Jackson S.D., Hargreaves J.S.J. Metal Oxide Catalysis
Подождите немного. Документ загружается.


Heterogeneous Catalysis by Uranium Oxides
Stuart H. Taylor
539
Metal Oxide Catalysis. Edited by S. David Jackson and Justin S. J. Hargreaves
Copyright © 2009 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim
ISBN: 978-3-527-31815-5
13
13.1
Introduction
The element uranium has been used, in the form of its natural oxide, since ancient
times. It was used as an orange - yellow coloring agent for ceramics dating from at
least 79 AD. The element was identifi ed by Martin Heinrich Klaproth in 1789
when it was discovered in natural minerals. The element was named in honor of
the recently discovered new planet Uranus. In 1841 Peligot showed that Klaproth ’ s
substance , previously believed to be the metal, was in fact the oxide UO
2
. Shortly
thereafter Peligot showed that it was possible to produce the metal by reduction
of uranium tetrachloride by the metals sodium and potassium. The construction
of the periodic table by Mendel é ev in 1872 focused attention on uranium as it was
then the heaviest of the known elements. This stimulated much research on the
element but it was not until 1896 that Antoine Becquerel recognized the radioac-
tive properties of uranium. A research program starting in 1934 and led by Enrico
Fermi resulted in the fi ssile properties of uranium being used for power genera-
tion and production of nuclear weapons. The isotope
235
U is still of primary
importance and is used for power generation in both metallic and oxide forms. It
is the only naturally occurring nuclide that undergoes nuclear fi ssion with thermal
neutrons.
The average concentration of uranium in the earth ’ s crust is somewhere in the
range 2 – 4 ppm, which is very similar to elements such as molybdenum and
approximately 40 times greater than silver. Estimates of uranium reserves suggest
that there are 4.7 million tons of easily accessible uranium minerals, and a further
35 million tons that could be recovered with further investment. In addition, a
further 4.6 billion tons is present in sea water. Uranium is obtained mainly from
the mineral uraninite, also called pitchblende, which consists largely of UO
2
.
Uranium - rich mineral resources are mined by a combination of open - cast and
underground extraction methods, whilst poorer grade deposits are often recovered
by leaching with acid or alkali.
Heterogeneous catalysis by compounds of uranium, and in particular the oxides
of uranium, is well established and has a long history. The versatility of uranium

540 13 Heterogeneous Catalysis by Uranium Oxides
oxide based catalysts is related to the rich and diverse properties of the wide variety
of phases and mixed phases that can be synthesized. The aim of this chapter is to
introduce the reader to the chemistry of uranium compounds and highlight their
uses as heterogeneous catalysts.
13.2
Structure of Uranium Oxides
Before examining the effi cacy of uranium oxides as catalysts it is benefi cial to
consider the structures of the oxides. The three main oxides of uranium are UO
2
(brown - black), UO
3
(orange - yellow) and U
3
O
8
(green - black). In addition to these
three compounds, a considerable number of oxides exist within the stoichiometric
range UO
2
–
UO
3
. The range of stoichiometries and structures that are possible for
the uranium – oxygen system make it the most complex of the Actinide elements,
and one of the most complicated of all the elements. Changing the oxidation state
of a given uranium ion is often accompanied by a modifi cation of the structure.
For example, within a limited temperature range, the uranium oxide structure
and the uranium oxidation state can be infl uenced by the atmosphere to which
it is exposed [1] . The complexity of the uranium – oxygen system is illustrated in
Figure 13.1 .
Allen and Holmes [2] have investigated the mechanism of transformation of
UO
2
to UO
3
and they have also summarized the intermediate phases that have
been identifi ed (Table 13.1 ).
