Jackson S.D., Hargreaves J.S.J. Metal Oxide Catalysis
Подождите немного. Документ загружается.

NMR spectroscopies, thermal analysis and inelastic neutron scattering as a func-
tion of the hydration state of the samples, as illustrated in Figure 14.7 and described
in Section 14.5.4 [116] .
An alternative way to determine acidity was to outgas the samples at increasing
temperatures and to follow the weight losses by thermogravimetric analysis ( TGA ).
Physisorbed water desorbs initially followed by water release from acid OH groups,
according to the equations
HPW O HO HPW O HO
31240 2 31240 2
⋅→ +nn
followed by
HPW O PW O HO
31240 12385 2
15→+
.
.
Another way to characterize acidity is to study the differential heat of adsorption
of a basic probe compound, such as ammonia or pyridine, by microcalorimetry as
a function of uptake. This technique yields the distribution of acid strength relative
to coverage, but unfortunately does not differentiate between Br ø nsted and Lewis
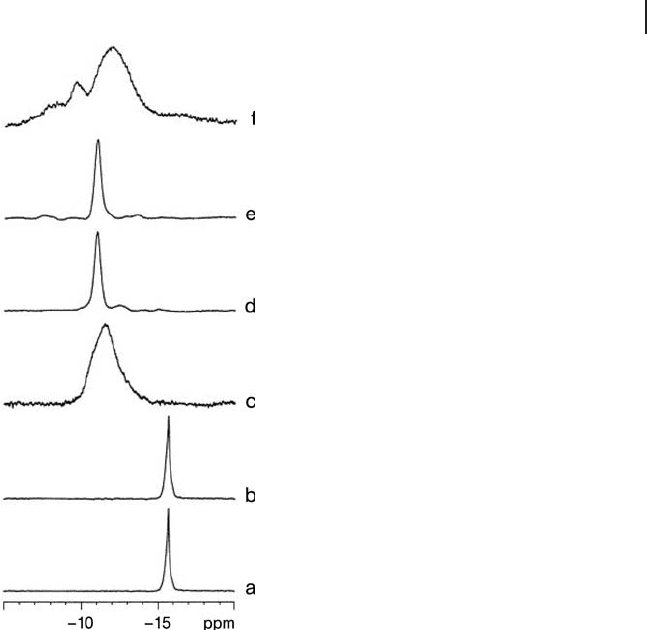
Figure 14.7 Room temperature MAS - NMR spectra of
H
3
PW
12
O
40
· n H
2
O as a function of dehydration under
dry N
2
fl ow for 2 h at: (a) 323 K, (b) 373 K, (c) 473 K,
(d) 573 K, (e) 673 K, (f) 873 K. (Taken from Ref. [116] ).
14.5 Characterization: Redox and Acid–Base Properties 579
580 14 Heteropolyoxometallate Catalysts for Partial Oxidation
acid sites. It should thus be coupled with IR spectroscopic study of adsorption of
the same basic probe molecule (Section 14.5.1 ).
X - ray and neutron diffraction and MAS - NMR techniques were successfully
applied to identify the structure of hydrated and/or partially dehydrated HPAs
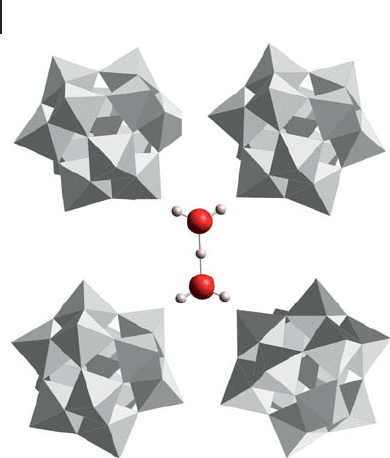
[117 – 119] . The structure of 12 - tungstophosphoric acid hexahydrate was solved
using X - ray and neutron diffraction. The proton was shown to be coordinated to
two water molecules in HO
52
+
species hydrogen bonded to four terminal W
=
O
t
oxide ions as shown in Figure 14.8 . The occupancy factor of the water O site was
found to be equal to 0.5, supporting the suggestion of formation of HO
52
+
, in the
inter - Keggin anion space.
17
O NMR has been applied to differentiate the types of oxide ions in the Keggin
anion [120] , except for the PO
4
tetrahedron, which did not give rise to O exchange.
