Jacobson M.Z. Atmospheric Pollution
Подождите немного. Документ загружается.

of such radiation. Their absorptivity increases from the visible to the UV spectra (e.g.,
Gillette et al., 1993; Sokolik et al. 1993).
7.1.3.2. The Imaginary Refractive Index
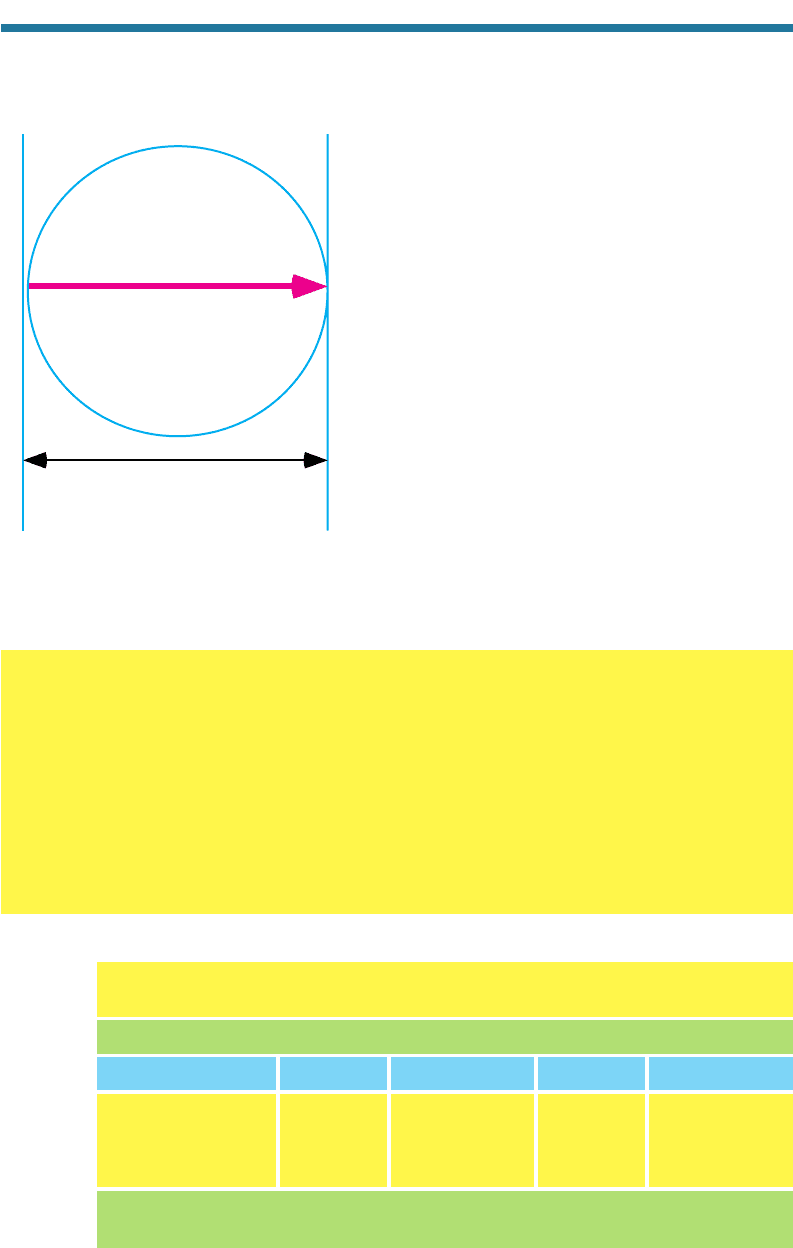
Figure 7.9 shows a possible path of radiation
through a single spherical aerosol-particle.
The attenuation due to absorption of incident
radiation of wavelength as it travels through a sin-
gle particle is
I I
0
e
4π (xx
0
)
(7.4)
where is the imaginary index of refraction, x x
0
is the distance through the particle, and I
0
is the ini-
tial radiation intensity. The imaginary index of
refraction is a measure of the extent to which a par-
ticle absorbs radiation. The term 4π is an
absorption extinction coefficient for a single aerosol-
particle. Table 7.2 gives imaginary refractive indices
for some substances at wavelengths of 0.50 and 10
m. Black carbon has the largest imaginary indices
of refraction among the substances shown.
188 ATMOSPHERIC POLLUTION: HISTORY, SCIENCE, AND REGULATION
x
0
x
I
0
I
dx
Figure 7.9. Attenuation of incident radiation
I
0
due to absorption by an aerosol particle.
Table 7.2. Real and Imaginary Indices of Refraction for Some Substances at
0.50 and 10.0 m
Real (n) Imaginary () Real (n) Imaginar y ()
H
2
O(aq)
a
1.34 1.0 10
9
1.22 0.05
Black Carbon (s)
b
1.82 0.74 2.40 1.0
Organic Matter(aq,s)
b
1.45 0.001 1.77 0.12
H
2
SO
4
(aq)
b
1.43 1.0 10
8
1.89 0.46
a
Hale and Querry (1973).
b
Krekov (1993).
0.5 m 10 m
EXAMPLE 7.2
Find the fraction of incident radiation intensity that transmits through a uniform particle of diameter
0.1 m at a wavelength of 0.5 m when the par ticle is composed of (a) black carbon and (b) water.
Solution
From Table 7.2, the imaginary refractive indices of black carbon and water at 0.5 m are 0.74
and 10
9
, respectively. From Equation 7.4, the transmission of light through (a) black carbon is
II
o
e
4π 0.74 0.1/0.5
0.16 and that through (b) liquid water is 0.999999997. Thus, a 0.1 m
black carbon particle absorbs 84 percent of incident radiation, whereas a 0.1 m water particle
absorbs only 0.0000003 percent of incident visible radiation passing thr
ough it. As such, black carbon
is a strong absorber of visible light, but liquid water is not.
