Jackson S.D., Hargreaves J.S.J. Metal Oxide Catalysis
Подождите немного. Документ загружается.

13.4 Catalysis by Uranium Oxides 549
the ability of UO
3
to oxidize olefi ns in a relatively facile manner owing to the lability
of lattice oxygen. However, in this case it must be taken into account that during
TPD there is no regeneration of surface reduced sites in contrast to steady - state
oxidation with oxygen present in the gas phase. On the other hand, the identifi ca-
tion of furan among the products shows that uranium oxides are active for carbon –
carbon bond formation in addition to carbon – oxygen selective bond formation.
Idriss and Madhavaram [41] have also studied the partial oxidation of ethanol to
furan over UO
3
. The oxides U
3
O
8
and UO
2
were also studied; UO
2
was inactive whilst
U
3
O
8
only exhibited very low activity. Using UO
3
, the maximum selectivity to furan
was 23% at 150 ° C with a conversion of 81%, with acetaldehyde being the other major
product. Comparing the reactions of ethylene and ethanol, the authors highlight two
points. Firstly, no ethylene was formed during the reaction of ethanol. Secondly,
acetaldehyde was formed from both ethylene and ethanol, and ethanol was formed
as a trace product from ethylene oxidation. These facts were used to propose a
mechanism in which the formation of furan was via an ethoxide intermediate [41] .
More commonly, uranium has been used as a catalyst component for mixed -
metal oxide catalysts for selective oxidation. Probably the most well known of these
mixed oxide catalysts are those based on uranium and antimony. The uranium –
antimony catalysts are exceptionally active and selective and they have been applied
industrially. An interpretation of the catalyst structure and reaction mechanism
has been reported by Grasselli and coworkers [42, 43] who discovered the catalyst.
The USb
3
O
10
mixed oxide has been extensively used for the oxidation/ammoxida-
tion reaction of propylene to acrolein and acrylonitrile. The selective ammoxida-
tion of propylene was investigated by Grasselli and coworkers [44] , and it has been
demonstrated that at 460 ° C a 62.0% selectivity to acrolein with a conversion of
65.2% can be achieved. Furthermore, Delobel and coworkers [45] studied the selec-
tive oxidation of propylene over USb
3
O
10
, which at 340 ° C gave a selectivity to
acrolein of 96.7%.
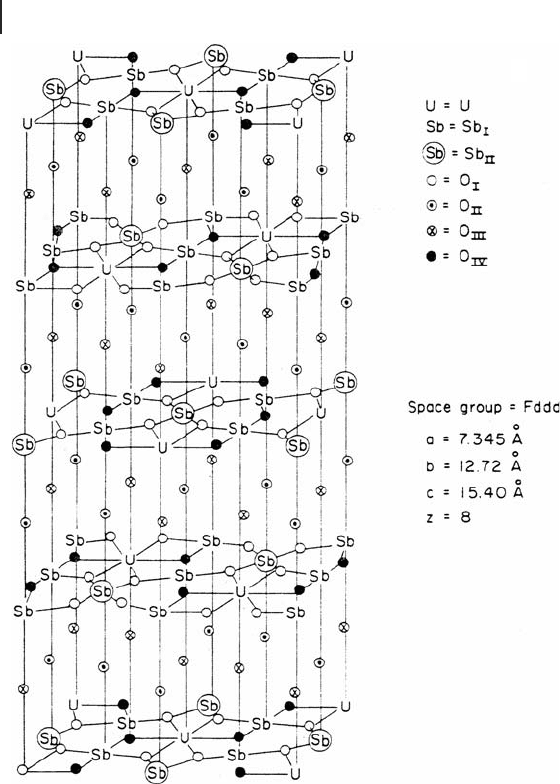
The structure of USb
3
O
10
is complex and it is crucial for selective oxidation
activity. USb
3
O
10
contains one type of uranium, two types of antimony and four
different types of oxygen. The unit cell is composed of eight formula weight units
( Z = 8). The structure is composed of layers that contain heavy atoms and oxygen
alternating with layers of oxygen only. Five layers of planes containing heavy metal
atoms are required to completely describe the unit cell of USb
3
O
10.
It was found
that there are two types of lattice oxygen in the structure, one giving high selectivity
Table 13.3 Products from TPD of ethylene adsorbed on UO
3
[33] .
