Jackson S.D., Hargreaves J.S.J. Metal Oxide Catalysis
Подождите немного. Документ загружается.

508 12 Vanadium Phosphate Catalysts
This has been attributed to the use of ammonia as a probe molecule, since this
cannot distinguish between Lewis and Br ø nsted acidity. Cornaglia and coworkers
[79] measured the acid sites using pyridine and acetonitrile. However, the pyridine
results showed no correlation between the activity and selectivity to maleic anhy-
dride and either the Lewis - to - Br ø nsted acid sites ratio (L/B acidity ratio) or the
Lewis acid site concentration.
Adsorption of acetonitrile enabled the strength of the Lewis sites to be measured
and a greater number of strong Lewis sites were found to be present in organically
made catalysts than in those prepared in an aqueous medium. Thus, the greater
the concentration of strong Lewis acid sites in the catalyst, the higher the maleic
anhydride yield. Cornaglia and coworkers suggest that the strong Lewis sites are
responsible for butane dehydrogenation.
12.3
Preparation of VPP Precursors
Vanadium phosphate catalysts are obtained by activating the catalyst precursor in
the reaction feedstock. After pre - treatment, the catalyst is equilibrated and catalytic
activity remains consistent throughout the lifetime of the catalyst. The activated
catalysts are formed topotactically from the precursors [86] . For this reason, a great
deal of research is based around the preparation of catalyst precursors with well
defi ned, favorable morphologies.
VOHPO
4
· ½H
2
O (Figure 12.4 ) is the catalytic precursor for (VO)
2
P
2
O
7
. A number
of preparation methods are commonly used to prepare VOHPO
4
· ½H
2
O. These
usually involve reacting V
2
O
5
and H
3
PO
4
in the presence of a reducing agent.
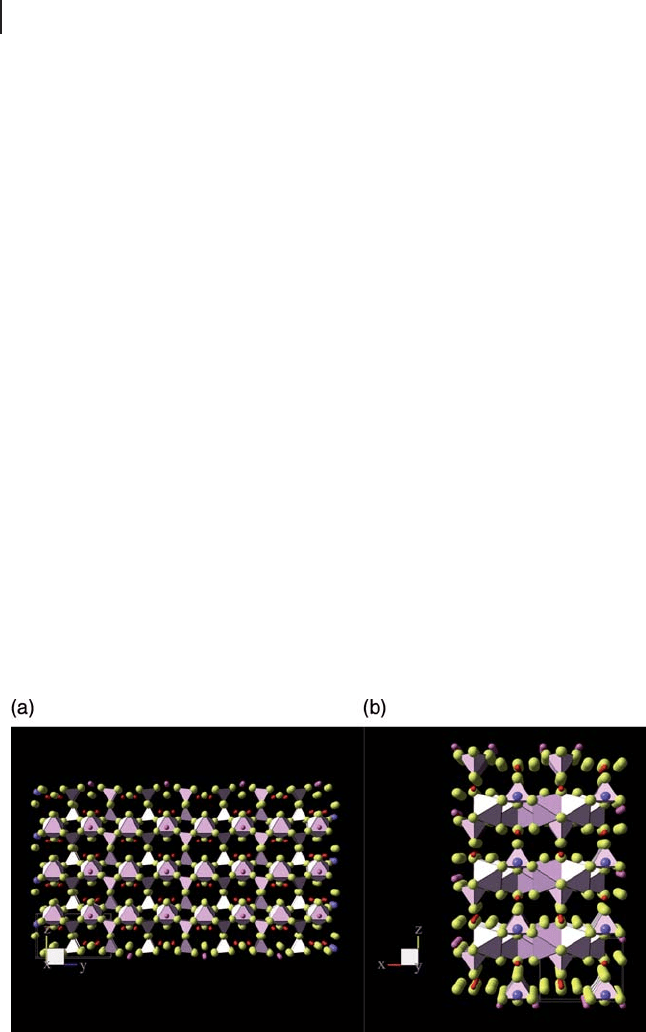
Figure 12.4 Schematic diagram showing the layered structure
of hemihydrate precursors in the (a) (100) and (b) (010)
directions (both orthogonal to the (001) layer normal
direction) [87] .