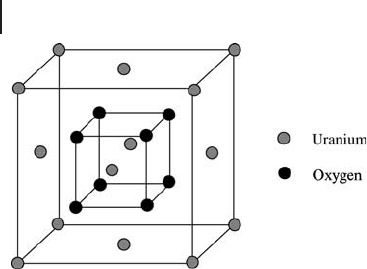
UO
2
has the fl uorite structure; it is a face centered cubic based structure with
each uranium ion coordinated to eight oxygen ions (Figure 13.2 ). The fact that
there are vacant coordination positions in the lattice is critical for the catalytic, and
other, properties of the oxide. This is because ion exchange is more effi cient by
Figure 13.1 Phase diagram of uranium oxides. ( Journal
of the Chemical Society, Dalton Transactions (1982), 2169;
reproduced by permission of The Royal Society of Chemistry).

Table 13.1 Crystallographic data for the known uranium oxides [2] .
Phase O : U ratio Structure Crystal class Unit cell dimensions ( Å ) Ref.
UO
2
2.00 fl uorite cubic a = 5.470 [3]
UO
2+ x
2.00 – 2.25 fl uorite cubic a = 5.470 – 5.445 [4]
α - U
4
O
9
2.45 – 2.25 fl uorite rhombohedral
a = 4 × (5.441 – 5.444); α = 90.078 °
( T = 20 ° C)
[5]
β - U
4
O
9
2.25 fl uorite cubic
a = 4 × 5.438 ( T = 65 ° C)
[6]
γ - U
4
O
9
2.25 fl uorite cubic
a = 4 × (5.47 – 5.50) ( T > 600 ° C)
[6]
α - U
3
O
7
2.27 – 2.33 fl uorite tetragonal a = 5.472; c = 5.397 [7]
β - U
3
O
7
2.33 fl uorite tetragonal a = 5.363; c = 5.531 [7]
U
16
O
37
( γ - U
3
O
7
)
2.31 fl uorite tetragonal a = 5.407; c = 5.497 [8]
U
8
O
19
( δ - U
3
O
7
)
2.375 fl uorite monoclinic a = 5.378; b = 5.559; c = 5.378;
β = 90.29 ° C
[9]
γ - U
2
O
5
2.50 fl uorite monoclinic a = 5.410; b = 5.481; c = 5.410;
β = 90.49 °
[8]
α - U
2
O
5
2.50 layered hexagonal a = 3.885; c = 4.082 [8]
β - U
2
O
5
2.50 layered hexagonal a = 3.813; c = 13.18 [8]
U
2
O
5
2.50 layered orthorhombic a = 8.29; b = 31.71; c = 6.73 [10]
α - U
3
O
8
2.660 – 2.667 layered orthorhombic a = 6.715; b = 11.96; c = 4.146 [11, 12]
β - U
3
O
8
2.67 layered orthorhombic a = 7.07; b = 11.45; c = 8.30 [13]
U
12
O
35
2.92 layered orthorhombic a = 6.91; b = 3.92; c = 4.12 [14]
α
–
UO
3
3.00 layered orthorhombic a = 6.84; b = 43.45; c = 4.12 [15]
β - UO
3
3.00 layered monoclinic
a = 10.34; b = 14.33; c = 3.91; β = 99.03
[16]
γ - UO
3
3.00 tetragonal a = 6.013; c = 19.975 [17]
δ - UO
3
3.00 ReO
3
- type cubic a = 4.16 [18]
ε - UO
3
3.00 layered triclinic
a = 4.002l; α = 98.10 ° ; b = 3.841;
β = 90.20 ° ; c = 4.165; γ = 120.17
[19]
η - UO
3
3.00 orthorhombic a = 7.511; b = 5.466; c = 5.224 [20]
13.2 Structure of Uranium Oxides
541
542 13 Heterogeneous Catalysis by Uranium Oxides
exchange through lattice vacancies. Another important factor is that the UO
2
fl uo-
rite structure is readily able to accommodate up to 10% additional oxygen in the
lattice without any change of the structure [21] .The study of the oxidation of
uranium oxides can be divided into two distinct regions: the oxidation of stoichio-
metric cubic UO
2
to orthorhombic U
3
O
8
and the subsequent oxidation of this
phase to UO
3
[22] .
