gram-negative type. Magnification x 9200. Bar: 1
µm. From Westergaard et al. (1978).
DENTAL PLAQUE AND CALCULUS • 97
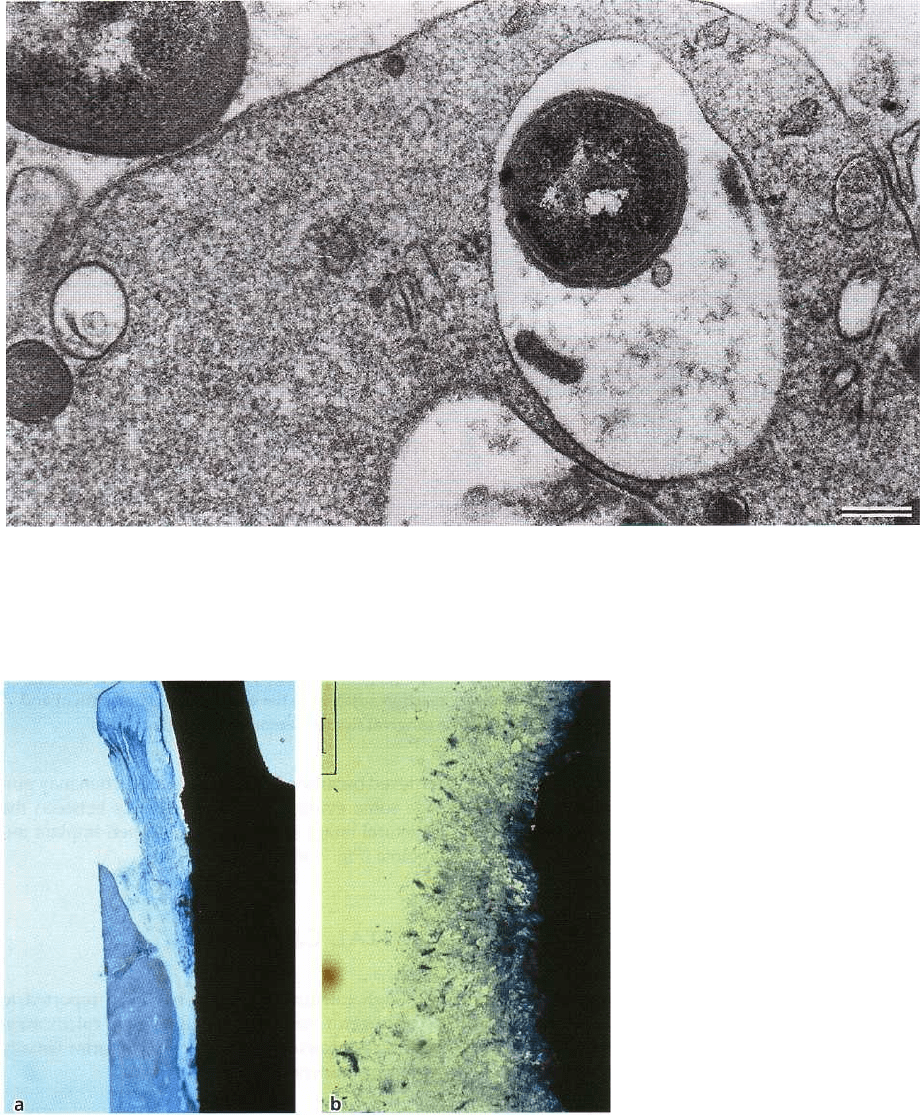
Fig. 3-35. Thin section of part of a leukocyte situated between subgingival plaque and the junctional epithelium of
the dog. The large membrane bound compartment of the leukocyte cytoplasm contains a phagocytized Gram-nega-
tive microorganism. Another bacterium is in close apposition to the cytoplasmic membrane of the leukocyte. Mag-
nification x 21 500. Bar: 0.5 km. From Theilade & Attstrom (1985).