The up - fi eld NMR shift of the O
t
resonance, upon dehydration of polycrystalline
H
3
PW
12
O
40
· x H
2
O was interpreted as an indication that protonation sites in the
anhydrous form of H
3
PW
12
O
40
are the terminal oxide ions. However, based on IR
spectroscopy data, the identifi cation of protonation sites in anhydrous POMs with
the Keggin structure resulted in a different interpretation. Both terminal oxygens
(M
=
O
t
) and/or bridged oxygens (M
–
O
–
M) were proposed [121, 122] . Additionally,
IR and Raman spectroscopies were used extensively to monitor the structural
variations against the nature of the heteroatom (X) and/or of the transition metal
element (M). Valuable information has been collected regarding both the assign-
ment of the absorption frequency of the M
=
O, M
–
O
–
M and X
–
O vibrations [123]
and the sensitivity of these vibrations to structural changes and/or hydration state
or partial replacement of protons by alkaline cations.
Figure 14.8 HO
52
+
species located between Keggin anions.

14.5.1
IR Spectroscopy
The IR spectra of polyoxometallates have already been comprehensively discussed
in the literature. The IR bands have been assigned previously [124 – 127] . The four
distinct oxygen sites in a Keggin unit are represented in Figure 14.2 (b) and corre-
spond to the following description:
• Four O
a
belong to the central tetrahedra PO
4
.
• Twelve O are terminal oxygens, to a lone addendum M atom.
• Twelve O
b
are involved in M
–
O
b
–
M bridges, between two different M
3
O
13
groups.
• Twelve O
c
are involved in M
–
O
c
–
M bridge, in the same M
3
O
13
groups.
The most relevant assignments are as follows: ν
as
(P
–
O
a
), (1080 – 1060 cm
− 1
),
ν
as
(M
–
O
t
) (990 – 960 cm
− 1
), ν
as
(M
–
O
b
–
M) (900 – 870 cm
− 1
) and ν
as
(M
–
O
c
–
M) (810 –
760 cm
− 1
). Some differences have been observed for lacunary Keggin - related com-
pounds α - [XM
11
O
39
]
n −
, which have a defect structure in which one metal atom and
its terminal oxygen atoms are missing. These anions have a hole surrounded by
fi ve oxygen atoms and behave as pentadentate ligands. A general splitting of P
–
O
stretching frequencies was observed and interpreted as a weakening of anion
cohesion. In particular, the decrease in frequency of asymmetric bridge stretching
is consistent with the lowering of M
–
Oc
–
M angles. The P
–
O stretching band for
[PM
11
O
39
]
7 −
is split into 1085 and 1040 cm
− 1
. This splitting is due to change of sym-
metry from T
d
(XM
12
) to C
s
(XM
11
) and may cause broadening of the band.
The protons were suggested to be localized on the most highly negatively charged
O atoms, namely the O
b
atoms. The ν
as
(M
–
O
b
–
M) mode is thus expected to be
sensitive to the degree of hydration owing to hydrogen bonding, which decreases
the M
–
O bond strength, thus increasing the bond length and decreasing the vibra-
tion frequency. Consequently, it is also sensitive to the nature of the cations
exchanging protons. An increase in the ν
as
(M
–
O
b
–
M) frequency is thus expected
upon dehydration as clearly shown in an in situ study of Cs
2.5
H
1.5
W
12
O
40
. Moreover,
when the M
–
O bond strength increases, the bond becomes more covalent, its
vibration frequency increases and the protons more free and thus more acidic.
Thus an increase in ν
as
(M
–
O
b
–
M) frequency may correspond to an increase in
Br ø nsted acidity strength and/or to a dehydrated state.