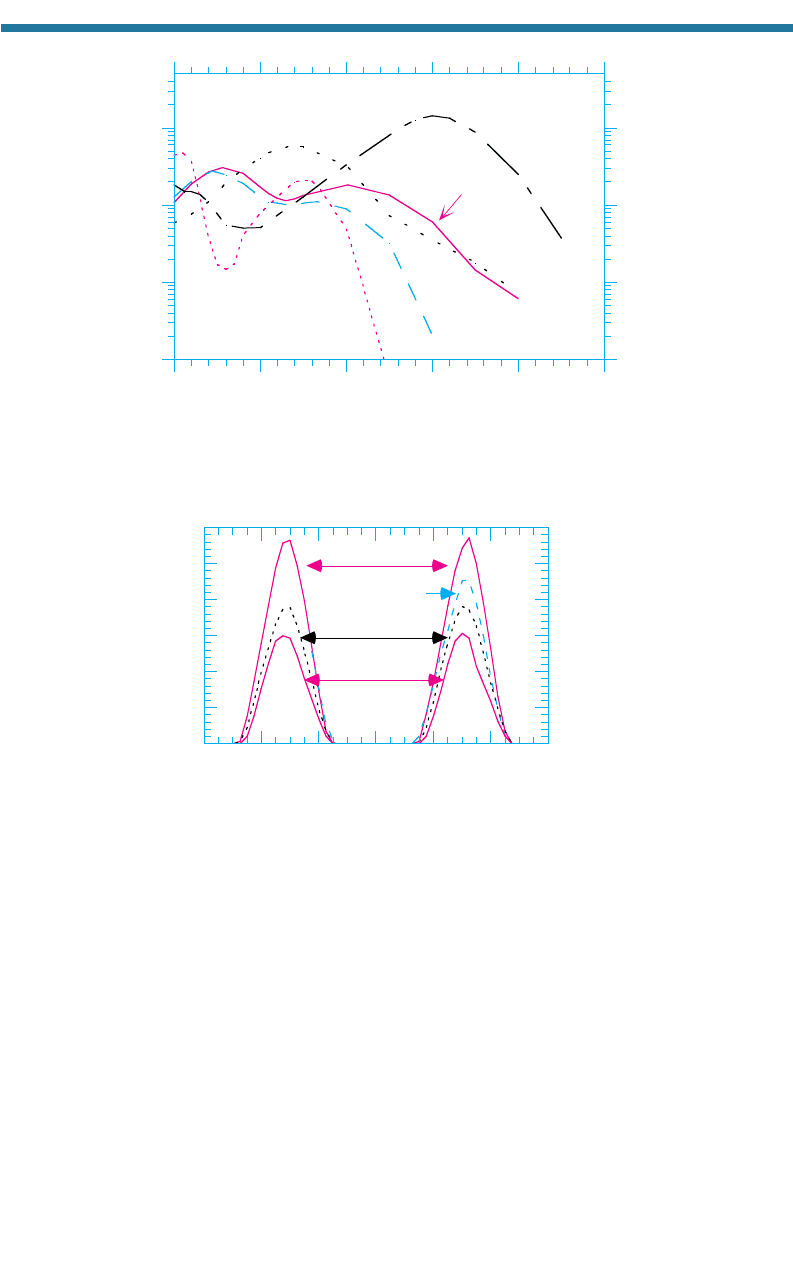
7.1.3.3. Effects of Aerosol-Particle Absorption on UV Radiation
Figure 7.10 shows the imaginary refractive indices of several liquid organic com-
pounds versus wavelength. The figure indicates that these organics preferentially
absorb UV radiation.
Absorption of UV radiation by organics in aerosol particles affects the amount of
UV radiation reaching the ground in smog. Measurements in the Los Angeles Basin in
1987, shown in Fig. 7.11, indicate that UV radiation 0.295 to 0.385 m was reduced
by 22 percent in central Los Angeles (near the coast), 33 percent at Claremont (further
inland), and 48 percent at Riverside (much further inland) in comparison with UV
radiation at Mount Wilson, 1.7 km above the basin. Measurements also indicated that
EFFECTS OF POLLUTION ON VISIBILITY, ULTRAVIOLET RADIATION, AND ATMOSPHERIC OPTICS 189
0.001
0.01
0.1
1.0
0.25 0.3 0.35 0.4 0.45 0.5
Imaginary refractive index
Wavelength (μm)
4-nitrophenol anion
3-nitrophenol
4-nitrophenol
2-nitrophenol
2-hydroxybenzaldehyde
Figure 7.10. Imaginary index of refraction of some liquid organics versus wavelength. From
Jacobson (1999c).
0
10
20
30
40
50
60
0 8 16 24 32 40 48
Downward UV intensity (W m
-2
)
Hour after first midnight
UV 295
-385 nm
Mt. Wilson
Central L.A.
Claremont
Riverside
Figure 7.11. Effect of pollution on UV radiation. The figure shows measurements of
downward UV radiation intensity at four sites in Los Angeles, California, on August 27–28,
1987. The three nonmountain sites were progressively further inland. The elevations of
the four sites were as follows: Mt. Wilson, 1.7 km; Central Los Angeles, 87 m; Claremont,
364 m; Riverside, 249 m. UV radiation between the mountain and surface was reduced
the most at Riverside, wher
e most pollution occurred. Measurements in Central Los
Angeles were available only on the second day. Data were provided by the California Air
Resources Board.

total solar radiation 0.285 to 2.8 m was reduced by only 8 to 14 percent between
Riverside and Mount Wilson. Some components of photochemical smog appeared to
have preferentially reduced UV radiation over total solar radiation.
Whereas some of the preferential UV reductions were due to gas absorption,
Rayleigh scattering, and aerosol-particle scattering, the sum of these effects could not
account for the up to 50 percent observed decreases in UV radiation at Riverside.
Thus, aerosol particle absorption most likely played a role in the reductions. Because
black carbon is not a preferential absorber of UV radiation (it absorbs UV and visible
wavelengths relatively equally), and because soil-dust concentrations were relatively
low, these UV absorbers did not account for the remainder of preferential UV reduc-
tions. Nitrated and aromatic aerosol components,
which preferentially absorb UV
(Fig. 7.10) may have accounted for a portion of the remainder of UV absorption.
Figure 5.14 shows that nitrate concentrations at Riverside, where the largest UV reduc-
tions were occurring, were high (up to 60 g m
3
); thus, it is plausible that organics at
Riverside were heavily nitrated and absorbed UV radiation.