In the fi rst region the initial stage is the oxidation step of UO
2
to UO
2.25
, where
gradual addition of oxygen leads to displacement of the ideal lattice positions until
the structure of U
4
O
9
(UO
2.25
) is reached. The addition of this oxygen has no effect
on the uranium sub - lattice as the oxygen is incorporated in interstitial sites.
Increasing the stoichiometry to UO
2.12
, the oxygen is distributed randomly in the
interstitial sites; however, as the O/U ratio increases further towards UO
2.25
, the
formation of clusters in the lattice takes place. The clusters develop to form
ordered cluster chains. The clusters are known as 2 : 2 : 2 clusters and contain two
interstitial oxygen atoms in the (110) direction, two vacancies in the oxygen sub -
lattice and two interstitial oxygen atoms in the (111) direction [23, 24] .
For the stoichiometry UO
2.25
, the structure can be simplifi ed to an arrangement
of 4 : 3 : 2 clusters [25] .These clusters are composed of four interstitial oxygen atoms
in the (110) direction with three oxygen vacancies and two interstitial oxygen atoms
in the (111) direction. The addition of this oxygen causes the expansion of the
cubic structure so that the cell dimension for U
4
O
9
is approximately four times
that of UO
2
[6] , although the cubic structure is retained.
Increasing the O/U ratio further from the stoichiometry U
4
O
9
to U
3
O
7
, the
structure of the oxide changes from the cubic crystal system to a variety of tetrago-
nal structures [7, 8] . Further oxidation from U
3
O
7
to U
2
O
5
results in a further
change of structure from the fl uorite structure to a layered structure that is close
to that observed for U
3
O
8
[8, 10] . The U
2
O
5
phase was fi rst shown conclusively to
exist by Rundle and coworkers in 1948 [10] . This work showed that the structure
was orthorhombic like U
3
O
8
, but the actual cell dimensions were larger [11 – 13] .
It is interesting that U
2
O
5
phases have been reported with a monoclinic fl uorite
structure [8] , with a hexagonal layered structure [8] and with an orthorhombic
layered structure similar to that for U
3
O
8
[10] . Allen and Holmes [2] suggest that
the β - U
2
O
5
could represent an intermediate bridging structure, since it shows
neither fl uorite - type nor U
3
O
8
- type structure.
Figure 13.2 Fluorite structure of UO
2
.
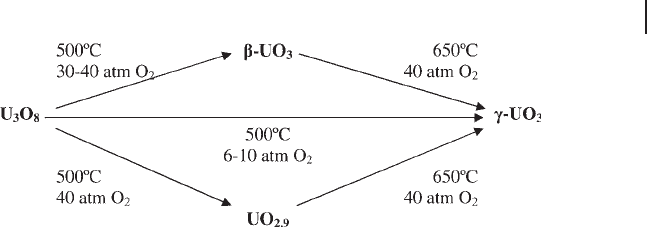
The second region of oxidation covers the addition of oxygen from U
3
O
8
to UO
3
.
Numerous phases have been identifi ed for UO
3
, with the majority showing layered
structures similar to U
3
O
8
[14 – 19] . UO
3
can be prepared from U
3
O
8
by heating
between 400 and 700 ° C; however, it must be prepared under pressures of up to
40 atmospheres [13] . The transformation of U
3
O
8
to UO
3
phases is summarized
in Figure 13.3 .
In UO
3
phases the uranium atom may be coordinated to six, seven or eight
oxygen atoms, leading to at least fi ve known modifi cations. For example the γ - UO
3
phase has two independent uranium atoms U (1) and U (2) in the structure [17] .
The coordination polyhedron around U (2) is a slightly distorted octahedron,
whereas U (1) is surrounded by eight oxygen atoms forming a somewhat distorted
dodecahedron. This chapter is not intended to provide a detailed understanding
of the multiple and complex phases of the uranium oxides, but it is clear even
from a brief survey that the structures and chemistry are diverse.