Product Desorption temperature
( ° C)
Carbon yield
(%)
Carbon selectivity
(%)
Ethylene 127 – 427 85.7 –
Acetaldehyde 207 8.3 58
Furan 277 6.0 42
CO
2
above 527 not calculated –
550 13 Heterogeneous Catalysis by Uranium Oxides
for partial oxidation, and the other, which was less active and tended to be less
selective, producing carbon oxides. Grasselli and coworkers [43] suggested that the
active oxygen was O(IV) and the less active one was O(I), as shown in Figure 13.4 .
The USbO
5
structure is very similar to that of USb
3
O
10
. USbO
5
has the heavy atoms
at positions close to those of USb
3
O
10
, but with a slight distortion of all atoms,
leading to a lower symmetry sub - group.
The USbO
5
phase was proposed as a precursor to the USb
3
O
10
phase, which at
high temperature decomposes to give USbO
5
. The decomposition of UO
2
(NO
3
)
2
and oxidation of the Sb
2
O
3
, from which the catalyst was prepared, led to the forma-
tion of UO
3
and Sb
2
O
4
respectively. On thermal treatment the antimony oxide
Figure 13.4 USb
3
O
10
structure. (Journal of Catalysis , 25 (1972),
273; reproduced by permission of Academic Press).

13.4 Catalysis by Uranium Oxides 551
penetrates the lattice of UO
3
and forms the USbO
5
phase, according to the
reactions
222
324 324 5
UO Sb O UO Sb O USbO+→⋅
[]
→
23 3 6
38 24 2 3 24 5
UO SbO O 6UO SbO USbO++→⋅
[]
→
The Sb
2
O
5
phase is stabilized by the presence of the USbO
5
phase and further
reaction proceeds to form the desired USb
3
O
10
catalytically active phase.
USbO Sb O USbO Sb O USb O
525 525 310
+→ ⋅
[]
→
USbO Sb O O USbO Sb O USb O
524 2 525 310
1
2
++→ ⋅
[]
→
Powder X - ray diffraction studies of USbO
5
, USb
3
O
10
and Sb
2
O
4
as a function of
calcination time and temperature allowed investigation of the mechanism of for-
mation of the phases, USbO
5
and USb
3
O
10
. Accordingly, USbO
5
, which was formed
at about 675 ° C, was considered to be the precursor of the USb
3
O
10
phase [43] . At
this temperature Sb
2
O
5
was also initially present and since it was not stable it
reacted with USbO
5
to produce USb
3
O
10
. The USb
3
O
10
phase increased in concen-
tration as the temperature was increased. At 980 ° C the USb
3
O
10
phase was decom-
posed to re - form USbO
5
.
The uranium – antimony oxide system remains as a basis of interest for catalysts.
The preparation of a new uranyl antimonate has been described and it was pre-
pared by hydrothermal synthesis from UO
3
, Sb
2
O
3
and KCl [46] . A detailed struc-
tural analysis was reported, but more importantly the UO
2
Sb
2
O
4
was selective for
the oxidation of propylene to acrolein.
Some further mixed - metal oxides containing uranium, such as bismuth ura-
nates, are known to be effective catalysts in certain selective oxidation reactions,
for instance toluene partial oxidation. The oxidative demethylation of toluene
using bismuth uranate as oxidant has been studied by Van deer Baan and cowork-
ers [47] in a non - steady - state reactor . The reaction conditions were: temperature,
480 ° C; pulse volume, 0.534 cm
3
; mole fraction of toluene, 0.023; gas fl ow,
25 cm
3
min
− 1
and catalysts used were samples with different Bi to U atomic ratios.
Results indicated that at the reaction temperature the most active catalyst was
Bi
2
UO
6
with a selectivity of 70% to benzene in the absence of gas - phase oxygen.
The experiments carried out showed that bismuth uranate is a very active but
unselective oxidant in the very early stages of the toluene oxidation reaction.
However, as soon as the catalyst starts to become depleted of oxygen the activity
decreased and the selectivity increased. The reduced uranate was easily reoxidized
by molecular oxygen in a second step. The mechanism proposed for this reaction
was a redox mechanism, which occurred via a benzoate - like intermediate. It was
stated that the oxidation of toluene gave two main products: benzaldehyde, which
dissociated to benzene and carbon monoxide and benzoic acid, which also dissoci-
ated to produce benzene and carbon dioxide.