7.1.3.4. Effects of UV-Radiation Reductions on Ozone
The effect of UV radiation loss on ozone in Los Angeles cannot be measured but it
can be examined by a model. A modeling study of 1987 air pollution in Los Angeles
found that decreases in UV radiation due to smog decreased photolysis rates,
decreas-
ing near-surface ozone mixing ratios by an average of 5 to 8 percent (Jacobson, 1998,
1997b). The study also found that
• in regions of the boundary layer where absorption of UV radiation by aerosol par-
ticles was strong, photolysis of UV-absorbing gases decreased and ozone decreased
• in regions of the boundary layer where UV scattering dominated UV absorption by
aerosol particles, photolysis of UV-absorbing gases increased and ozone increased
In a study of relatively nonaborbing aerosols in Maryland, Dickerson et al. (1997)
found that highly scattering aerosol particles increased ozone, consistent with the sec-
ond result.
Although reduced UV radiation and ozone may appear to be ironic benefits of cer-
tain smogs, the cause of UV reductions is heavy particle loadings. Particles,
particularly small ones, cause harmful health effects that far outweigh the benefits of
reduced UV radiation or the small level of reduced ozone that they might trigger. In
addition, although ozone mixing ratios slightly decrease in the presence of absorbing
particles, the mixing ratios of other pollutant gases increase.
7.1.4. Aerosol and Hydrometeor Particle Scattering
Particle scattering is the redirection of incident energy by a particle without a loss of
energy to the particle. Particle scattering is really the combination of several processes,
including reflection, refraction, and diffraction. These processes are discussed next.
7.1.4.1. Reflection
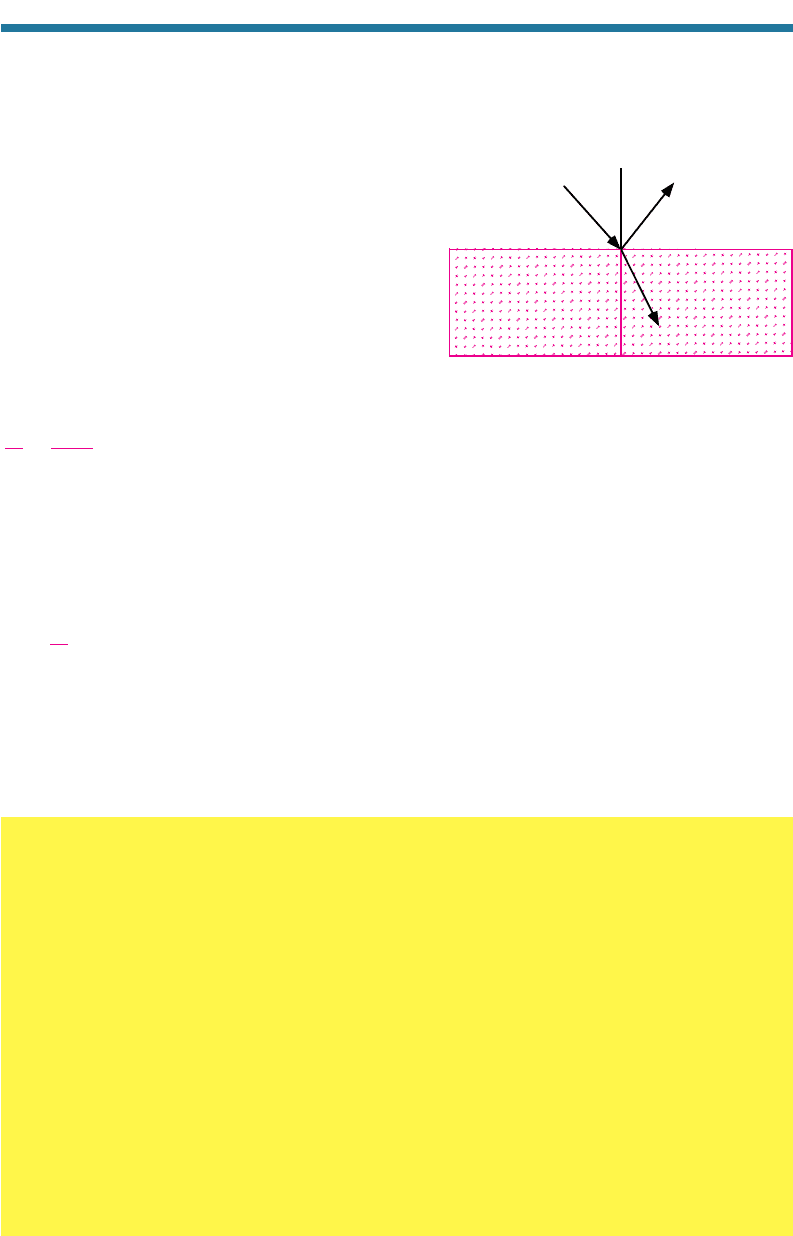
Reflection occurs when radiation bounces off an object at an angle equal to the
angle of incidence. No energy is lost during reflection. Figure 7.12 shows an example
of reflection. Radiation can reflect off of aerosol particles, cloud drops, or other surfaces.
The colors of most objects that we see are due to preferential reflection of certain
190 ATMOSPHERIC POLLUTION: HISTORY, SCIENCE, AND REGULATION
wavelengths by the object. For example, an apple appears red because the apple’s skin
absorbs blue and green wavelengths and reflects red wavelengths to our eye.
7.1.4.2. Refraction
Refraction occurs when a wave or photon
leaves a medium of one density and enters a medium
of another density. In such a case, the speed of the
wave changes, changing the angle of the incident
wave relative to a surface normal, as shown in
Fig. 7.12. If a wave travels from a medium of one
density to a medium of a higher density, it bends
(refracts) toward the surface normal (the vertical line
in Fig. 7.12). The angle of refraction is related to the
angle of incidence by Snell’s law,
(7.5)
In this equation, n is the real index of refraction
(dimensionless), is the angle of incidence or refraction, and subscripts 1 and 2 refer to
media 1 and 2, respectively. The real index of refraction is the ratio of the speed of light
in a vacuum (c 2.99792 10
8
m s
1
) to that in a different medium (c
1
,m s
1
). Thus,
(7.6)
Because light cannot travel faster than its speed in a vacuum, the real index of refrac-
tion of a medium other than a vacuum must exceed unity. The refractive index of air at
a wavelength of 0.5 m is 1.000279. Real refractive indices of some liquids and solids
were given in Table 7.2.
n
1
c
c
1
n
2
n
1
sin
1
sin
2
EFFECTS OF POLLUTION ON VISIBILITY, ULTRAVIOLET RADIATION, AND ATMOSPHERIC OPTICS 191
Water
θ
1
Air
Incident Reflected
Refracted
θ
3
θ
2
Figure 7.12. Examples of reflection and
refraction. During reflection, the angle of inci-
dence (
1
) equals the angle of r
eflection (
3
).