13.3
Historical Uses of Uranium Oxides as Catalysts
Uranium compounds have historically been used as catalysts for many years,
dating back to the initial development of catalysis as a recognized scientifi c disci-
pline. In 1922, James published a paper detailing the vapor - phase low - temperature
catalytic oxidation of fuel oil and other crude petroleum fractions [26] . It was con-
cluded that to obtain satisfactorily high yields of suffi cient quality for industrial
use a catalyst must be employed. An apparatus was developed to test catalysts on
a pilot - plant scale and it was found that the most effective confi guration consisted
of three catalyst layers, called catalyst screens. The most effective catalyst system
for the reaction was an initial bed composed of uranium oxide and two subsequent
beds containing molybdenum oxide. The uranium oxide was the best catalyst for
the oxidation to aldehyde - type compounds and was particularly favored when acids
were the desired products, as the higher yields of aldehyde were converted to acids
over the molybdenum oxide screens. Typically the catalyst was supported on
asbestos, although it is unclear whether the asbestos was a support in the way
conventionally associated with a catalyst or merely acted as gauze to hold the
Figure 13.3 Summary of preparation of γ - UO
3
from U
3
O
8
. (Adapted from [14] ).
13.3 Historical Uses of Uranium Oxides as Catalysts 543

544 13 Heterogeneous Catalysis by Uranium Oxides
catalyst bed in place. Air was used as oxidant and this was fed independently before
each catalyst bed, which ensured that the oxygen concentration laterally through
the catalyst screens was relatively low and probably helped to reduce over -
oxidation. The production of acids was typically carried out at 280 ° C, whilst
increasing the reaction temperature to ca. 400 ° C resulted in the production of
hydrocarbons and lower molecular weight oxygenates, which were suitable for use
as fuels. The data presented are relatively scant; however, the publication is one
of the fi rst to indicate that uranium oxide is a potentially important oxidation
catalyst.
Early work has also demonstrated that uranium oxide catalysts show promising
activity for the oxidation of hydrocarbons in the liquid phase. A patent granted to
the Dow Chemical Company describes a process for the manufacture of phenol
from the partial oxidation of benzene [27] . The Dow Chemical patent claims that
oxides of vanadium, molybdenum, tungsten and uranium were all effective cata-
lysts for the oxidation of benzene to phenol in the presence of aqueous sodium
hydroxide. The process was operated at approximately 200 atm under air in the
temperature range 320 – 400 ° C with an alkali solution of 20 – 25%. Sodium benzoate
was formed in the aqueous phase and the unreacted benzene remained as an
immiscible organic phase. Phenol was liberated by acidifi cation of the aqueous
phase. The process was 100% selective to the mono phenol product, and the unre-
acted benzene was easily recycled. It is stated that the uranium oxide catalyst gave
the best results, although these results are not specifi ed. Furthermore, the nature
of the uranium oxide catalyst is not clear, but the patent acknowledges the exis-
tence of several different oxides and it is implied that all have been investigated
and there is no differentiation in their activity.
The vapor phase oxidation of aromatic hydrocarbons using uranium oxide cata-
lysts is also discussed in a patent fi led shortly after the liquid - phase process and
was assigned to the Barrett Corporation of New Jersey [28] . Studies concentrated
mainly on the oxidation of toluene, and a large range of metal oxides were inves-
tigated. Air was used as the oxidant and it was pre - mixed with toluene before
passing over the catalyst maintained at standard test conditions. The conditions
are not specifi cally stated although it is thought that they are similar to 14/1 air/
toluene by weight, pressure slightly elevated above atmospheric and a temperature
of 500 ° C. The reactivity was classed into four groups, these are described below
and the oxides contained in them are also listed:
Group 1: Relatively high benzaldehyde production and relatively low combustion
oxides of tantalum, tungsten, zirconium and molybdenum.
Group 2: Relatively high benzaldehyde production and relatively high combus-
tion oxides of manganese, chromium, copper, nickel, thorium and
uranium.