Originally, catalyst precursors were prepared in aqueous media, most commonly
using hydrochloric acid as the reducing agent [80, 88 – 91] . This is commonly
referred to as the VPA route:
2. H
3
PO
4
1. ∆, 2h
V
2
O
5
+ HCl VOHPO
4
·½H
2
O
(12.2)
Alternative aqueous routes have been used by a number of groups to prepare
VOHPO
4
· ½H
2
O. Oxalic acid [88, 92] , lactic acid [93] , phosphorous acid [93] and
NH
2
OH.HCl [75] have all been investigated as reducing agents in place of hydro-
chloric acid.
There has also been investigation into alternative vanadium sources. Poli and
coworkers [88, 92] used NH
4
VO
3
as the vanadium source in conjunction with
H
3
PO
4
and oxalic acid, and Harouch Batis and coworkers [91] used a VCl
3
/V
2
O
5
mixture instead of vanadium pentoxide. Mizuno and coworkers [95] reported a
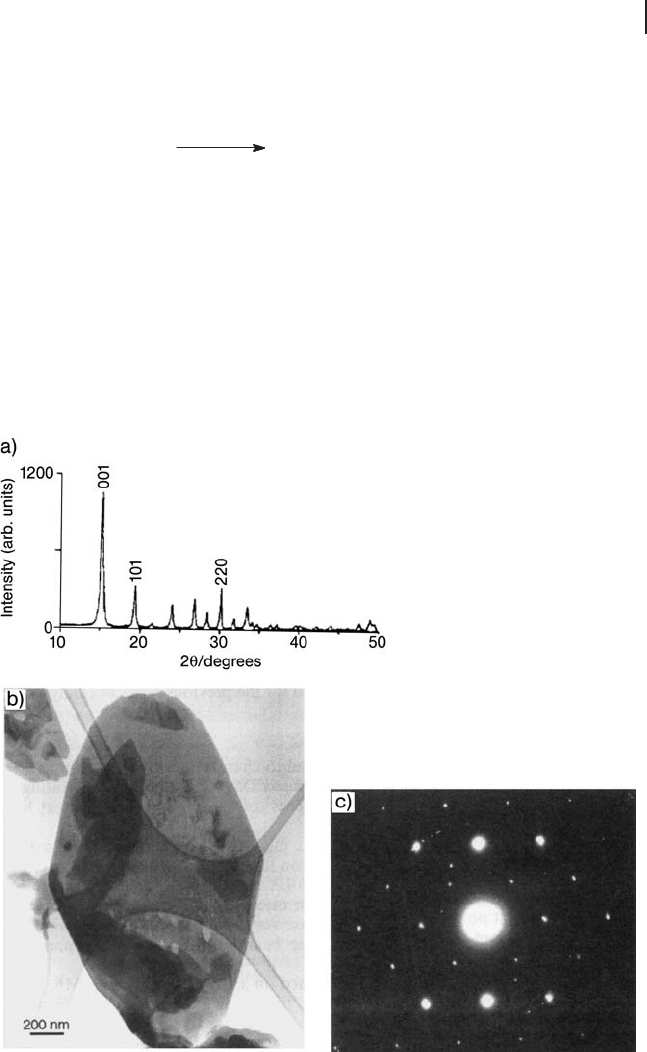
Figure 12.5 (a) XRD pattern, (b) bright - fi eld image and
(c) selected area diffraction pattern from the hemihydrate
precursor material [94] . (Reproduced with permission).
12.3 Preparation of VPP Precursors 509
510 12 Vanadium Phosphate Catalysts
preparative route using vanadium metal to reduce vanadium pentoxide. They
heated a mixture of phosphoric acid, cetyltrimethylammonium chloride, vana-
dium and vanadium pentoxide in an autoclave, at 200 ° C for 48 h. Schimoda and
coworkers [75] have also reported the direct reaction of V
2
O
4
and H
3
PO
4
.