During refraction, the angles of incidence and
refraction are related by Snell’s law. The line
perpendicular to the air–water interface is the
surface normal.
EXAMPLE 7.3
(a) Suppose light at a wavelength of 0.5 m travels between the atmosphere (medium 1) and liquid water
(medium 2). Suppose also that the angle between the incident light and the surface normal is
1
45°. By
how man
y degrees is the light bent toward the surface normal when it enters medium 2? (b) Do the same
calculation for light traveling between outer space (medium 1) and the atmosphere (medium 2).
Solution
(a) At a wavelength of 0.5 m, the real index of refraction of air is n
1
1.000279 and that of
liquid water is n
2
1.335. From Equation 7.5, the angle between the light and the surface
normal in medium 2 is
2
32°; thus, the light is bent by 13° toward the surface normal
when it enters water from the atmosphere.
(b) At a wavelength of 0.5 m, the real index of refraction of a vacuum is n
1
1.0. From
Equation 7.5, the angle between the light and the surface normal in medium 2 is
2
44.984°; thus, the light is bent by 0.016° toward the surface normal when it enters the
atmosphere from space.
In sum, the angle of refraction between the atmosphere and water is much greater than that between
space and the atmosphere.
Because the real index of refraction is wavelength dependent, different wave-
lengths are refracted by different angles when they pass from one medium to
another. For instance, when visible light passes from air to liquid water at an inci-
dent angle of
1
45, wavelengths of 0.4 and 0.7 m are bent by 13.11 and
12.90, respectively. Thus, refraction bends short
(blue) wavelengths of visible light more than it
bends long (red) wavelengths. Separation of white
visible light into individual colors by this selective
refraction is called dispersion (or dispersive
refraction). When Sir Isaac Newton separated
white light into multiple colors by passing it
through a glass prism (Section 7.1), he discovered
dispersive refraction.
As shown in Figs. 7.6 and 7.8, refraction between
space and the atmosphere is responsible for twi-
light, which is the sunlight we see after the sun sets
and before the sun rises. Such refraction also caus-
es stars to appear positioned where they are not, as shown in Fig. 7.13. Layers of air
at different densities in the Earth’s atmosphere cause starlight to refract multiple
times and, thus, flicker, twinkle, or scintillate.
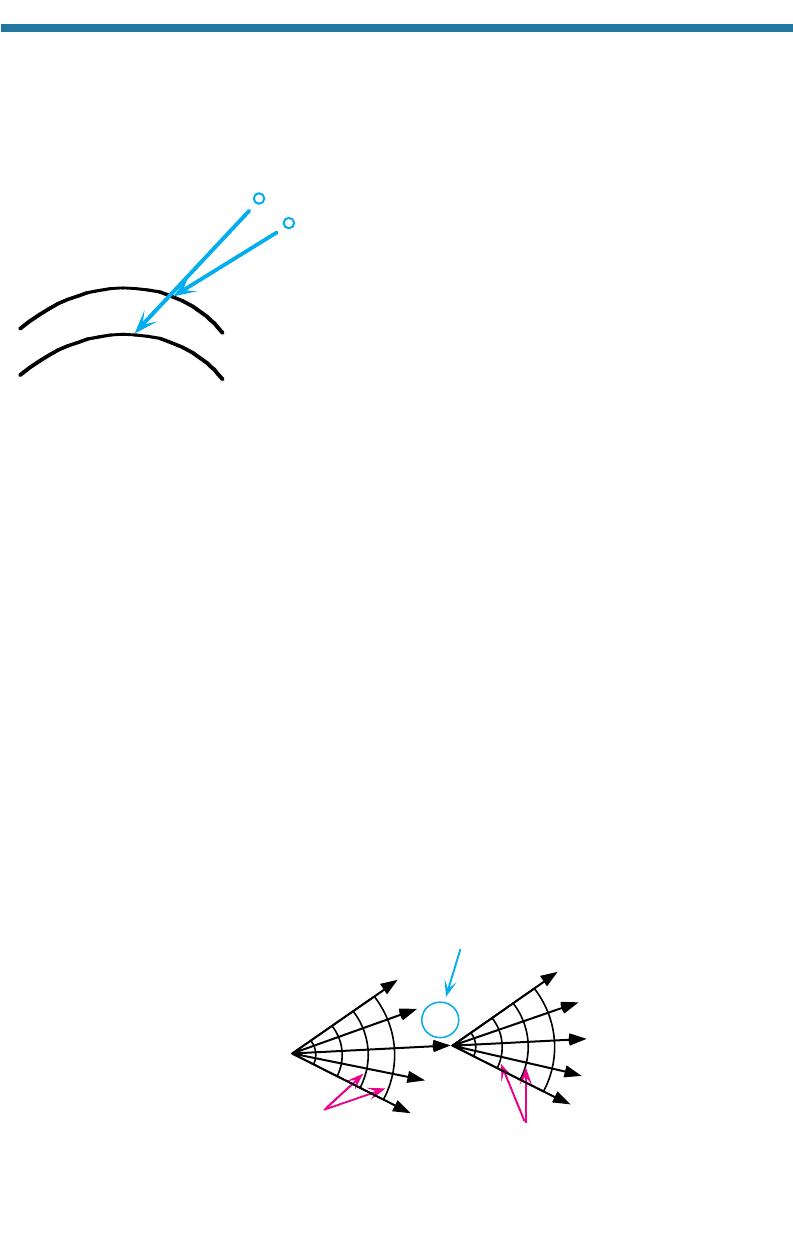
7.1.4.3. Diffraction
Diffraction is a process by which the direction of propagation of a wave changes
when the wave encounters an obstruction. In terms of visible wavelengths, it is the
bending of light as it passes by the edge of an obstruction. In the air, waves diffract as
they pass by the surface of an aerosol particle, cloud drop, or raindrop.