Group 3: Relatively low benzaldehyde production and relatively high combustion
oxides of cobalt and cerium.
Group 4: Relatively low benzaldehyde production and relatively low combustion
oxides of titanium, bismuth and tin.

The activity demonstrated by uranium and molybdenum was signifi cantly better
than the other catalysts in the respective groups, whilst vanadium oxide was not
classifi ed in any of the groups as it produced quantities of maleic acid and benzoic
acid in addition to benzaldehyde and carbon oxides. The catalyst performance was
considerably enhanced by synthesizing catalysts containing mixtures of the oxides.
The catalysts were prepared by a type of impregnation technique which involved
placing a support, usually pumice or asbestos, in a solution of the metal salts
before evaporating the solution to dryness.
Parks and Katz [29] also carried out early studies into the oxidation of toluene
to benzaldehyde and benzoic acid over a range of catalysts, concentrating on
uranium, tungsten and molybdenum oxides. Reactions used an air/toluene
mixture with a gas hourly space velocity ranging from ca 400 – 1500 hour
− 1
and
reaction temperatures 350 – 520 ° C. Catalytic activity was expressed in terms of
oxygen consumption to total and partial oxidation products. Selected data are pre-
sented in Table 13.2 . A considerable range of catalysts were tested, and although
specifi c yields of products are not available it is clear that the catalysts are active
for oxidation. Appreciable yields of selective oxidation products were obtained
when uranium oxide was used in combination with other oxides that are recog-
nized as selective oxidation components.
Table 13.2 Catalytic data for toluene oxidation using uranium oxide based catalysts [6] .
Catalyst
GHSV (h
− 1
)
Toluene : air Temp ( ° C) O
2
consumed in
total oxidation
(%)
O
2
consumed
in partial
oxidation (%)
UO
2
WO
4
1455 0.72 445 7.8 4.8
480 23.8 14.2
545 50.5 26.2
UO
2
WO
4
+ Al
2
O
3
1455 0.72 405 30.9 21.4
545 62.8 28.8
UO
2
MoO
4
(no support)
414 0.63 406 6.7 8.6
430 15.2 12.4
524 74.3 23.8
U(MoO
4
)
2
500 0.28 385 7.1 7.1
415 27.6 16.2
450 68.6 29.5
U(MoO
4
)
2
510 73.3 24.7
492 0.16 390 15.2 8.6
420 36.1 12.4
470 75.3 16.2
GHSV, gas hourly space velocity.
13.3 Historical Uses of Uranium Oxides as Catalysts 545

546 13 Heterogeneous Catalysis by Uranium Oxides
In another patent also granted to the Barrett Corporation of New Jersey [30] , the
partial oxidation of ethanol to acetaldehyde by oxide catalysts was investigated. The
patent details a process for reducing combustion by controlling the reaction tem-
perature by means of effi cient heat removal from the functioning catalyst. This
was achieved by packing the catalyst in a series of tubular reactors, and is the pre-
cursor to the multi - tubular design that is used so effectively by the modern chemi-
cal industry. The majority of results are concerned with vanadium oxide as the
catalyst, which at 300 ° C and 0.39 sec contact time produced 70 parts acetaldehyde
per 100 parts of ethanol. Acetic acid (10 parts) was also produced and only approxi-
mately 3% of the ethanol feed was combusted to carbon dioxide. No specifi c data
were presented but it was acknowledged that many oxides, including those of
uranium, were active. Cobalt, tin, cerium and titanium oxides only showed low
acetaldehyde yields; all the other oxides showed reasonable yields although they
were all lower than vanadium oxides. It was also highlighted that oxides of uranium,
chromium, manganese and copper showed higher levels of combustion. The study
was extended to include mixed oxide catalysts: one such system consisted of 93%
uranium oxide and 7% molybdenum oxide, which yielded almost exclusively acet-
aldehyde with virtually no carbon dioxide and only a small amount of acetic acid
products.