The acidic properties can also be estimated by NH
3
thermal desorption, although
NH
3
can also act as a reductant at high temperature [128] and does not differentiate
between Br ø nsted and Lewis acid sites. The acid subjected to evacuation at room
temperature for 30 min showed a strong ν
OH
absorption at 3209 cm
− 1
with a promi-
nent shoulder at 3355 cm
− 1
. In addition, the δ
H2O
absorption band appeared at 1708
cm
− 1
as previously reported [129 – 134] . TGA performed under the same conditions,
vacuum at ambient temperature for 30 min, showed that the HPA sample corre-
sponded to H
3
PW
12
O
40
· 5 H
2
O, a state close to the stable hexahydrate form. The
former absorption, at 1708 cm
− 1
, is typical of the presence of the protonated water
clusters, probably the di - aqua - hydrogen ion HO
52
+
, as demonstrated to be present
14.5 Characterization: Redox and Acid–Base Properties 581
582 14 Heteropolyoxometallate Catalysts for Partial Oxidation
in H
3
PW
12
O
40
· 6 H
2
O by X - ray and neutron diffraction studies [135] , and/or
hydroxonium ion which has been recently identifi ed by inelastic neutron scatter-
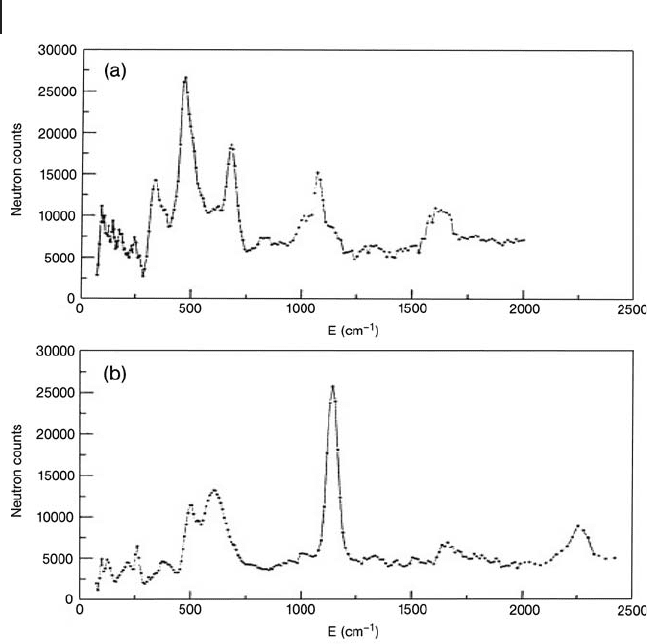
ing ( INS ) in partially dehydrated 12 - tungstophosphoric acid [116, 136] . INS data
as a function of hydration state of H
3
PW
12
O
40
are illustrated in Figure 14.9 . At
4K HO
52
+
, H
3
O
+
and H
+
(lone protons) were identifi ed. Cs
1.9
H
1.1
PW
12
O
40
was also
characterized in the same way by INS.
14.5.2
Photoacoustic Spectroscopy
This technique is complementary to IR spectroscopy and well suited for dark
colored samples. As for IR, it has been employed for structural determination
Figure 14.9 4 K INS spectra of H
3
PW
12
O
40
· n H
2
O as a
function of dehydration under dry N
2
fl ow for 2 h at:
(a) 473 K (H
3
O
+
species) and (b) 573 K (lone protons).
(Taken from Ref. [116] ).

using fi ve or six bands below 1100 cm
− 1
that are characteristic of POMs and for
water species such as H
3
O
+
( ∼ 1710 cm
− 1
) and water (broad bands at ∼ 3200 cm
− 1
, at
∼ 2240 cm
− 1
due to H - bonded crystal water) [137] .
14.5.3
UV - V isible Spectroscopy
Electronic spectroscopy of Keggin - type heteropoly compounds has been used for
structural and quantitative analyses. POMs mainly absorb in the 180 – 270 nm
region. The incorporation of a transition metal in the framework gives rise to
additional bands in the UV - Vis region, depending on its nature and oxidation state.
Postulation of reaction mechanisms, especially for oxidation reactions by perform-
ing in situ studies of the reaction mixture, has been based upon application of the
technique. The changes observed in the spectra can be correlated to the formation
of active intermediates formed due to the interaction between the active center in
the catalyst and the oxidant, and the change in oxidation state of the transition
metal elements.
14.5.4
Nuclear Magnetic Resonance Spectroscopy
NMR spectroscopy is a valuable tool in the study of the electron density distribu-
tion which results in large chemical shifts induced by both paramagnetic atoms
and electron transfer between atoms in diamagnetic HPAs. Some paramagnetic
contributions may exist depending on the presence of paramagnetic elements,
such as V
IV
, Mo
V
, Cr
3+/V
, Fe
3+
, Ni
2+
, Co
2+
, and so on (d
n
ions, n ≠ 0 or 10) and leads
to variations of the observed chemical shift. Several important elements can be
studied easily. The most widely investigated nuclei include
31
P,
183
W,
51
V,
1
H,
13
C
and
17
O. Studies of the electron density distribution in both diamagnetic and
paramagnetic species are important for understanding the nature of chemical
bonding in POMs and their role in chemical reactions.