Diffraction can be explained in terms of Huygens’s principle, which states that
each point of an advancing wavefront may be considered the source of a new series of
secondary waves. If a stone is dropped in a tank of water, waves move out horizontally
in all directions, and wavefronts are seen as concentric circles around the stone. If a
point source emits waves in three dimensions, wavefronts are concentric spherical sur-
faces. When a wavefront encounters the edge of an obstacle, such as in Fig. 7.14,
waves appear to bend (diffract) around the obstacle because a series of secondary con-
centric waves is emitted at the edge of the obstacle.
192 ATMOSPHERIC POLLUTION: HISTORY, SCIENCE, AND REGULATION
Actual
position
Apparent
position
Earth
Atmosphere
Figure 7.13. Refraction of starlight by the
atmosphere makes stars appear to be where
they are not.
Primary
wavefronts
Source
Particle
Secondary
wavefronts
Diffracted rays
A
Figure 7.14. Diffraction around a spherical particle. Any point along a wavefront may be taken
as the source of a new series of secondary waves. Rays emitted from point A appear to
cause waves from the original source to bend around the particle.
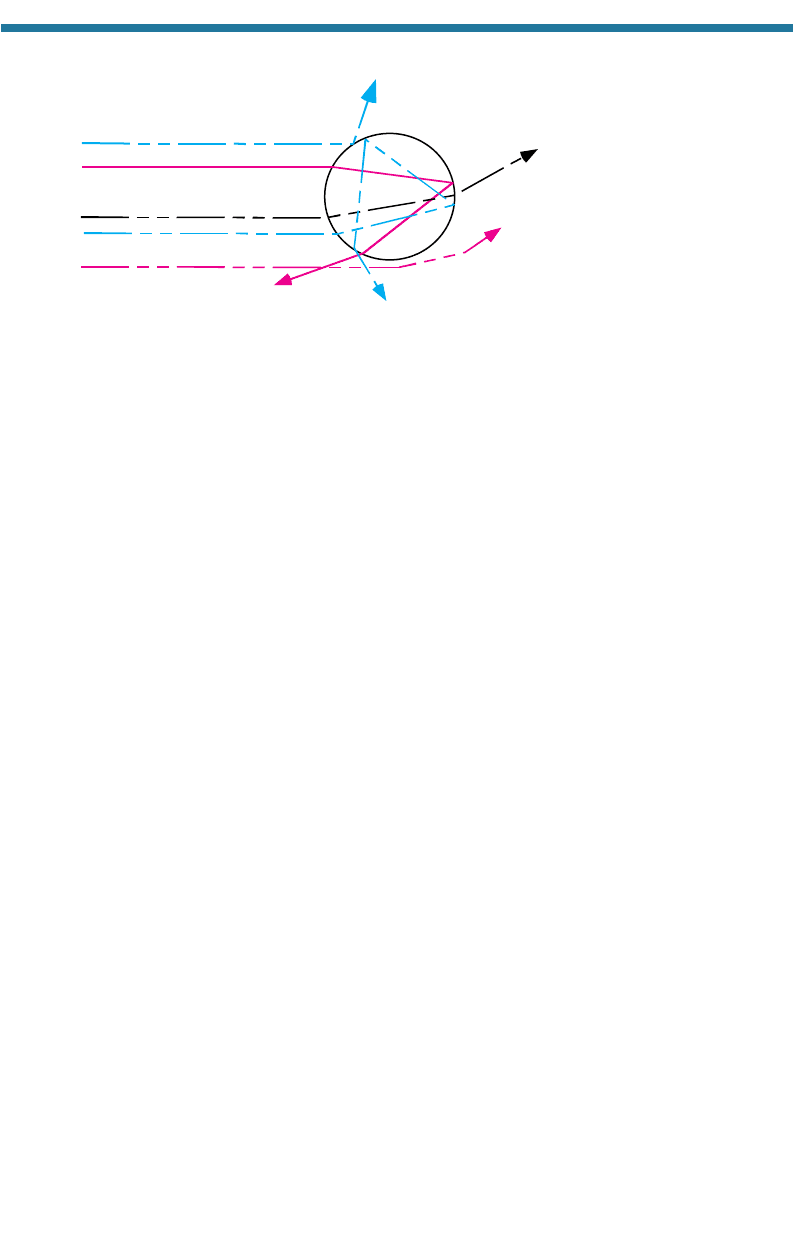
7.1.4.4. Summary of Particle Scattering
Particle scattering is the combination of the effects of reflection, refraction, and
diffraction. When a wave approaches a spherical particle, such as a cloud drop, it can
reflect off the particle,
diffract around the edge of the particle, or refract into the parti-
cle. Once in the particle, the wave can be absorbed, transmit through the particle and
refract out, or reflect internally one or more times and then refract out. Figure 7.15
illustrates these processes, except for absorption, which is not a scattering process. The
processes that affect particle scattering the most are diffraction and double refraction,
identified by rays C and B,
respectively. Thus, particles scatter light primarily in the
forward direction. They also scatter some light to the side and in the backward direction.
Backscattered light results primarily from a single internal reflection (ray E). The
light rays seen in Fig. 7.16 are the result of light scattering off of cloud drops in the
forward and sideward directions.
7.1.4.5. Rainbows
A rainbow results from two light-scattering processes, dispersive refraction and
reflection, and can be seen only if the sun is at the viewer’s back and raindrops are
falling in front of the vie
wer. The seven most prominent colors in a rainbow are red,
orange, yellow, green, blue, indigo, and violet. In a primary rainbow, red appears on
the top and violet appears on the bottom. Figure 7.17 shows an example of a primary
rainbow. In a secondary rainbow, sometimes seen faintly above a primary rainbow,
violet appears on top and red appears on the bottom. For convenience, the discussion
of rainbows below considers only red, green, and blue.
Figure 7.18 shows how light interacts with raindrops to form a primary rainbow.