In 1932 Wietzel and Pfaundler [31] described the use of a uranium oxide based
catalyst to produce valuable hydrocarbons of low boiling point from various sources
including coal, tar and mineral oils. One example describes the use of uranium
oxide in a process in which a fraction of mineral oil (BPt > 270 ° C) was passed with
excess hydrogen over the catalyst at 450 ° C and 200 at. The actual catalytic material
was fi ne aluminum granules activated with 1 – 2% of uranyl nitrate. The catalyst
produced aromatics of boiling point less than 200 ° C in a yield of over 80%.
Uranium oxide was also cited for an example to convert bituminous coal tar (BPt
300 – 420 ° C). The pressure was the same as in the experiments described above,
with the temperature raised to 480 ° C. The catalyst in this example was formed by
heating aluminum gauze in ammonium vanadate and uranyl nitrate in hydrochlo-
ric acid. The resulting uranium – vanadium – aluminum catalyst was able to convert
the coal tar to 70% oil.
Thus, historically, uranium oxides have been used as catalysts, and more
often they have been used as catalyst components in combination with other
metal oxides. Often it is diffi cult to identify the catalysts unambiguously: there
is little characterization data in the studies, and it is most likely that the specifi c
stoichiometries of uranium oxides quoted as catalysts are not correct. There are
many other examples of the use of uranium oxides for heterogeneous catalysis
and the few examples presented in this section are typical of some of the earli-
est uses. It is interesting to note that, although some of the work highlighted
was carried out over 80 years ago, some of the aims, such as selective hydro-
carbon oxidation, are still major research aims for heterogeneous catalysis
today.

13.4 Catalysis by Uranium Oxides 547
13.4
Catalysis by Uranium Oxides
13.4.1
Total Oxidation
One of the earlier studies investigating the total oxidation of uranium oxides con-
centrated on the oxidation of carbon monoxide [32] . The oxide U
3
O
8
was the most
active catalyst probed for the formation of carbon dioxide by oxidation by molecular
oxygen. Comparison was made with V
2
O
5
, MoO
3
and WO
3
; although these oxides
are not recognized as high - activity catalysts for carbon monoxide oxidation the
results indicated that U
3
O
8
was a potential catalyst for total oxidation.
The oxidative destruction of volatile organic compounds ( VOCs ) over uranium
oxides has been studied by Hutchings, Taylor and coworkers [33, 34] . Studies have
shown that U
3
O
8
is a highly active catalyst for the destruction of a wide range of
chemically diverse VOCs. In the case of benzene oxidation over U
3
O
8
a conversion
of 100% at 400 ° C was reached, with selectivities of 27% and 73% for CO and CO
2
respectively. Comparing these results with the total oxidation activity of Co
3
O
4
,
a well known active combustion catalyst, it was found that even at 450 ° C the con-
version of benzene over Co
3
O
4
was only 90%. The uranium oxide U
3
O
8
was par-
ticularly active for the total oxidation of chlorinated VOCs [33] . For example,
investigating the oxidation of chlorobenzene, 99.7% conversion was reached at
only 350 ° C with selectivity to CO
x
of 100%. Furthermore, oxidation of chlorobu-
tane at 350 ° C led to 100% selectivity to CO
x
with a conversion higher than 99.5%.
No catalyst deactivation was observed for the oxidation of the VOCs, even for pro-
longed oxidation of chlorinated compounds [35] .
Short - chain linear alkanes are amongst the most diffi cult of VOCs to destroy.
A study has investigated the catalytic activity of uranium oxide catalysts for the
destruction of alkanes in the C
1
–
C
4
range [36] . Uranium oxide, U
3
O
8
, showed rela-
tively low activity for the combustion of methane and ethane and moderate activity
for propane and n - butane. Catalyst activity was improved by supporting the
uranium oxide on silica and further improvements were achieved by the addition
of chromium. X - ray Diffraction ( XRD ), X - ray Photoelectron Spectroscopy ( XPS )
and Temperature - Programmed Reaction ( TPR ) characterization data indicated
that supporting the U
3
O
8
phase and adding chromium modifi ed the structure and
chemistry of the oxide. This modifi cation may culminate in an increase of the
detect structure of the oxide, resulting in the increased oxidation activity.