552 13 Heterogeneous Catalysis by Uranium Oxides
In a more detailed study of bismuth uranate reduction by toluene using pulsed
fl ow and steady - state fl ow, further insight into the mechanism of oxidation was
obtained [48] . In the temperature range 455 – 527 ° C, Bi
2
UO
6
was reduced by toluene
to metallic bismuth and UO
2
. The reaction rate was related to the surface reaction
rate and the rate of diffusion of oxygen through the lattice. It was apparent that
Bi
2
UO
6
contained two types of reactive lattice oxygen. The fi rst was a nonselective
species that was ca. 2% of the total lattice oxygen and it showed activation for dif-
fusion of ca. 107 kJ mol
− 1
. The second type was a species responsible for selective
oxidation and had a diffusion energy of ca. 261 kJ mol
− 1
at 470 ° C. Evidence for the
formation of shear planes in the Bi
2
UO
6
structure was presented and the relatively
small concentration of nonselective oxygen was thought to be from positions in
the shear planes.
The oxidation of carbon monoxide to carbon dioxide using similar bismuth
uranate catalysts has been reported by Derouane and coworkers [49] . The work on
carbon monoxide oxidation confi rmed that the bismuth uranate catalyst operated
by a redox mechanism. These studies on bismuth uranates highlight the important
role played by oxygen transfer via the lattice, and reinforce the importance of the
ability of uranium to exhibit relatively facile redox behavior.
The selective oxidation of toluene has been studied over a number of catalysts
based on metal oxides, with the U/Mo oxide system being one of the most active
and selective [50, 51] . The main products in the oxidation of toluene, excluding the
non - oxidative coupling products, were benzaldehyde, benzoic acid, maleic anhy-
dride, benzene, benzoquinone, CO and CO
2
. Under the same reaction conditions
toluene may also yield coupling products such as phthalic anhydride, methyldi-
phenylmethane, benzophenone, diphenylethanone and anthraquinone, as shown
by Zhu and coworkers [51] . A range of different uranium - based oxides were tested
[51] and the results obtained are shown in Table 13.4 .
Table 13.4 Conversion and selectivity data for toluene
oxidation using U - based mixed oxides at 500 ° C [51] .
Catalyst
(atomic ratio)
Contact time
(s)
Conversion
(%)
Benzaldehyde
selectivity (%)
Benzaldehyde
yield (mol%)
U alone 0.065 21.4 90 19.3
0.200 47.6 69 32.1
0.320 66.0 49 32.0
0.400 77.4 47 35.8
U : P (8 : 2) 0.032 37.8 47 17.6
0.065 54.2 38 15.4
U : W (9 : 1) 0.032 38.4 69 26.4
U : V (9 : 1) 0.032 48.4 54 26.4
U : Sb (1 : 4) 1.3 11.0 50 5.6

13.4 Catalysis by Uranium Oxides 553
Under the reaction conditions used, a U
3
O
8
catalyst demonstrated appreciable
selective oxidation activity. The best results, in terms of both activity and selectivity
to benzaldehyde, were obtained with the mixed oxides with U : Mo atomic ratios
in the range 8 : 2 to 9 : 1. The maximum yield of benzaldehyde was 40 mol%. On
the other hand, antimony - based uranium oxides were not found to be effective
as catalyst for this reaction. U
–
Mo and Bi
–
Mo mixtures also exhibited promising
activity and selectivity to benzaldehyde. Bi
–
Mo and Bi
–
Mo
–
P
–
Si catalysts were
also tested. Qualitatively there was little difference between the product distribu-
tions from the two catalysts. The major products formed were benzaldehyde,
benzene and carbon oxides, as well as traces of anthraquinone and benzoic
acid.