Fig. 3-36. Peri-implant infection. (
a) Human explant of an ITI
©
den-
tal implant affected by a peri-im-
plantitis with an infrabony lesion.
Adhering plaque closely resem-
bles the structure of subgingival
microbiota encountered in ad-
vanced periodontitis. (b) Higher
magnification of plaque adhering
to the implant surface.
found between the epithelial cells. Evidence of phago-
cytosis (by polymorphonuclear leukocytes) is fre-
quently encountered (Fig. 3-35).
Although subgingival plaque formation in the dog
may not develop identically to that in man, the dog may
still serve as a convenient model for investigating the
basic phenomena governing the formation of sub-
gingival plaque (Schroeder & Attstrom 1979).
In summary, there are four distinct subgingival
ecologic niches which are probably different in their
composition:
1. the tooth (or implant) surface
2. the gingival exudate fluid medium
3. the surface of epithelial cells and
4. the superficial portion of the pocket epithelium.
Fig. 3-34. Thin section of old subgingival plaque in a dog with long-standing gingivitis. The most apical colony
consists primarily of spirochetes attached to a dense cuticle and surrounded by migrated leukocytes. Single micro-
organisms are seen between them (arrows). Magnification x 2800. Bar: 1 µm. From Theilade & Attstrom (1985).
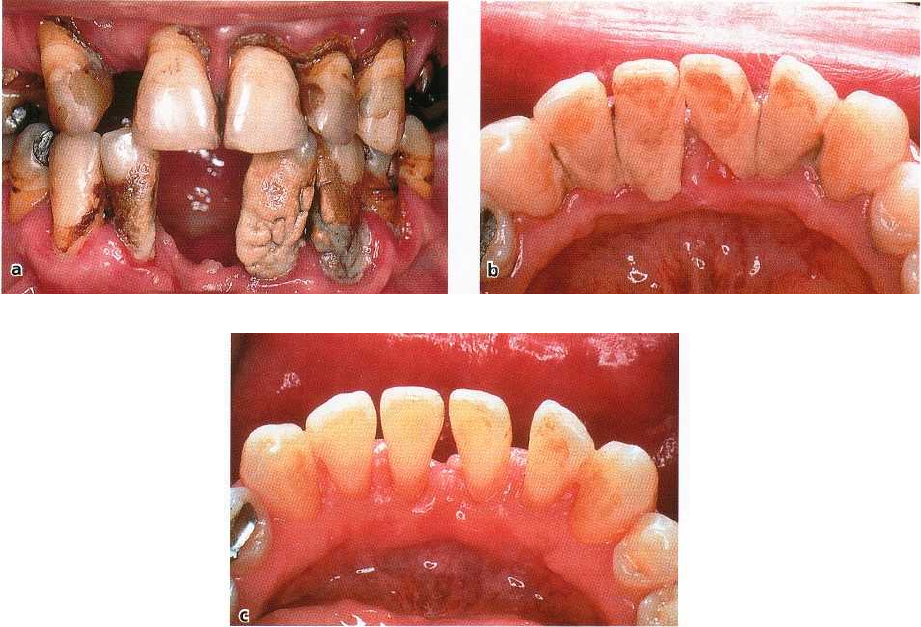
98 • CHAPTER 3
Fig. 3-37. Abundance of supragingival calculus deposits. (a) Gross deposits as a result of long-term neglect of oral
hygiene. Two mandibular incisors have been exfoliated. (b) Supragingival plaque usually covering the lingual as-
pect of mandibular incisors. Note the intense inflammatory reaction adjacent to the deposits. (c) Same patient and
region as in Fig. 3-37b following removal of the calculus. The gingival tissues demonstrate healing.
The composition of the bacteria in these niches has still
not been completely investigated. The influence of the
different bacterial compartments on the pathogenesis
of the disease process is generally unknown.
retrieved because of a peri-implant infection may pro-
vide some evidence for the similarity between the
structural image of the submucosal peri-implant mi-
crobiota (Fig. 3-36).