The effect of water addition on the complete oxidation of benzene and propane
VOCs by uranium oxide catalysts has been investigated [37] . Benzene oxidation
was studied using a silica - supported U
3
O
8
catalyst. Complete oxidation was pro-
moted by the addition of 2.6% water compared with the reactivity when no water
was added to the reactant feed. Increasing the water concentration to 12.1%
resulted in a suppression of oxidation activity. Investigation of propane oxidation
using U
3
O
8
showed a dramatic promotion of activity. Propane conversion was ca
50% at 600 ° C without added water, whilst it increased to 100% at 400 ° C with the

548 13 Heterogeneous Catalysis by Uranium Oxides
addition of 2.6% water. Comparison of total oxidation activity with Mn
2
O
3
showed
that any level of water addition suppressed conversion, and this was in clear con-
trast to the U
3
O
8
catalyst. In situ powder XRD studies showed that the bulk U
3
O
8
structure was stable under all the reaction conditions. The origin of the increased
activity is not clear but it may be due to modifi cation of the catalyst surface, pos-
sibly aiding the activation of the VOCs by increased hydroxylation.
A Temporal Analysis of Products ( TAP ) reactor has been used to investigate the
mechanism of oxidation by uranium oxide catalysts [35, 38] . A combination of TAP
pulse experiments with oxygen present and absent in the gas phase indicated that
the lattice oxygen from the catalyst was responsible for the total oxidation activity.
It was proposed that the catalyst operates by a redox mechanism using lattice
oxygen and the high activity shown by U
3
O
8
was due to the facile uranium redox
couple and the non - stoichiometry of the oxide. Isotopically labeled oxygen studies
of carbon monoxide oxidation confi rmed that lattice oxygen was the active
oxidant.
In a much earlier patent, the removal of organics from exhaust gases by oxida-
tion over a supported uranium oxide catalyst was reported by Hofer and Anderson
[39] . The catalyst was 4% U
3
O
8
supported on alumina spheres. The authors used
the incipient wetness technique to impregnate alumina with uranyl nitrate solu-
tion. In this case the catalyst precursors were calcined at 700 ° C for 3 h to decom-
pose the uranium salt. The use of other uranium compounds as starting materials
was mentioned and these included uranyl acetate, uranium ammonium carbonate
and uranyl chloride. The alumina - supported catalyst had a surface area of ca
400 m
2
g
− 1
and further added components, such as copper, chromium and iron,
were highlighted as effi cient additives to increase activity.
The catalysts were evaluated by exposure to a simulated automobile exhaust gas
stream composed of 0.2% isopentane, 2% carbon monoxide, 4% oxygen and
a balance of nitrogen. The temperature required to oxidize the isopentane and
carbon monoxide was used to compare catalyst performance. The chromium -
promoted catalyst oxidized isopentane at the lowest temperature, and a mixed
chromium/copper - promoted catalyst proved the most effi cient for oxidizing carbon
monoxide and isopentane. It is interesting to note that the test rig used a stationary
engine with 21 pounds of catalyst. Although the catalyst was very effective it is
diffi cult to envisage uranium oxide catalysts employed for emission control of
mobile sources.
13.4.2
Selective Oxidation
Uranium oxides have been investigated as catalysts and catalyst components for
selective oxidation. They are more commonly used as catalyst components, but
there are also reports of uranium oxide alone as a selective oxidation catalyst. The
oxidation of ethylene over UO
3
has been studied by Idriss and Madhavaram [40]
using the technique of temperature programmed desorption ( TPD ). Table 13.3
shows the desorption products formed during TPD after ethylene adsorption at
room temperature on UO
3
. The production of acetaldehyde from ethylene indicates