Perhaps one of the most demanding selective oxidation reactions is the oxidation
of methane to the oxygenates formaldehyde and methanol. Although the use of
uranium as a catalyst component for selective methane partial oxidation is rela-
tively rare, there is one notable attempt. Dowden and Walker reported the activity
of a range of two - component catalysts, one component of which was molybdenum
[52] . They developed these catalysts based on the principles outlined by the pro-
posal of a virtual mechanism [53] . Results were reported for MoO
3
/ZnO, (MoO
3
)
4
/
Fe
2
O
3
, MoO
3
/VO
2
and MoO
3
/UO
2
supported on 1 : 3 Al
2
O
3
: SiO
2
with a low area
of ca. 0.1 m
2
g
− 1
, containing ca. 5% active oxide. Experimental conditions were 30
bar pressure with a CH
4
: O
2
ratio of 97 : 3 in the temperature range 430 – 500 ° C. In
order to maintain high selectivity to CH
3
OH the reactor effl uent was cooled to
below 200 ° C within 0.03 s of leaving the heated catalyst bed, by the injection of
cooling water. Although the most successful catalyst was the mixed oxide of molyb-
denum and iron, which gave yields of 869 g (kg cat)
− 1
h
− 1
and 100 g (kg cat)
− 1
h
− 1
of
CH
3
OH and HCHO respectively, these were only marginally greater than the
yields from the molybdenum – uranium oxide catalyst.
A study has been undertaken to compare the effectiveness of molybdenum and
uranium oxide and iron sodalite catalysts with the homogeneous gas - phase oxida-
tion of methane [54] . Catalyst performance was evaluated in a high - pressure
annular reactor and data were compared to the reactivity of the empty reactor. It
was concluded that none of the catalysts gave any advantage over the homogeneous
reaction. Indeed, using a catalyst only reduced the selectivity to the desired partial
oxidation products. Similar conclusions have been reached for many catalysts used
for the partial oxidation of methane, and therefore it is perhaps not surprising that
uranium oxide catalysts are no different.
Catalysts using molybdenum and uranium oxides have also been used for
selective oxidation of other alkanes. The partial oxidation of isobutane over MoO
3
–
UO
3
–
SiO
2
was studied by Corma and coworkers [55] , and led to the formation of
a range of oxygenated hydrocarbons. The primary products formed in this reaction
were methallyl alcohol, methacrolein, acetone and biacetyl. In addition, a large
number of secondary products were obtained, such as acetic acid, ethanal, formic
acid, methanal, and methacrylic acid. Total oxidation to carbon oxides was also
determined. The best performance of the silica - supported MoO
3
/UO
3
catalyst was
obtained at 380 ° C, with an oxygen : isobutene ratio of 12 : 1, to produce a 45%
selectivity to methacrolein and 10% to acetone.

554 13 Heterogeneous Catalysis by Uranium Oxides
Taylor and coworkers demonstrated that catalysts of iron and uranium oxide were
effective for the selective oxidation of propane and propene to formaldehyde [56] .
Catalysts were prepared by co - precipitation and were most effective when prepared
with Fe : U ratios of 0.5 : 3 and 1 : 3. It was possible to achieve 44% selectivity towards
formaldehyde at 450 ° C with a 42% conversion. The balance of the products was
carbon oxides. Characterization of the catalysts showed that they were composed of
iron oxide highly dispersed on UO
3
, and it was a combination of the highly dispersed
iron oxide and the UO
3
that was responsible for the selective oxidation. The mecha-
nism of formaldehyde production is not clear, but there are other studies in the lit-
erature reporting similar observations. Oxidation of propane over highly dispersed
iron oxide supported on SiO
2
showed the formation of formaldehyde [57] . Lee and
coworkers have also shown that formaldehyde is produced during the oxidation of
isobutene using a urania – titania catalyst [58] . Supporting the uranium oxide on
titania modifi ed the redox behavior of the uranium component and this modifi ca-
tion was responsible for the enhanced selective oxidation function.
In recent years, interest in the use of gold - based catalysts has expanded consider-
ably. In particular, the potential of gold catalysts for selective oxidation now receives
signifi cant attention. Gold - based catalysts supported on a wide range of materials
have been prepared and tested for a wide range of reactions. Choudhary and cowork-
ers have used nanoparticles of gold supported on U
3
O
8
for the selective oxidation of
benzyl alcohol to benzaldehyde by molecular oxygen in solvent - free conditions [59] .
Variations of catalyst preparation conditions and reaction conditions were studied.
The best catalysts were those with high gold loadings and small gold particles.
Increasing the reaction temperature or time increased benzyl alcohol conversion,
whilst benzaldehyde selectivity decreased and benzyl benzoate selectivity increased.
The addition of a range of solvents was deleterious for catalyst performance.