17
O NMR spectroscopy provides information about the bonding nature of the
oxygen atoms. There is a correlation between the downfi eld shift and the
decreasing number of metal atoms to which the oxygen atom is bonded. Unfor-
tunately,
17
O NMR spectroscopy is not often used, owing to the low natural
abundance of
17
O. In contrast,
1
H NMR is an important and widely used tool
for the detection of the different types of protons present in the heteropoly
compound, especially because there is a change in the spectra with the number
of hydration water molecules.
31
P NMR is widely used for structure description,
especially in mixed addenda heteropoly compounds owing to the presence of a
number of structural isomers, which become more numerous as the number of
addenda atoms increases. It is greatly dependent on the degree of hydration in
12 - phosphotungstic acid, containing n H
2
O, values being − 15.1 to − 15.6 ppm for
n = 6 and − 11.1 to − 10.5 ppm for n = 0 as shown in Figure 14.7 . The difference
14.5 Characterization: Redox and Acid–Base Properties 583

584 14 Heteropolyoxometallate Catalysts for Partial Oxidation
is explained as follows: the former band is assigned to protonated water,
HHO
2
2
()
+
, connected with the heteropolyanion by hydrogen bonding at terminal
oxygen and the latter band is assigned to the protons directly attached to the
oxygen atoms of the polyanion.
1
H MAS - NMR spectra of H
3
PW
12
O
40
have been
recorded at room temperature as a function of dehydration state [116] as for
31
P
spectra in Figure 14.7 . Samples a to c showed a single peak at about 7 ppm,
samples d and e showed a broad peak at 7.5 ppm while sample f showed a peak
at 6.6 ppm. The assignment of the different peaks was as follows: the peak at
10.5 ppm for bare protons, at 9.7 ppm for H
3
O
+
, at 7.5 ppm for HO
52
+
and at
7 ppm for HHO
2
()
+
n
. A similar study of
31
P and
1
H on a Cs
1.9
H
1.1
PW
12
O
40
· n H
2
O
sample showed that the dehydration state was reached at a temperature about
100 K lower [116] .
51
V NMR has also been widely used for structural elucidation of vanadium - con-
taining mixed addenda heteropoly compounds, owing to the large natural abun-
dance of
51
V nuclei. The number of structural isomers increases with the increase
in the number of addenda atoms and their position in the Keggin structure is
confi rmed by
51
V NMR spectroscopy [138] .
14.5.5
Electron Spin Resonance ( ESR ) Spectroscopy
Electron Spin Resonance ( ESR ) spectroscopy is well suited to the study of electron
delocalization problems and spin density distribution, but it is limited to systems
containing unpaired electrons. It gives information about the site symmetry and
electronic structure of paramagnetic metal ions. ESR spectra may give informa-
tion about mixed valence structure of reduced heteropoly compounds. The pres-
ence of unpaired spin in reduced HPA species gives rise to an ESR spectrum
whose pattern depends on the number of atoms acquiring the unpaired electrons
and also the temperature. For axial MO
6
complexes, to which the reduced form
of HPA containing V
IV
, Mo
V
or W
V
belong, an anisotropic ESR spectrum is
observed [139] . The spectrum can become symmetrical at high temperature owing
to molecular tumbling and electron delocalization over several atoms. The pres-
ence of a non - degenerate d
xy
orbital for each octahedron allows these HPAs to be
reversibly reduced by one or more electrons with retention of the original struc-
ture. Reduction of the anion results in the blue color arising from the intervalence
band. The one - electron - reduced species gives rise to ESR spectra that have been
interpreted to show complete delocalization of the unpaired spin over the twelve
metal atoms. The ESR spectra at different temperatures have shown that one
electron is trapped at quite low temperatures with only a partial delocalization,
whereas it is completely delocalized at higher temperatures. This suggests that
the spin is partially localized in the ground state but is involved in rapid thermal
hopping from one site to another at elevated temperatures. This unpaired electron
resides on a more easily reduced metallic atom. The degree of electron delocaliza-
tion in the ground state is determined by the extent of interaction in the bridges
[138] .

14.5.6
Electrochemistry of Keggin Heteropoly Compounds
Heteropoly compounds can act as redox active materials when changing the
heteroatom transition metal, without affecting the basic Keggin structure. The
vast chemistry of heteropolyanion involves oxidation/reduction of addenda or
the active transition metal. The electrochemical analysis of such species forms
the basis of identifi cation of redox active species. Thus electrochemical tech-
niques such as cyclic voltammetry are means to understand the reactivity and
mechanistic behavior of heteropolyanions as redox agents and redox catalysts in
the liquid phase. The wide range of counter - cations assists in the study of the
redox behavior of these anions, inducing solubility in a variety of solvents from
water to a wide range of organic solvents. The heteropolyanions undergo rapid
one - and two - electron reversible reduction to produce the heteropolyblues and
further irreversible reduction can lead to their concomitant decomposition.