In the 1970s, catalyst precursors prepared in organic media became increasingly
popular. The most common route (the VPO route, Figure 12.5 ), is a one - pot
method using alcohol as both the solvent and the reducing agent:
V
2
O
5
+
∆, 16h
H
3
PO
4
+ alcohol VOHPO
4
·½H
2
O
(12.3)
A number of alcohols have been used in this preparation, isobutanol being the
most common [96] . Another common organic route uses a mixture of isobutanol
and benzyl alcohol. V
2
O
5
is refl uxed in the alcohol for an hour before H
3
PO
4
is
added, and the mixture refl uxed for a further hour [96, 97] .
Johnson and coworkers [86] described a method for the preparation of
VOHPO
4
· ½H
2
O by reduction of VOPO
4
· 2H
2
O with alcohol. This is known as the
VPD route. This route was investigated more fully by Horowitz and coworkers [98]
for short - chain alcohols, and Ellison and coworkers [99, 100] for longer chain
alcohols. VOPO
4
· 2H
2
O is prepared by heating an aqueous solution of V
2
O
5
and
H
3
PO
4
under refl ux conditions for 16 h. This is then reduced with alcohol to yield
VOHPO
4
· ½H
2
O:
H
2
O
∆, 16h∆, 16h
alcohol
VOHPO
4
·½H
2
OV
2
O
5
+ H
3
PO
4
VOPO
4
·2H
2
O
(12.4)
Alternative vanadium sources have been investigated using organic solvents.
Doi and Miyake [101, 102] used V
4
O
9
as the vanadium source. V
2
O
5
was initially
reduced to V
4
O
9
by isobutanol. The V
4
O
9
was then reacted with H
3
PO
4
with a range
of alcohols as the solvent. As with their aqueous preparations Harouch Batis and
coworkers [91] used a VCl
3
/V
2
O
5
mixture instead of vanadium pentoxide.
Guilhaume and coworkers [103] investigated the effect of the reactant V
2
O
5
morphology on the morphology of the fi nal catalysts. They found a correlation
between the size of the V
2
O
5
grains and the preferentially exposed planes of
(VO)
2
P
2
O
7
. This study was only carried out on a few V
2
O
5
samples, and further
research on this aspect of the preparation is needed.
Investigations comparing organically and aqueously prepared VOHPO
4
· ½H
2
O,
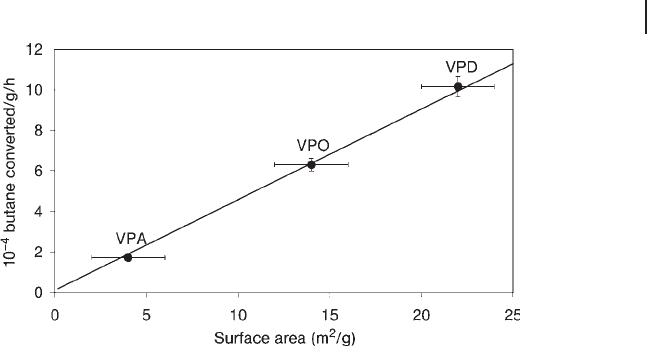
found that the fi nal catalysts had very similar specifi c activities [89, 91] However,
the organic preparation routes tend to give precursors with a higher surface area
than those prepared in aqueous solution. As the morphology of the precursor is
retained in the active catalyst, the organically prepared catalysts show the better
catalytic performance (Figure 12.6 ).