As a beam of visible light enters a raindrop, all wavelengths bend toward the surface
normal due to refraction. Blue light bends the most as a result of dispersive refrac-
tion. When light hits the back of the drop, much of it reflects internally. When
reflected light reaches the front edge of the drop, it leaves the drop and refracts away
from the surface normal. The angles of the blue and red wavelengths that reach a
viewer’s eye are 40 and 42, respectively, in relation to the incident beam. Only one
wavelength from each raindrop impinges on a viewer’s eye. Thus, a rainbow appears
when individual waves from many raindrops hit the viewer’s eye. As seen in
EFFECTS OF POLLUTION ON VISIBILITY, ULTRAVIOLET RADIATION, AND ATMOSPHERIC OPTICS 193
Figure 7.15. Radiative scattering by a sphere. Ray A is reflected; B is refracted twice; C is dif-
fracted; D is refracted, internally reflected twice, then refracted; and E is refracted, reflected
once, then refracted. Rays A, B, C, and D scatter in the forward or side
ward direction;
E scat-
ters in the backward direction.
A Sidescattering
B
C
D Sidescattering
E
Backscattering
Forward
scattering

194 ATMOSPHERIC POLLUTION: HISTORY, SCIENCE, AND REGULATION
Figure 7.17. Rainbow over an Alaskan lake in September 1992. Photo by Commander John
Bortniak, NOAA Corps, available from the NOAA Central Library.
Figure 7.16. Forward- and side-scattering of sunlight by a cloud. The thickness of the cloud
prevents most sunlight from being transmitted.
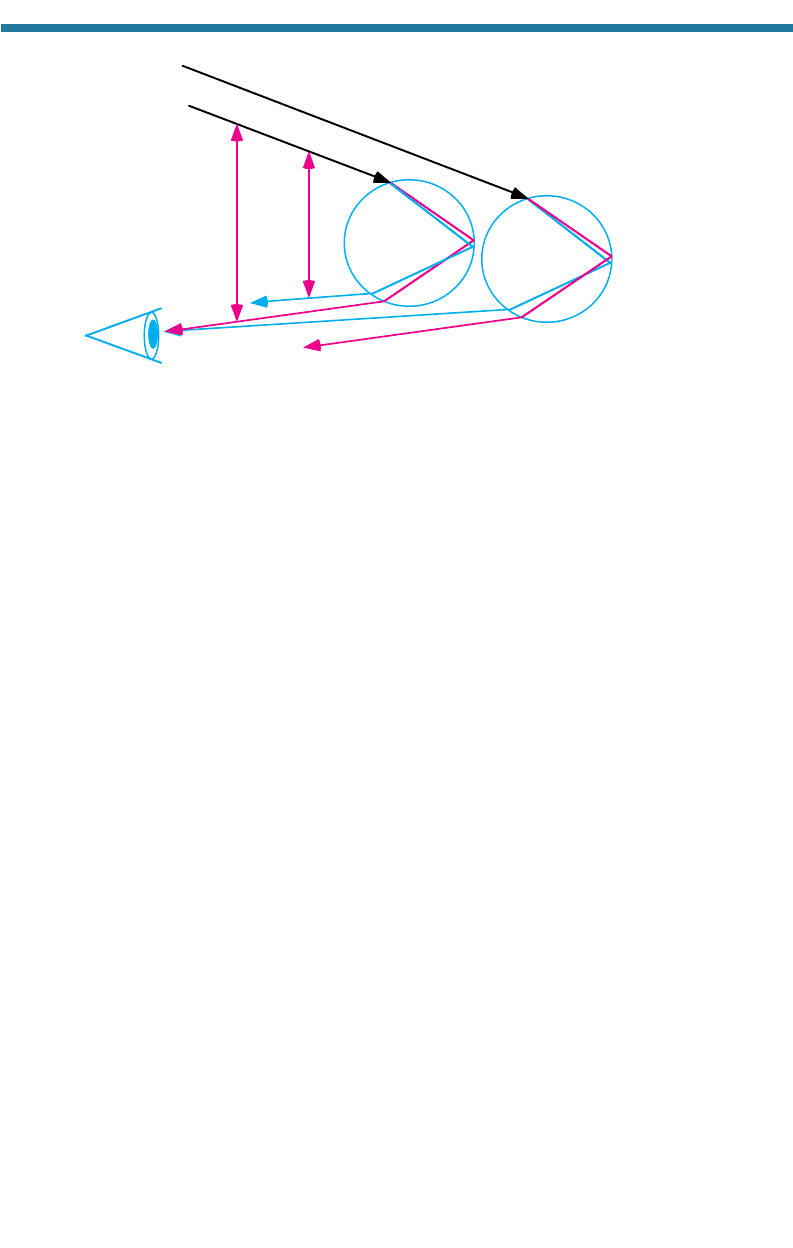
Fig. 7.18, red appears on the top of a primary rainbow. A secondary rainbow occurs if
a second reflection occurs inside each raindrop.
Because winds at midlatitudes originate from the west or southwest (Fig. 6.5) and
a rainbow appears only when the sun is at a viewer’s back, sailors at midlatitudes
knew that if they saw a rainbow in the morning, the rainbow was to the west and the
winds were driving the storm creating the rainbow toward them. If they saw a rainbow
in the evening, the rainbow was to the east and the winds were driving the storm creat-
ing the rainbow away from them. These factors led to the rhyme,
Rainbow in the morning, sailors take warning
Rainbow at night, sailor’s delight.
7.1.5. Particle Scattering and Absorption Extinction Coefficients
The quantification of particle scattering and absorption is more complex than is that of
gas scattering or absorption due to the variety of sizes and compositions of aerosol
particles. Aerosol particle absorption and scattering extinction coefficients (cm
1
) at a
given wavelength can be estimated with
(7.7)
respectively, where the summations are over N
B
particle sizes, n
i
is the number concen-
tration (particles per cubic centimeter of air) of particles of radius r
i
(cm), r
i
2
is the
actual cross section of a particle assuming it is spherical (cm
2
per particle), and Q
a,i
and Q
s,i
are single-particle absorption and scattering efficiencies (dimensionless),
respectively.
A single-particle scattering efficiency is the ratio of the effective scattering cross
section of a particle to its actual cross section. The scattering efficiency can exceed
unity because a portion of the radiation diffracting around a particle can be intercepted
and scattered by the particle. Scattering efficiencies above unity account for this addi-
tional scattering.