Peri-implant plaque
Biofilms form not only on natural teeth, but also on
artificial surfaces exposed to the oral environment. As
a consequence, the formation of bacterial plaque on
oral implants deserves some attention. Although a
number of studies have characterized the plaque de-
posits of the human peri-implant sulcus or pocket
using either dark field microscopy (Mombelli et al.
1988, Quirynen & Listgarten 1990) or microbiologic
culturing techniques (Rams et al. 1984, Mombelli et al.
1987, 1988,
Apse et al. 1989, Leonhardt et al. 1992), no
studies have attempted to document the structure of
the supramucosal or the peri-implant (submucosal)
microbiota. However, the similarities between peri-
implant and subgingival microbial deposits have
clearly been demonstrated in cross-sectional (Mom-
belli et al. 1987, 1995) and longitudinal studies (Mom-
belli et al. 1988, Pontoriero et al. 1994), and it may be
anticipated that the structure of peri-implant plaque
deposits may resemble that encountered in the sub-
gingival environment. Micrographs from an implant
DENTAL CALCULUS
Although calculus formation has been reported to
occur in germ-free animals as a result of calcification
of salivary proteins, dental calculus or tartar usually
represents mineralized bacterial plaque.
Clinical appearance, distribution and clinical
diagnosis
Supragingivally, calculus can be recognized as a
creamy-whitish to dark yellow or even brownish mass
of moderate hardness (Fig. 3-37). The degree of calcu-
lus formation is not only dependent on the amount of
bacterial plaque present but also on the secretion of
the salivary glands. Hence, supragingival calculus is
predominantly found adjacent to the excretion ducts
of the major salivary glands, such as the lingual aspect
of the mandibular anterior teeth and the buccal aspect
of the maxillary first molars, where the parotid gland
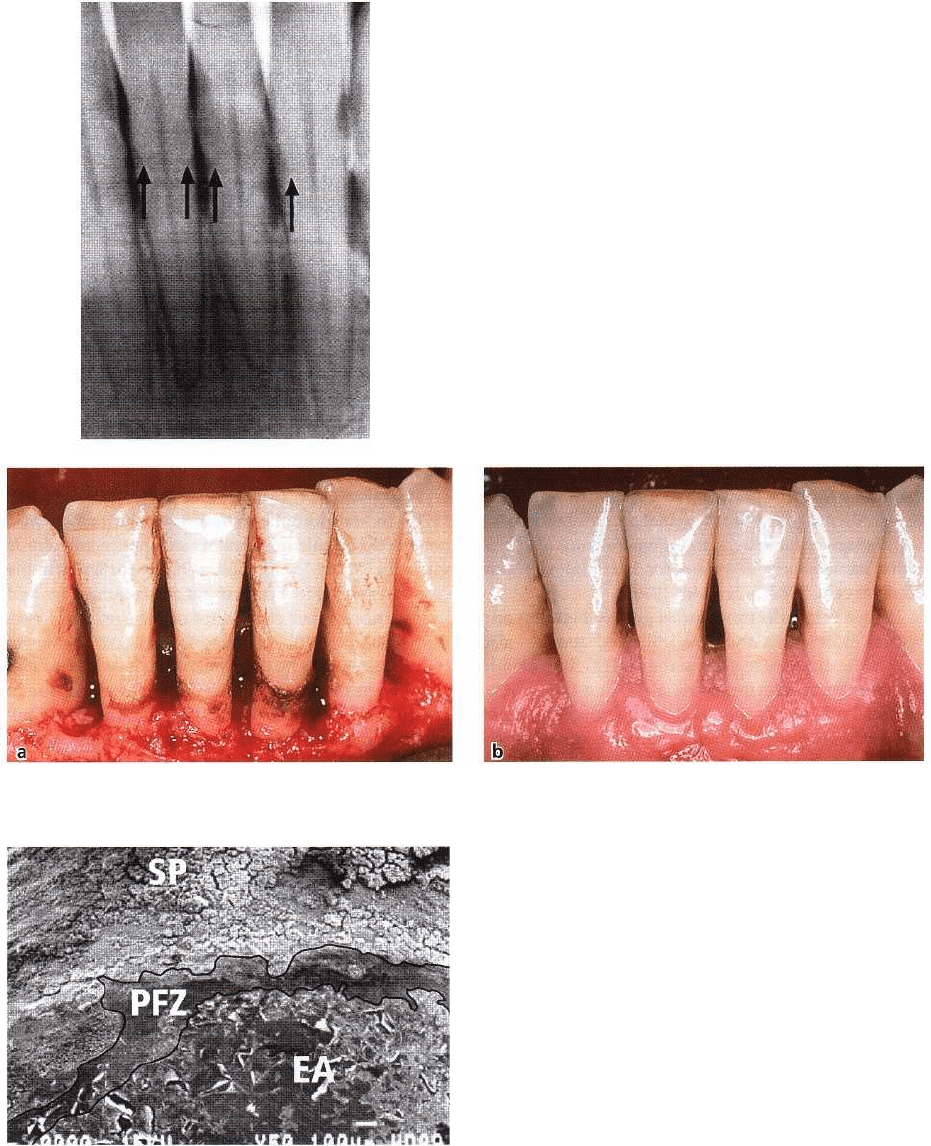
DENTAL PLAQUE AND CALCULUS • 99
Fig. 3-39. Subgingival calculus presents as a black-brownish hard mass if the gingival margin is retracted or re-
flected during a surgical procedure (a). Healing of the site following removal of all hard deposits (b).