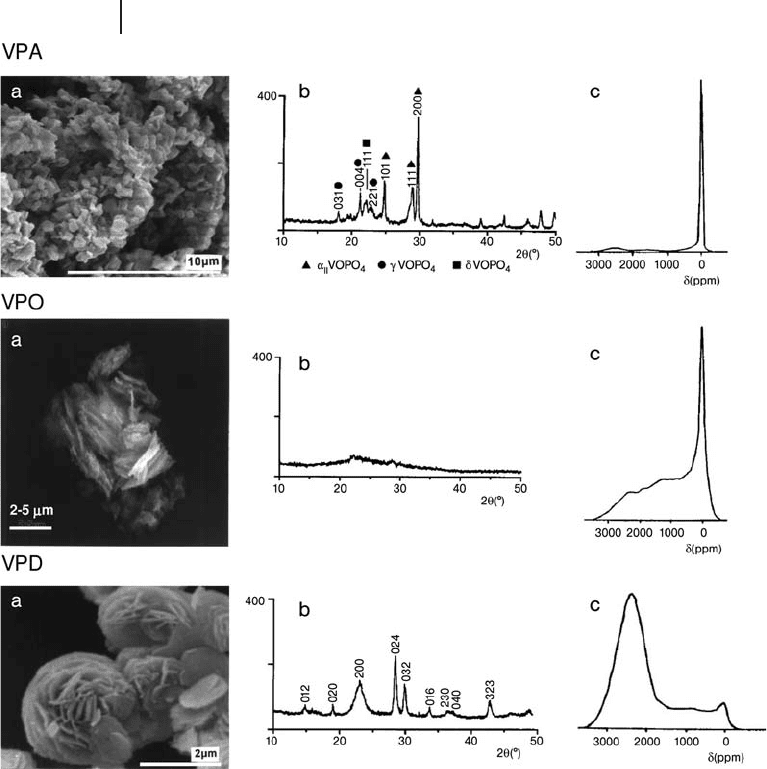
Hutchings and coworkers [89] investigated VPA, VPO and VPD catalysts, pre-
pared with a range of alcohols (Figure 12.7 ). VPA catalysts were found to have a
cubic morphology and a low surface area and VPO catalysts were found to have a
platelet morphology. However, VPD catalysts prepared with primary alcohols gave
a high surface area catalyst with a rosette structure, characterized by an X - ray dif-
fraction pattern with only one peak corresponding to the (220) refl ection. VPD
catalysts prepared with secondary alcohols had a similar morphology and surface
area to VPO catalysts and have a characteristic X - ray diffraction pattern containing
many peaks, with the (001) refl ection as the dominant feature. The catalysts with
rosette morphology were found to have a considerably higher activity than the
platelets. This is probably due to the increased surface area, as all the VOHPO
4
· ½H
2
O
catalysts are reported to have similar specifi c activities (the activity to maleic anhy-
dride per unit area).
Horowitz and coworkers [98] investigated a range of preparations in organic
solvents. They reported the rosette structure for catalysts prepared by a VPD
method with isobutanol and straight - chain alcohols, as well as a VPO type prepara-
tion using a 1 : 10 mixture of benzyl alcohol and isobutanol or 1 - butanol. This study
found that the platelet catalysts were more selective than the rosettes. They suggest
this is due to the rosette structure obscuring the active plane. The greater selectivity
of thick platelets has been confi rmed by other researchers [26] .
Okuhara and coworkers [104 – 110] have modifi ed the preparations using inter-
calated and exfoliated VOPO
4
· 2H
2
O. Using this technique, intercalating com-
pounds such as amines, amides, alcohols or carboxylic acids can replace the water
between the vanadium phosphate layers. These materials can then be delaminated
in a polar organic solvent and the exfoliated VOPO
4
reduced to give V
4+
vanadium
phosphates with unusual morphologies. It is thought that this process can occur
in an alcohol, which leads to the formation of the rosette structures found by
reduction of VOPO
4
· 2H
2
O with a primary alcohol [106] .
The crystallization of VOHPO
4
· ½H
2
O from V
2
O
5
and H
3
PO
4
has been studied
in detail by O ’ Mahony and coworkers [111, 112] using time - resolved X - ray diffrac-
tion. They found that an intermediate phase was formed initially but this then
disappeared as VOHPO
4
· ½H
2
O was detected. Concurrent focused ion beam
Figure 12.6 Relationship between catalyst activity and surface
area for standard vanadium phosphate catalysts for the
oxidation of n - butane [89] . (Reproduced with permission).
12.3 Preparation of VPP Precursors 511
512 12 Vanadium Phosphate Catalysts
microscopy showed rosette structures forming from delaminated plates as the
reaction proceeded (Figure 12.8 ).