A single-particle absorption efficiency is the ratio of the effective absorption
cross section of a particle to its actual cross section. The absorption efficiency can
s,p
N
B
i1
n
i
r
i
2
Q
s,i
a,p
N
B
i1
n
i
r
i
2
Q
a,i
EFFECTS OF POLLUTION ON VISIBILITY, ULTRAVIOLET RADIATION, AND ATMOSPHERIC OPTICS 195
Blue
Red
Blue
Re
d
42° 40°
Visible
radiation
Figure 7.18. Geometry of a primary rainbow.
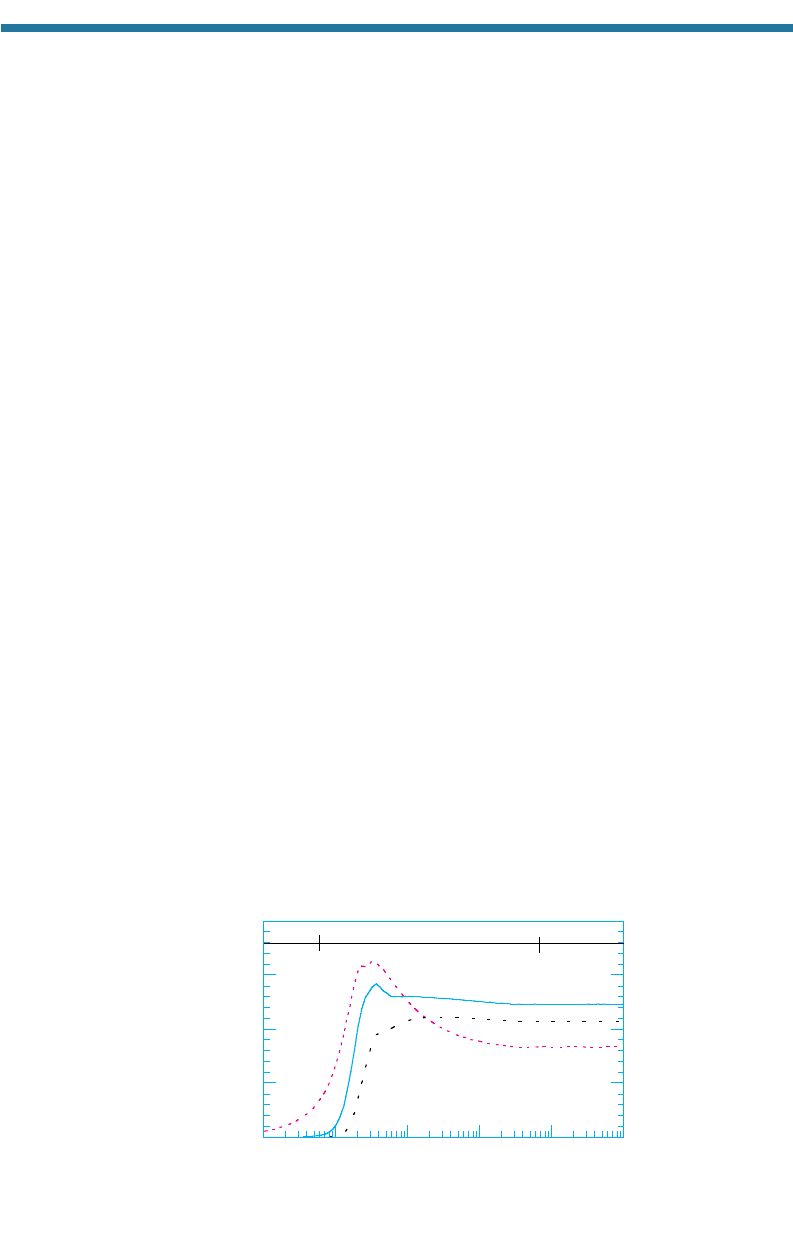
exceed unity because a portion of the radiation diffracting around a particle can be
intercepted and absorbed by the particle. Absorption efficiencies above unity account
for this additional absorption. The larger the imaginary index of refraction of a parti-
cle, the greater its absorption efficiency.
Single-particle absorption and scattering efficiencies vary with particle size, radia-
tion wavelength, and refractive indices. Figures 7.19 and 7.20 show Q
a,i
and Q
s,i
for
black carbon and liquid water, respectively, at a wavelength of 0.5 m. The figures
also show the single-particle forward scattering efficiency Q
f,i
, which is the efficien-
cy with which a particle scatters light in the forward direction. The forward scattering
efficiency is always less than is the total scattering efficiency. The proximity of Q
f,i
to Q
s,i
in both figures indicates that aerosol particles scatter strongly in the forw
ard direction.
The difference between Q
s,i
and Q
f,i
is the scattering efficiency in the backward direction.
When a particle’s diameter (D) is much smaller than the wavelength of light ()
(e.g., when (D0.03), the particle is in the Rayleigh regime and is called a
Tyndall absorber or scatterer. John Tyndall (1820–1893) was an English experi-
mental physicist who demonstrated experimentally that the sky’s blue color results
from scattering of visible light by gas molecules and that a similar effect occurs with
small particles.
When a particle’s diameter is near the wavelength of light (0.03 D < 32), the
particle is in the Mie regime. Gustav Mie (1868–1957) was a German physicist who
derived equations describing the scattering of radiation by particles in this re
gime
(Mie, 1908).
When a particle has diameter much larger than the wavelength of light (D32),
the particle is in the geometric regime. Figures 7.19 and 7.20 show the diameters cor-
responding to these regimes for a wavelength of 0.5 m.
Figure 7.19 shows that visible-light absorption efficiencies of black carbon par-
ticles peak when the particles are 0.2 to 0.4 m in diameter. Such particles are in
the accumulation mode with respect to particle size and in the Mie regime with
respect to the ratio of particle size to the wavelength of light. Figure 7.20 shows that
water particles 0.3 to 2.0 m in diameter scatter visible light more efficiently than
do smaller or larger particles. These particles are also in the accumulation mode
with respect to particle size and in the Mie regime with respect to the ratio of parti-
cle size to the wavelength of light. Because the accumulation mode contains a
196 ATMOSPHERIC POLLUTION: HISTORY, SCIENCE, AND REGULATION
0
0.5
1.0
1.5
2.0
0
0.5
1.0
1.5
2.0
0.01 0.1 1 10 100 1,000
Particle diameter (μm)
Q
a
Q
s
Q
f
Mie regime
Geometric
regime
Rayleigh regime
Figure 7.19. Single-particle absorption (Q
a
), total scattering (Q
s
), and forward scattering (Q
f
)
efficiencies of black carbon particles of different sizes at 0.50 m (n 1.94, 0.66).