Fig. 3-40. Plaque- and calculus-free zone coronal to the
epithelial attachment. SP: Subgingival plaque bacteria.
PFZ: Plaque-free zone. EA: Remnants of junctional epi-
thelium.
ducts open into the oral vestibule. The duct openings
of the submandibular glands are located in the former
region. It should be noted that calculus continually
harbors a viable bacterial plaque (Zander et al. 1960,
Theilade 1964, Schroeder 1969).
Subgingivally, calculus may be found by tactile
exploration only, since its formation occurs apical to
the gingival margin and, hence, is usually not visible
to the naked eye. Occasionally, subgingival calculus
may be visible in dental radiographs provided that the
deposits present an adequate mass (Fig. 3-38). Small
deposits or residual deposits following root instru-
mentation may barely be visualized radiographically.
If the gingival margin is pushed open by a blast of air
or retracted by a dental instrument, a brownish to
black calcified hard mass with a rough surface may
become visible (Fig. 3-39). Again, this mineralized
mass reflects predominantly bacterial accumulations
mixed with products from gingival crevicular fluid and
blood. Consequently, subgingival calculus is found in
most periodontal pockets, usually extending from the
cemento-enamel junction and reaching close
Fig. 3-38. Subgingival calculus may be visible (arrows)
on radiographs if abundant deposits are present.

100 • CHAPTER 3
Fig. 3-41. Seven-day-old calcified plaque. Observe the
isolated calcification centers indicated by the black ar
eas (van Kossa stain).
to the bottom of the pocket. However, a band of ap-
proximately 0.5 mm is usually found coronal to the
apical extension of the periodontal pocket (Fig. 3-40).
This zone appears to be free from mineralized deposits
owing to the fact that gingival crevicular fluid is
exudating from the periodontal soft tissues and acting
as a gradient against the microbial accumulation. Like
supragingival calculus, subgingival calculus also pro-
vides an ideal environment for bacterial adhesion (
Zander et al. 1960, Schroeder 1969).