A number of groups have also studied VO(H
2
PO
4
)
2
as a catalyst precursor
[113 – 118] . Mount and Raffl eson [119] prepared VO(H
2
PO
4
)
2
by heating V
2
O
5
and H
3
PO
4
with H
3
PO
3
in an autoclave at 150 ° C. They found this material decom-
posed at 360 ° C to yield VO(PO
3
)
2
. Hannour and coworkers [120 – 122] prepared
VO(H
2
PO
4
)
2
by heating an aqueous solution of V
2
O
5
, H
3
PO
4
and oxalic acid. This
was calcined in air to give β - VO(PO
3
)
2
. They also prepared α - VO(PO
3
)
2
by heating
V
2
O
5
in a large excess of H
3
PO
4
. Both of these catalysts were poorly selective to
maleic anhydride, with carbon oxides being the major products.
Figure 12.7 (a) An SEM micrograph, (b) an XRD pattern,
(c)
31
P NMR spin echo mapping spectrum from: activated
VPA, activated VPO, and activated VPD [5] . (Reproduced with
permission).
This is consistent with previous studies that have shown VO(PO
3
)
2
is not as
catalytically active as (VO)
2
P
2
O
7
. Hutchings and Higgins [123] found benefi cial
results by removing VO(H
2
PO
4
)
2
by solvent extraction, from the VOHPO
4
· ½H
2
O.
This yielded a higher surface area precursor and a more active catalyst after activa-
tion. Most VOHPO
4
· ½H
2
O preparations include boiling in water as a fi nal step
to remove water soluble impurities.
12.3.1
The Preparation of Novel Vanadium Phosphates
Bordes and Courtine [124] prepared a number of precursors that required
calcination in air, oxygen or nitrogen to give the fi nal catalyst. NH
4
(VO
2
)
2
PO
4
,
(NH
4
)
2
[(VO
2
)
2
C
2
O
4
(HPO
4
)
2
] · 5H
2
O and NH
4
HVPO
6
gave fi nal catalysts mainly
comprising (VO)
2
P
2
O
7
, VO(PO
3
)
2
or V(PO
3
)
3
, depending on the activation
conditions.
The synthesis of new vanadium phosphate precursors as reported by Benziger
and coworkers [125] consisted of intercalated n - alkyl amine pillars inserted between
the layers of VOHPO
4
· ½H
2
O. The (VO)
2
P
2
O
7
catalysts derived from these precur-
sors show an increase in selectivity which is attributed to stacking faults created
by the pillars. Vanadyl phosphonates with the formula VOC
n
H
2 n +1
PO
3
· x H
2
O, ( n = 0
to 4, x = 1 or 5) were also synthesized. These could be converted into (VO)
2
P
2
O
7
at considerably lower temperatures than VOHPO
4
· ½H
2
O and produced catalysts
with higher surface areas and increased yields of maleic anhydride.
The gas - phase synthesis of VOPO
4
· 2H
2
O has been reported [126] . A gas stream
of VOCl
3
, POCl
3
and H
2
O with N
2
as carrier, was passed through a furnace where
the powdered VOPO
4
· 2H
2
O was collected. This was converted to α - and β - VOPO
4
by calcination in nitrogen, before in situ activation gave the (VO)
2
P
2
O
7
catalyst.
Figure 12.8 A focused ion beam image of the samples
recovered 2 min after reaction of V
2
O
5
and H
3
PO
4
in alcohol.
Rosette - shaped hemihydrate particles appear to grow out
from the basal plane of the platelets [112] . (Reproduced with
permission).
12.3 Preparation of VPP Precursors 513

514 12 Vanadium Phosphate Catalysts
Michalakos and coworkers [127] prepared vanadium phosphate catalysts using
an aerosol process. The aerosol was created with aqueous solutions of NH
4
VO
3
and H
3
PO
4
(with air as the carrier) and sprayed into a furnace. The solid was
collected at the reactor exit on a cooled fi lter. The compound was found to be
VOPO
4
· n H
2
O which was converted to α
I
- VOPO
4
with a small amount of
VO(H
2
PO
4
)
2
by calcining. The catalyst was found to be more active than VPA cata-
lysts, despite having a lower surface area.