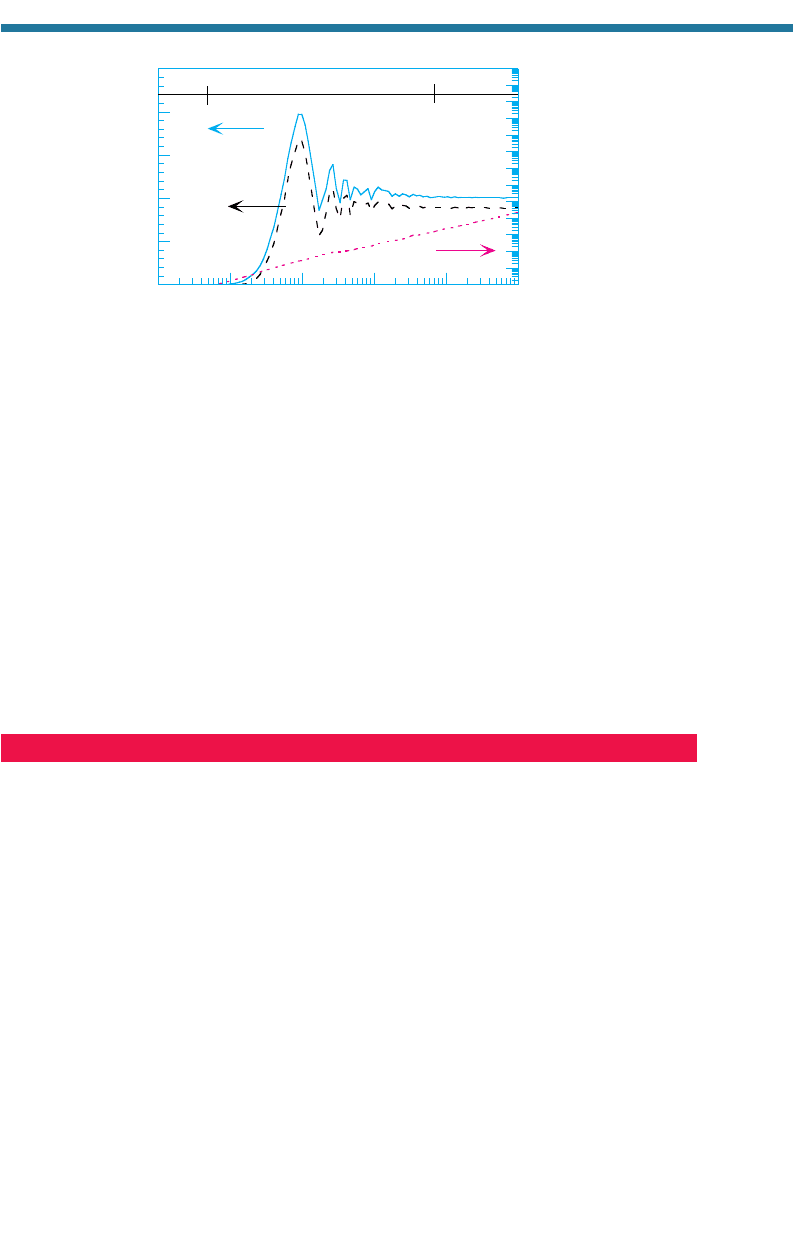
relatively high particle number concentration, and because particles in this mode
have high scattering and absorption (with respect to black carbon) efficiencies, the
accumulation mode almost al
ways causes more light reduction than do the nucle-
ation or coarse particle modes (Waggoner et al., 1981). In many urban regions, 20 to
50 percent of the accumulation mode mass is sulfate. Thus, sulfate is correlated
with particle scattering more closely than is any other particulate species, aside
from liquid water.
Figure 7.20 shows that liquid w
ater hardly absorbs visible light until particles are
raindrop-size (larger than 1,000 m in diameter). The absorptivity of rain drops causes
the bottoms of precipitating clouds to appear gray or black.
7.2. VISIBILITY
Visibility is a measure of how far we can see through the air. Even in the cleanest air,
our ability to see along the Earth’s horizon is limited to a few hundred kilometers by
background gases and aerosol particles. If we look up through the sky at night, however,
we can discern light from stars that are millions of kilometers away. The difference
between looking horizontally and vertically is that more gas molecules and aerosol
particles lie in front of us in the horizontal than in the vertical.
Several terms describe maximum visibility. Two subjective terms are visual range
and prevailing visibility. Visual range is the actual distance at which a person can
discern an ideal dark object against the horizon sky. Prevailing visibility is the great-
est visual range a person can see along 50 percent or more of the horizon circle
(360), but not necessarily in continuous sectors around the circle. It is determined by
a person who identifies landmarks known distances away in a full 360 circle around
an observation point. The greatest visual range observed over 180 or more of the cir-
cle (not necessarily in continuous sectors) is the prevailing visibility. Thus, half the
area around an observation point may have visibility worse than the prevailing visibil-
ity, which is important, because most prevailing visibility observations are made at
airports. If the visual range in a sector is significantly different from the prevailing
visibility, the observer at an airport usually denotes this information in the observa-
tion record.
EFFECTS OF POLLUTION ON VISIBILITY, ULTRAVIOLET RADIATION, AND ATMOSPHERIC OPTICS 197
0
1
2
3
4
5
10
-9
10
-7
10
-5
10
-3
10
-1
10
1
10
3
0.01 0.1 1 10 100 1,000
Particle diameter (μm)
Q
a
Q
s
Q
f
Mie regime
Geometric
regime
Rayleigh regime
Figure 7.20. Single-particle absorption (Q
a
), total scattering (Q
s
), and forward scattering
(Q
f
) efficiencies of liquid water drops of different sizes at 0.50 m (n 1.335,
1.0 10
9
).