13.4.3
Reduction
The versatility of uranium oxides in functioning as catalysts for a range of reactions
is demonstrated by their ability to catalyze a range of reduction reactions, although
primarily they have been used for oxidation. The reduction of acetaldehyde on
UO
2
, prepared by reduction of UO
3
with hydrogen, has been studied by Idriss and
coworkers [40] using TPD. The formation of C
4
olefi ns indicated the ability of UO
2
surfaces to abstract large amounts of oxygen from carbonyl species bound to the
surface. This functionality was due to the ability of the fl uorite structure of UO
2
to readily abstract and accommodate a considerable quantity of oxygen in vacant
sites in the oxide lattice. As discussed in Section 13.2 , this behavior is well known
and is a specifi c feature of the structure of UO
2
.
More recently, Madhavaram and Idriss studied the reactions of acetaldehyde
over the oxides UO
2
, α - U
3
O
8
and β - UO
3
[60] . The products were strongly depen-
dent on the U : O ratio of the oxide. In agreement with earlier work, UO
2
showed
activity for reductive coupling to produce C
4
olefi ns, whilst U
3
O
8
produced pre-
dominantly crotonaldehyde by aldol condensation. It was possible to produce both

13.4 Catalysis by Uranium Oxides 555
furan and crotonaldehyde over UO
3
, and stoichiometric reactions using TPD
demonstrated that the type of product was related to the surface coverage. At low
surface coverage of acetaldehyde, furan was predominant and the product distribu-
tion shifted to furan and crotonaldehyde as the surface coverage increased. The
mode of acetaldehyde adsorption was studied by FTIR, and distinctly different
modes were identifi ed depending on the uranium oxide catalyst. The differences
in products over the oxides have been explained in terms of the possible adsorption
modes and the variation in the semiconductor properties of the different oxides.
Two further reduction reactions of interest are the TPD of acetone over U
3
O
8
,
the principal product being isobutene, and over UO
2
forming mainly propylene
[61] . Again a signifi cant difference between the two uranium oxides used for these
reactions resides in the fact that, in the fi rst case, U
3
O
8
reacted leading to C
–
C
bond formation to give a C
4
olefi n. However, over UO
2
the main product was pro-
pylene, once again showing the ability of UO
2
to accommodate excess oxygen in
its fl uorite structure.
The dehydrogenation of ethylbenzene is an important process used for styrene
manufacture, and uranium oxide catalysts have been investigated for this reaction.
A catalyst of uranium dioxide supported on alumina showed high selectivity to
styrene of 96% at high conversion [62, 63] . The catalyst was synthesized as a higher
oxide of uranium and initially it was not UO
2
. Consequently, over the initial on -
stream period only carbon dioxide and water were observed, as the catalyst pro-
duced total oxidation products. However, as the reaction preceeded the uranium
oxide was reduced in situ by the ethylbenzene and hydrogen to form the active
UO
2
phase. It was only when the uranium oxide was fully reduced to UO
2
that
styrene was produced with high selectivity.
Nickel catalysts supported on uranium oxides have been reported for the hydro-
genation of carbon dioxide to methane [64, 65] . The catalysts were selective below
500 ° C, as CO was the major product at higher temperatures. The nickel was
deposited on the catalysts by evaporation and the reduction characteristics of the
catalysts were complex, depending on the calcination conditions and the metal
content. The uranium oxide support had a crucial role in maintaining the high
dispersion of the active nickel by preventing sintering. The most active catalysts
were those with the highest stable nickel surface areas.
Uranium oxide catalysts have largely been employed for the reduction of organic
species but, in a series of interesting studies, a uranium oxide catalyst has also
been used for the reduction of NO
x
and simultaneous oxidation of CO [66] . Studies
showed that NO
x
was converted to N
2
with 100% selectivity under favorable reac-
tion conditions. Using a mixture of 4%NO, 4%CO with a balance of He, different
uranium oxides were tested in a fi xed bed micro - reactor. The results obtained are
shown in Table 13.5 , and compared with a conventional supported Pt catalyst.
At lower temperatures, reduction of NO produced N
2
O as the major product,
whereas an increase of reaction temperature not only enhanced NO conversion
but generally improved selectivity, as the only product obtained was N
2
.