A number of groups have prepared vanadium phosphate catalysts using hydro-
thermal synthesis [92, 93, 128 – 130] . Using standard reaction mixtures, Dong and
coworkers [128] showed that at elevated temperatures and pressures different
materials are synthesized from those obtained under refl ux conditions. Pressure
did not seem to affect the product formed, but as the temperature increased to
> 200 ° C further reductions occurred and V
3+
products formed. However, these
materials were not found to have enhanced catalytic activity compared to tradition-
ally prepared materials. At lower temperatures, hydrothermal syntheses have
produced catalysts with comparable activity to those prepared under standard
conditions [92, 93, 129, 130] . Taufi q - Yap and coworkers [129] found an enhance-
ment in activity for hydrothermally prepared catalysts and suggested this was due
to a modifi cation in the redox behavior of the catalysts evidenced by TPO/TPR
experiments.
Hydrothermal syntheses have also been used to prepare new porous materials.
Doi and Miyake [131] obtained a mesoporous vanadium phosphate compound by
intercalating a surfactant ( n - tetradecyltrimethyl ammonium chloride) between the
layers of VOHPO
4
· ½H
2
O. Furthermore, Bu and coworkers [132] have also reported
a new mesoporous vanadium phosphate compound synthesized with an organic
template molecule. NaVO
3
, V, H
3
PO
4
, H
2
O and the template piperazine were
mixed in a molar ratio of 1.0 : 0.5 : 5.17 : 476 : 0.71. A dark blue gel was formed after
15 minutes stirring, and the mixture was then heated at 170 ° C for seven days in
an autoclave. Light blue, needle - like crystals were observed and then recovered by
fi ltration. The catalytic activity of these mesoporous vanadium phosphates have
not been reported.
12.4
Activation of the Catalyst Precursors
Research into the activation of precursors falls into two categories: the study of
structural and morphological changes during the activation period, and the effect
of different activation methods on the fi nal catalytic behavior.
12.4.1
Activation Procedures
The catalyst precursor VOHPO
4
· ½H
2
O must be activated to the (VO)
2
P
2
O
7
cata-
lyst. This is usually done in situ with the reaction feedstock of 1.5% butane in air.
A number of pre - treatments have been claimed to speed up the activation process.
These can involve heating the catalyst in an inert atmosphere, a reducing environ-
ment or an oxidizing environment (Table 12.1 ).
The effects of standard activation procedures (adopted by industry) and fast
activation procedures (often reported in the literature) have been investigated by
Lombardo and coworkers [80] . In the standard activation procedure, the catalyst
was heated in air up to reaction temperature, followed by introduction of the
butane in three steps, up to 1.5%. The gas hourly space velocity ( GHSV ) was
increased in four steps to 2500 h
− 1
. This procedure could take up to 380 hours.
In the fast activation procedure the catalyst was heated under 1.0% butane in
air up to reaction temperature, at a GHSV of 900 h
− 1
. This was held for 3 h before
the butane concentration was increased to 1.5% and the GHSV to 2500 h
− 1
. Lom-
bardo and coworkers found that the standard activation procedure gave fi nal cata-
lysts that were more crystalline, had less V
5+
phases present and were far more
active than the fast activated catalysts.
Albonetti and coworkers [133] have compared equilibrated and non - equilibrated
catalysts. Non - equilibrated catalysts, which had only been on - line for 100 hours,
were found to be poorly crystalline, with an oxidation state of +4.36 and a surface
area of 11 m
2
g
− 1
. The equilibrated catalysts that had been on - line for 1000 hours
were more crystalline, had an increased surface area (23 m
2
g
− 1
), and an oxidation
state of +4.00. The equilibrated catalyst was found to be considerably more active
and selective than the non - equilibrated catalyst.