Plaque mineralization varies greatly between and
within individuals and — as indicated above — also
within the different regions of the oral cavity. Not only
the formation rate for bacterial plaque (amount of
bacterial plaque per time and tooth surface), but also
the formation rate for dental calculus (time period
during which newly deposited supragingival plaque
with an ash weight of 5-10% becomes calcified and
yields an ash weight of approximately 80%) is subject
to great variability In some subjects, the time required
for the formation of supragingival calculus is 2 weeks,
at which time the deposit may already contain ap-
proximately 80% of the inorganic material found in
mature calculus (Fig. 3-41) (Miihlemann & Schneider
1959, Mandel 1963, Muhlemann & Schroeder 1964). In
fact, evidence of mineralization may already be pre-
sent after a few days (Theilade 1964). Nevertheless, the
formation of dental calculus with the mature crystal-
line composition of old calculus may require months
to years (Schroeder & Baumbauer 1966). Supragingi-
val plaque becomes mineralized saliva and subgingi-
val plaque in the presence of the inflammatory
exudate in the pocket. It is, therefore, evident that
subgingival calculus represents a secondary product
of infection and not a primary cause of periodontitis.
Attachment to tooth surfaces and implants
Dental calculus generally adheres tenaciously to tooth
surfaces. Hence, the removal of subgingival calculus
may be expected to be rather difficult. The reason for
this firm attachment to the tooth surface is the fact that
the pellicle beneath the bacterial plaque also calcifies.
This, in turn, results in an intimate contact with
enamel (Fig. 3-42), cementum (Fig. 3-43) or dentin
crystals (Fig. 3-44) (Kopczyk & Conroy 1968, Selvig
1970). In addition, the surface irregularities are also
penetrated by calculus crystals and, hence, calculus is
virtually locked to the tooth. This is particularly the
case on exposed root cementum, where small pits and
irregularities occur at the sites of the previous inser-
tion of Sharpey's fibers (Bercy & Frank 1980). Uneven
root surfaces may be the result of carious lesions and
small areas of cementum may have been lost due to
resorption, when the periodontal ligament was still
invested into the root surface (Moskow 1969). Under
such conditions it may become extremely difficult to
remove all calculus deposits without sacrificing some
hard tissues of the root.
Although some irregularities may also be encoun-
tered on oral implant surfaces, the attachment to com-
mercially pure titanium generally is less intimate than
to root surface structures. This in turn, would mean