More - detailed studies of uranium - based catalysts for NO
x
reduction and CO
oxidation have been published and concentrate on catalyst characterization [67]
556 13 Heterogeneous Catalysis by Uranium Oxides
and catalyst performance [68] . Catalysts were prepared from the precursors uranyl
nitrate and uranium(IV) chloride, which were supported on γ - Al
2
O
3
, SiO
2
and
mesoporous SiO
2
. Both the support and the uranium oxide precursor were found
to infl uence the nature of the catalyst. Calcination of the mesoporous SiO
2
sup-
ported material at 800 ° C resulted in signifi cant extrusion of the uranium from the
support, resulting in the formation of large orthorhombic domains of U
3
O
8
. The
formation of a U
3
O
8
phase was promoted on the mesoporous support and by the
presence of chloride. On the silica and alumina supports hexagonal U
3
O
8
was
formed, with the presence of chloride inhibiting the growth of larger uranium
oxide domains on all the catalysts.
Calcination of the uranium oxide mesoporous SiO
2
supported catalyst resulted
in sintering of the active uranium oxide phase into larger particles and this was
detrimental to catalytic activity. However, preparing the mesoporous supported
catalysts from uranyl nitrate using thermal treatment in dilute CO/O
2
or CO/NO
resulted in the best catalysts with high activity comparable to a Pt/Al
2
O
3
catalyst.
The rate expression for the best catalyst was zero order with respect to NO and
showed an order of 1.4 with respect to CO. This was in clear contrast to the reac-
tion over bulk U
3
O
8
, which was dependent solely on the NO concentration. For
the majority of catalysts the presence of residual chloride resulted in lower activity.
The exception was the γ - Al
2
O
3
supported catalyst prepared from the chloride pre-
cursor and thermally treated at 600 ° C in dilute CO/O
2
. It was possible to correlate
the catalytic activity with the residual chloride content and the average crystallite
size of the supported uranium oxide.
13.4.4
Steam Reforming
Nicklin, with others, fi led several early patents describing the use of uranium
oxides as steam reforming catalysts [69] . U
3
O
8
was used along with nickel oxide as
the basis of a steam reforming catalyst, and it was modifi ed with potassium species
(potassium hydroxide, potassium oxide and/or potassium carbonate), all supported
on either alumina or a mix of alumina and magnesium oxide. The uranium and
nickel catalysts proved to be extremely effi cient for steam reforming.
In later work Nicklin describes a reduction – oxidation cycle that was used to treat
the catalysts prior to use [70] . Four to six cycles were performed, with the oxidation
Table 13.5 NO conversion and selectivity to N
2
over uranium oxide catalysts [66] .
Catalyst Conversion (%) Selectivity (%) Temperature ( ° C)
U
3
O
8
100 100 800
U
3
O
8
/ γ - Al
2
O
3
800 ° C
a)
100 100 400
U
3
O
8
/ γ - Al
2
O
3
450 ° C
a)
100 100 400
Pt/ γ - Al
2
O
3
10 100 250
100 35 400
a Calcination temperature.

13.4 Catalysis by Uranium Oxides 557
taking place at 600 – 650 ° C and the reduction at temperatures no higher than 600 ° C
(preferably not above 550 ° C). The gases used for these processes were hydrogen
and oxygen for the reduction and oxidation steps respectively. The catalyst studied
in this patent differed from the previous work, as additional UO
3
was present in
the catalyst. The reduction – oxidation step was added to enhance catalyst activity
compared to the earlier work. The authors observed that during the oxidation part
of the process cycle the amount of oxygen used must be reduced with each suc-
cessive oxidation to prevent the catalyst becoming highly pyrophoric.
A later patent presents more details of catalyst performance and specifi c details
of the preparation and composition of the catalyst [71] . The supported catalyst used
was composed of nickel (23.20%), uranium (11.45%) and potassium (0.23%).
A naphtha stream was used to determine catalyst activity. A steam : naphtha
ratio of 3.8 : 1 was employed at a pressure of ca. 11 bar and a gas hourly space
velocity of 2058 h
− 1
. The temperature of the inlet gas was 465 ° C, whilst the
temperature of the outlet gas was 756 ° C. The outlet of the reactor contained
methane, carbon monoxide, carbon dioxide and hydrogen in the proportions
CH
4
: CO : CO
2
: H
2
= 6.7 : 12.5 : 14.6 : 66.0.