Reduction is accompanied by an increase in the charge density and basicity.
Reduction can also be accompanied by protonation depending on the p K
a
of the
produced oxometallate. The reduction potentials of the Keggin - type heteropoly
tungstates and heteropoly molybdates are controlled by factors such as the
nature of isomers. The reducibility increases in the sequence α - , β - , γ - . The
reduction potential of one - electron waves is found to decrease linearly with a
decrease in the valence of the central metal or an increase in the negative charge
of the heteropolyanion. Transition metals incorporated into heteropolyanions
reside in an octahedral environment, with one coordination site occupied by a
solvent molecule, most commonly water. They can be oxidized to the corre-
sponding oxometal, hydroxometal or peroxometal derivative depending on the
nature of the metal and the oxidant. These species play an important role in
oxidation catalysis.
The formal redox potential depends on a number of factors such as the nature
of the heteroatom and addenda atom. The redox potential of the metal increases
with increasing formal charge on the central atom. For a given oxidation state, the
redox potential increases with the size and decreasing electronegativity of the
central metal atom. The electrolytic conditions such as pH, counter - cation and
the nature of the solvent also play an important role in determining the formal
redox potential. By varying the addenda atom, the electrochemical character of the
polyoxometallates can be changed. The addenda atoms can be arranged in decreas-
ing oxidizing ability in the following order V
V
> Mo
VI
> W
VI
. In case of one - elec-
tron - reduced mixed addenda heteropolyanions, the added electron is localized on
the more reducible atom at room temperature [140] . For HPA - n = H
3+ n
PMo
12 − n
V
n
O
40
,
E
Red
< E
HPA - n
< E
O2
, where Red indicates reduced The reduction potential E
HPA - n
equals about 0.7 V versus standard hydrogen electrode (SHE) for HPA - n with
n = 1 – 4 at pH = 1 and the standard reduction potential of oxygen E
O2
equals 1.23 V
at 298 K.
14.5 Characterization: Redox and Acid–Base Properties 585

586 14 Heteropolyoxometallate Catalysts for Partial Oxidation
14.5.7
Thermal Analysis
TGA, Differential Thermal Analysis ( DTA ) and Differential Thermogravimetric
Analysis ( DTG ) measurements have been employed to determine the number and
nature of water molecules present in the POMs. The results of TGA show the
presence of two types of water in POM compounds, namely “ water of crystalliza-
tion ” and “ constitutional water molecules. ” The loss of the former usually occurs
at temperatures below 443 – 473 K [141] . At temperatures exceeding 543 K for
H
3
PMo
12
O
40
and 623 K for H
3
PW
12
O
40,
the constitutional water molecules (the
acidic protons bound to the oxygens of the polyanion) are lost, according to the
literature and for the acid forms [140] . For example the thermolysis of H
3
PMo
12
O
40
proceeds in two steps as schematized below:
H PMo O H O H PMo O H O below K
312402 31240 2
543⋅→ +nn
HPMo O H PMo O HO above K
3 12 40 3 12 40 2 2
2 623→+
()
−−xx
x
When protons are exchanged with metal cations, some water molecules physi-
sorbed on these cations may be released at temperatures higher than 623 K, which
unfortunately makes the assignment of water release above 270 ° C to constitutional
water, and therefore to protonic sites, questionable [142] .
Thermal desorption of basic molecules such as ammonia and pyridine has been
used to characterize acidic properties. For instance, ammonia has been observed
to desorb at about 573 K on SiO
2
–
Al
2
O
3
against > 773 K on acid zeolites and > 873 K
on H
3
PW
12
O
40
and its (NH
4
)
x
H
1 − x
and Cs
2.5
salts. Moreover it has also been shown
that decomposition of ammonia to N
2
, H
2
, H
2
O, as detected by MS analysis on
line, has occurred during desorption at high temperature, which precludes the
assignment to acid strength.