that calculus may be chipped off from oral implants (
Fig. 3-45) without detriment to the implant surface (
Matarasso et al. 1996).
Fig. 3-42. Thin section of enamel surface (E) with overlying calculus. The enamel and calculus crystals are in inti
mate contact, and the latter extends into the minute irregularities of the enamel. Magnification x 37 500.
Bar:
0.1 µm. From Selvig (1970).
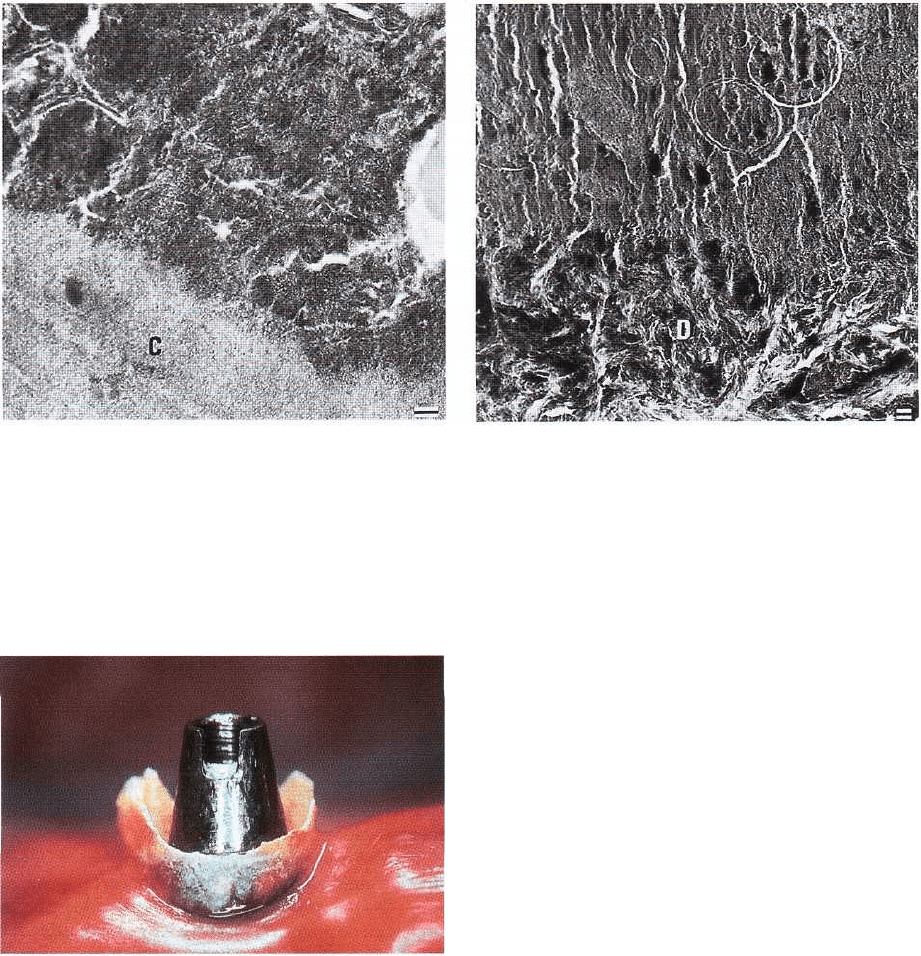
DENTAL PLAQUE AND CALCULUS • 101
Fig. 3-43. Thin section of cementum surface (C) with
overlying calculus. The calculus is closely adapted to
the irregular cementum and is more electron-dense and
therefore harder than the adjacent cementum. To the
right in the illustration, part of an uncalcified micro-
organism. Magnification x 32 000. Bar: 0.1 µ.m. From
Selvig (1970).
Mineralization, composition and structure
The mineralization starts in centers which arise in-
tracellularly in bacterial colonies (Fig. 3-46) or ex-
tracellularly from matrix with crystallization nuclei (
Fig. 3-47). Recent and old calculus consists of four
different crystals of calcium phosphate (for review see
Schroeder 1969):
1. CaH (PO4) x 2 H
2
O = Brushite (B)
2. Ca4H (PO4)3 X 2 H2O = Octa calcium phosphate
(
OCP)
3. Ca5 (PO4)3 X OH = Hydroxyapatite (HA)
4. a-Ca3 (PO4)2 = Whitlockite (W)
Supragingival calculus is clearly built up in layers and
yields a great heterogeneity from one layer to another
with regard to mineral content. On average, the min-
eral content is 37%, but ranges from 16% to 51%, with
Fig. 3-44. Thin section of dentin (D) surface with overly-
ing calculus. The interface between the calculus and
dentin cannot be precisely determined because the cal-
culus crystals fill the irregularities of the dentin sur-
face, which is devoid of cementum as a result of a pre-
vious scaling of the root surface. The circular profiles in
the calculus completely surround calcified bacteria.
Magnification x 19 000. Bar: 1 gm. From Selvig (1970).
Fig. 3-45. Calculus deposit on an oral implant in a pa-
tient without regular maintenance care.
some layers yielding a maximal density of minerals of
up to 80% exceptionally (Kani et al. 1983, Friskopp &
Isacsson 1984). The predominant mineral in exterior
layers is
OCP,
while HA is dominant in inner layers of
old calculus. W is only found in small proportions (
Sundberg & Friskopp 1985). B is identified in recent
calculus, not older than 2 weeks, and appears to form
the basis for supragingival calculus formation. The
appearance of the crystals is characteristic for
OCP
as
forming platelet-like crystals, for HA as forming
sandgrain or rod-like crystals, while W presents with
hexagonal (cuboidal, rhomboidal) crystals (Kodaka et
al. 1988).