Results of experiments varying the ratio of uranium to nickel showed that the
ratios giving the largest surface area and catalyst volume were in the range 0.45 –
0.76 (U : Ni). These two characteristics were the most important for activity for
these reactions. The catalysts were in a reduced state, which could explain the
addition of the reduction – oxidation step in the previous patent. A further reason
for using catalysts in the 0.45 – 0.74 U : Ni range was that the catalyst demonstrated
greatest resistance to coke deposition at a ratio of 0.71 : 1.
Gavin [72] has studied similar catalysts to Nicklin and coworkers for steam
reforming. It has been suggested that the nickel and uranium combine to form a
nickel uranate phase (NiO · 3UO
3
). Once the catalysts were reduced, the active
components were nickel from excess nickel oxide, nickel from the nickel uranate
and tetra - uranium oxide (U
4
O
9
). Gavin described how the composition of the cata-
lyst affected the amount of NiO · 3UO
3
. The relationship between the catalyst
composition and activity for steam reforming is shown in Table 13.6 .
Table 13.6 Activity of nickel – uranium oxide catalysts for steam reforming of naphtha [72] .
Catalyst composition (%) Relative amount
of NiO
· 3UO
3
Conversion
(%)
Ni U Ba K
11.0 7.0 0 0 9.1 100.0
11.7 7.6 1.5 0 4.6 100.0
11.7 7.7 1.6 0 5.2 99.0
11.3 7.4 2.11 0 5.0 98.5
10.9 6.7 2.12 0 3.6 95.6
10.6 7.2 0 0.59 3.6 93.0
12.0 7.9 1.72 0.50 2.0 90.0

558 13 Heterogeneous Catalysis by Uranium Oxides
The presence of the alkaline components is essential and they are converted into
carbonates by heating in carbon dioxide during the catalyst preparation. It is
argued that as the concentration of the alkaline component increased there was
increased likelihood that the nickel uranate phase would react to produce barium
uranate (BaU
2
O
7
) and NiO. The barium and potassium were also thought to reduce
the tendency of coking on the catalyst surface, and their concentration is a balance
between their effi cacy for producing the most active phases and reducing coke
formation.
Despite the earlier patent reports of the suitability of nickel - based catalysts
incorporating uranium oxide for steam reforming, uranium did not become a
component of commercial steam reforming catalysts. Nevertheless, interest has
continued in assessing the effi cacy of uranium oxide as a steam reforming cata-
lyst component. Gordeva and coworkers prepared relatively porous oxides of UO
2
and U
3
O
8
as supports for nickel and ruthenium as catalysts for methane steam
reforming [73] .The catalysts were designed so that they could be used for conver-
sion of nuclear energy to chemical energy by the production of hydrogen. High
production rates of hydrogen were observed at 1 bar pressure and temperatures
of 600 – 700 ° C. Under operating conditions in a nuclear reactor, fi ssile products
would be expected to contaminate the syngas. In order to try and limit contami-
nation, studies also investigated containing the uranium oxide within a thin
coating of MgO/Al
2
O
3
. The infl uence of the coating on catalyst activity is not
clear and catalysts to be used for such advanced processes would clearly need
further development. However, uranium oxide based catalysts are ideal for appli-
cations of this type, owing to their fi ssile properties and the knowledge base
that is already in place because of the use of uranium oxides by the nuclear
industry.
13.5
Conclusions
Uranium oxides have been used as catalysts and catalyst components for a rela-
tively wide range of reactions. The oxides of uranium are numerous, with the main
oxides being UO
2
, U
3
O
8
and UO
3
. The structures of the oxides can be complex, as
can the relationship between the phases. However, the oxides have many proper-
ties that make them versatile catalysts and catalyst components. Uranium oxides
have been most widely applied as catalysts for oxidation reactions, and these
include total oxidation and partial oxidation. Uranium oxide based catalysts have
demonstrated excellent performance for selective oxidation and have been used
commercially, although this is no longer the case. Uranium oxides have also been
employed as catalysts for a range of reduction reactions and for steam reforming.
The use of uranium oxides as catalysts may be a controversial issue; however,
depleted uranium oxide is relatively widely available. The main concern in using
uranium as a catalyst component is associated with its toxicity, which is compara-
ble with that of lead, and it must be handled accordingly.