The effect of oxidation pre - treatments on the catalyst have been investigated by
A ï t - Lachgar and coworkers [134] . Pure VPP catalysts were obtained by heating
VOHPO
4
· ½H
2
O in a fl ow of N
2
at 750 ° C for 72 h. This was then oxidized in a
fl ow of O
2
for between 0.5 and 24 h. The catalyst oxidized for 1 h showed the
highest selectivity to maleic anhydride, although all the pre - treated catalysts were
more selective than pure (VO)
2
P
2
O
7
. This is thought to be due to the introduction
of V
5+
phases. Previous studies have found V
5+
to be detrimental to catalysts, many
researchers suggesting they may be responsible for the total oxidation of butane
[22 – 24, 80, 133] .
Cheng and Wang [135] have studied the effect of calcining catalysts in air,
N
2
and CO
2
. VOHPO
4
· ½H
2
O could be converted into an amorphous V
5+
phase,
Table 12.1 I n fl uence of activation conditions on unpromoted V
–
P
–
O catalysts [135] .
Entry Activation
atmosphere
Temp ( ° C) Time (h) MA
selc
@400 ° C
(%)
Conv @400 ° C
(%)
Ref.
1 1% Bu/Air 380 100 50 20 [133]
2 1% Bu/Air 380 1000 80 20 [133]
3 O
2
a)
500 1 84 ca. 12 [134]
4 30% O
2
in N
2
400 3 62.3 98.2 [135]
a Sample heated in N2 (750 ° C, 72 h) then heated in O
2
.
12.4 Activation of the Catalyst Precursors 515

516 12 Vanadium Phosphate Catalysts
crystalline VOPO
4
or (VO)
2
P
2
O
7
with varying crystallinity, depending on the calcin-
ing temperature and atmosphere. The calcining environment that resulted in the
best catalyst was different for promoted and unpromoted catalysts, depending on
how easily they were oxidized. It was found that for unpromoted VOHPO
4
· ½H
2
O
the best environment was 30% O
2
in N
2
. This gave a catalyst that was composed
of dispersed V
5+
species on a (VO)
2
P
2
O
7
matrix.
The addition of water to the reaction feed was investigated [136] . This led to two
signifi cant effects being noted. The selectivity to maleic anhydride increased (with
increased yields of acetic and acrylic acids). There was also an increase in the
surface area of the catalysts activated with the n - butane/water/air feed compared
to the dry activated catalyst. Arnold and Sundaresan excluded the possibility of the
water vapor acting as a diluent by performing experiments with an n - butane/N
2
/air
feed. Instead they proposed that water is adsorbed onto the surface, blocking sites
that are responsible for over - oxidation of the products.
Contractor and coworkers [137] confi rmed the effects of water vapor in the
gas feed, using a riser reactor. They proposed that the water and oxygen are in
direct competition for catalytic sites. Adsorbed water decreases the amount
of activated oxygen available, which decreases the activity, but prevents over -
oxidation, which in turn increases the selectivity. It is suggested that lattice
oxygen is responsible for the selective oxygen, while adsorbed or gaseous oxygen
(which is inhibited by the steam) forms the total oxidation products. Further-
more, Vedrine and coworkers [138] proposed that water plays an important role
in maintaining a hydrated catalyst surface, which allows a redox mechanism to
occur easily.
Centi and Perathoner [139] have discussed the benefi ts of small amounts of
sulfur dioxide in the reaction feed. The SO
2
is thought to adsorb on the surface to
form stable VOSO
4
, blocking the redox activity of surface V
5+
sites (considered
to be responsible for over - oxidation). This results in an increase in selectivity to
maleic anhydride, particularly at high conversions.
Recently a very detailed study of activation conditions was carried out by
Patience and coworkers [140] . A number of parameters were investigated includ-
ing temperature, pressure, time and gas - phase composition with respect to O
2
and H
2
O. They found that standard conditions (390 ° C and atmospheric pressure)
gave the best performance although the activation time could be shortened by
increasing the pressure. Water had a deleterious effect on the catalyst even at
low concentrations and this was proposed to be due to an increase in the V
5+
observed.