Subgingival calculus appears somewhat more ho-
mogeneous since it is built up in layers with an equally
high density of minerals. On average the density is
58% and ranges from 32% to 78%. Maximal values of
60-80% have been found (Kani et al. 1983, Friskopp &
Isacsson 1984). The predominant mineral is always W,

102 • CHAPTER 3
Fig. 3-46. Thin section of old plaque. A degenerating or
ganism is surrounded by intermicrobial matrix in
which initial mineralization has started by the deposi-
tion of small needle-shaped electron-dense apatite crys
tals. Magnification x 26 500. Bar: 0.5 gm. From
Zander et al. (1960).
although HA has been found (Sundberg & Friskopp
1985). W contains small proportions (3%) of magnesia
(McDougall 1985).
In the presence of a relatively low plaque-pH and a
concomitant high Ca/P-ratio in saliva, B is formed
which may later on develop into
HA
and W. When
supragingival plaque mineralizes, OCP forms and is
gradually changed into
HA.
In the presence of alkaline
and anaerobic conditions and concomitant presence
of magnesia (or Zn and CO
3
), large amounts of W are
formed, which are a stable form of mineralization.
Clinical implications
Although strong associations between calculus de-
posits and periodontitis have been demonstrated in
experimental (Wrhaug 1952, 1955) and epidemiol-
ogic studies (Lovdal et al. 1958), it has to be realized
that calculus is always covered by an unmineralized
layer of viable bacterial plaque. It has been debated
whether or not calculus may exert a detrimental effect
on the soft tissues owing to its rough surface. How-
ever, it has clearly been established that surface rough
ness alone does not initiate gingivitis (Wa?rhaug
1956). On the contrary, in monkeys a normal epithelial
attachment with the junctional epithelial cells forming
hemidesmosomes and a basement membrane on cal-
culus could be established (Listgarten & Ellegaard
1973) if the calculus surface had been disinfected us-
ing chlorhexidine (Fig. 3-48). Furthermore, it has been
demonstrated that autoclaved calculus may be encap-
sulated in connective tissue without inducing marked
Fig. 3-47. Thin section of old mineralizing plaque. The
intermicrobial matrix is totally calcified, and many mi
croorganisms show intracellular crystal deposition.
Magnification x 9500. Bar: 1 gm. From Theilade (1964).
inflammation or abscess formation (Allen & Kerr
1965).
These studies clearly exclude the possibility of
dental calculus being a primary cause of periodontal
diseases. The effect of calculus seems to be secondary
by providing an ideal surface configuration condu-
cive to further plaque accumulation and subsequent
mineralization.
Nevertheless, calculus deposits may have devel-
oped in areas with difficult access for oral hygiene or
may – by the size of the deposits – jeopardize proper
oral hygiene practices. Calculus may also amplify the
effects of bacterial plaque by keeping the bacterial
deposits in close contact with the tissue surface,
thereby influencing both bacterial ecology and tissue
response (Friskopp & Hammarstrom 1980).
Well-controlled animal (Nyman et al. 1986) and
clinical (Nyman et al. 1988, Mombelli et al. 1995) stud-
ies have shown that the removal of subgingival plaque
on top of subgingival calculus will result in healing of
periodontal lesions and the maintenance of healthy
gingival and periodontal tissues, provided that the
supragingival deposits are meticulously removed on a
regular basis. One of these studies (Mombelli et al.
1995) clearly demonstrated that the diligent and com-
plete removal of subgingival plaque on top of miner-
alized deposits after chipping off gross amounts of
calculus showed almost identical results in the com-
position of the microbiota and the clinical parameters
to those obtained with routine removal of subgingival
calculus by root surface instrumentation. Again, it has
to be realized that meticulous supragingival plaque
control guarantees the depletion of the supragingival