Mechanochemistry has been proposed as an activation method [141 – 146] . This
involves milling the catalyst precursor in a solvent, usually ethanol, prior to its
conversion to the active catalyst. Experiments have shown that this procedure
promotes the exposure of the (100) plane in the catalyst, which is considered to
be the active crystal face [141 – 143, 146] , and can reduce the particle size thus
increasing the surface area [141, 144 – 146] . Experiments with promoters show that
this procedure gives more active and selective catalysts than those prepared by
either chemical means or mechanical mixing [93, 141, 146] .
12.4.2
Structural Transformations during Activation
The importance of precursor morphology in determining catalyst performance
was reported by Johnson and coworkers [86] . This is due to the topotactic trans-
formation (Figure 12.3 ), so the (VO)
2
P
2
O
7
catalyst retains the morphology of the
VOHPO
4
· ½H
2
O precursor. Insight into how the transformation occurs can help
in the design of pre - treatments and activation procedures that will produce cata-
lysts with improved properties.
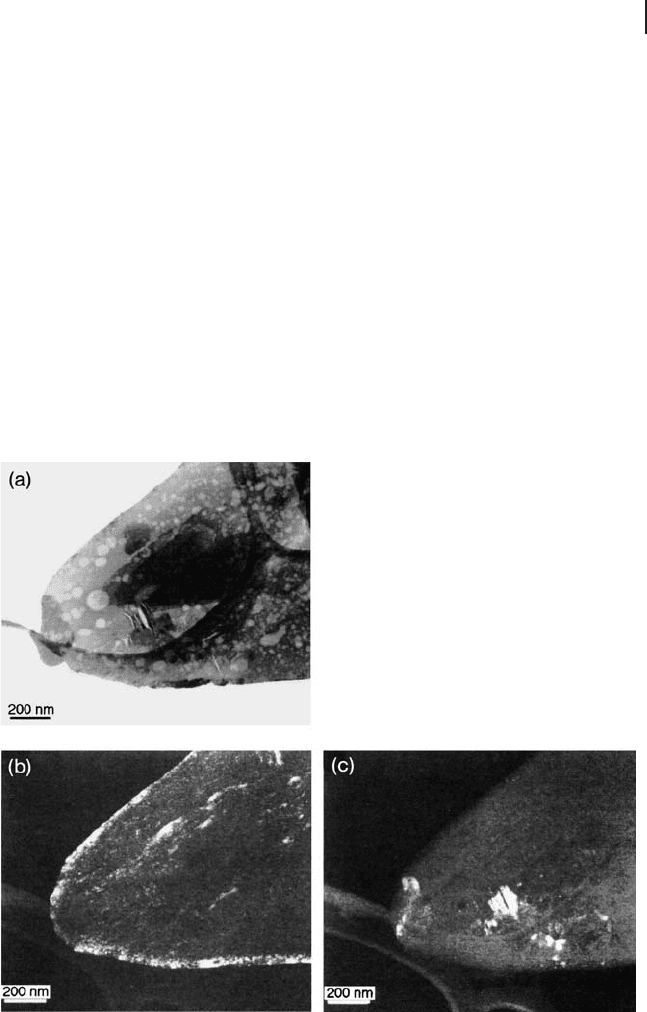
Kiely and coworkers [94] investigated the differences in activated catalysts which
were obtained from VOHPO
4
· ½H
2
O precursors prepared via VPA, VPO and VPD
routes. The VPA catalyst XRD pattern shows a mixture of VOPO
4
phases, with
(VO)
2
P
2
O
7
present as a minority phase, and there is a weak V
4+
peak seen in the
31
P NMR spin echo mapping spectrum. TEM revealed α
II
- VOPO
4
, δ - VOPO
4
,
(VO)
2
P
2
O
7
and some amorphous material (Figure 12.9 ). The (VO)
2
P
2
O
7
widely
considered to be the active phase constituted only 10% of the catalyst.
Figure 12.9 Bright - fi eld image showing a typical platelet from
the VPO - 0.1 sample. Corresponding dark - fi eld micrographs
taken in (b) the g
pyro
= (024) refl ection of (VO)
2
P
2
O
7
and (c)
the g
delta
= (022) refl ection of δ - VOPO
4
( g = diffraction vector)
[94] . (Reproduced with permission).
12.4 Activation of the Catalyst Precursors 517