14.5.8
Microcalorimetry of Acid or Basic Probe Adsorption
The technique has been fruitfully used to characterize acid and basic sites in
many catalysts, in particular for zeolites and metal oxides [143] . It has also been
applied for POMs [144] . It consists of measuring the differential heats of adsorp-
tion when adsorbing successive increments of a basic probe molecule such as
ammonia or pyridine for acidity characterization or of an acid probe molecule
such as CO
2
or SO
2
to characterize basicity. The technique produces a histogram
of the acid – base strength as a function of coverage, in particular when heteroge-
neity in strength exists. The data should then be compared with ammonia or
pyridine desorption data from IR and thermal desorption experiments (see
above).
14.6
Conclusions and Perspectives in Polyoxometallate Application in Heterogeneous
Oxidation Catalysis
Owing to their multifunctional properties and easy preparation methods, wheel - or
ball - shaped nanostructured POMs appear promising for applications in many
fi elds, especially in heterogeneous catalysis for acid - and oxidation - type reactions.
Industrial applications have already been established as mentioned at the end of
Section 14.3 . The problem of relatively weak stability under catalytic conditions
has been partly circumvented by using alkali metal salts such as those of Cs
+
.
However, further progress is required and the stabilization of POMs in reaction
conditions for long periods of time remains a key issue in their industrial develop-
ment, and a real challenge for the future. The tertiary structure is modifi ed under
oxidation reaction conditions with a systematic decrease of the surface area, but
more crippling is the observed change in the primary and consequently the sec-
ondary structures. For example, it was shown using Raman spectroscopy that the
Keggin anion structure of H
4
PVMo
11
O
40
was relatively unstable upon heat treat-
ment [145] . Vanadyl and molybdenyl species are expelled from the anions of the
fi rst layers of the compounds during oxidation reactions and defective Keggin
structures are formed. These defective structures further disintegrate, presumably
to form Mo
3
O
13
triads. In time, these fragments oligomerize to molybdenum
oxygen clusters comparable to hepta - or octamolybdates, and fi nally to MoO
3
- type
oxides. The presence of water in the gas feeds, thought to be positive for the sta-
bilization of the proton - containing phases, turned out to promote degradation of
the anion. As stated above, only the presence of alkali metal cations, such as
cesium, as counter - cations led to a partial stabilization of the structure. It has been
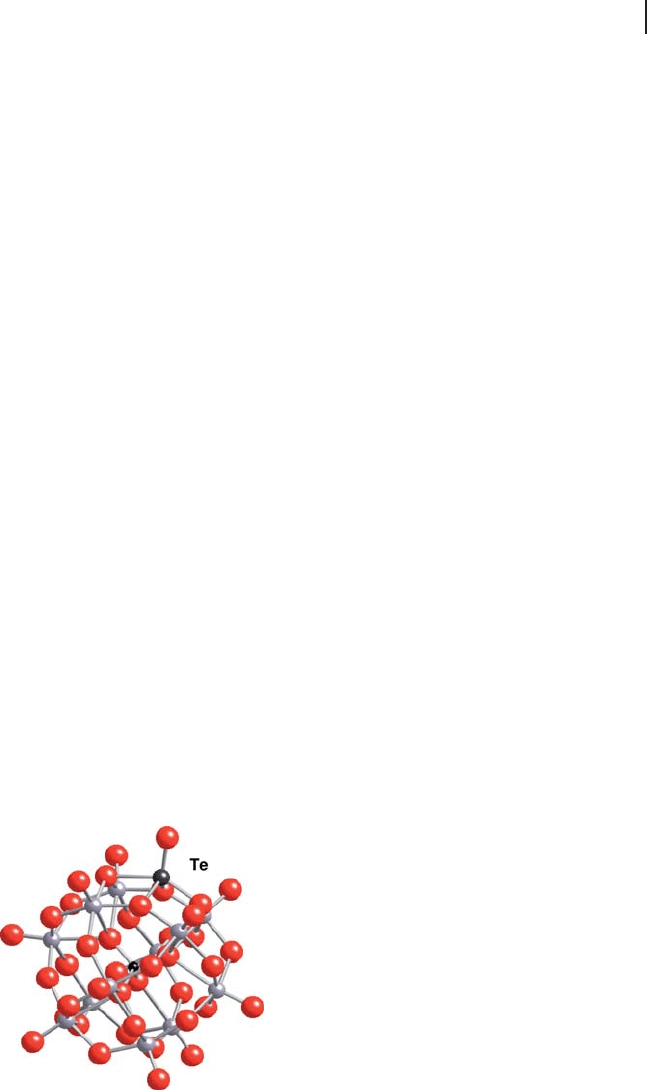
shown that pure molybdophosphoric Keggin anions with Te or V cations capping
these anions, as schematized in Figure 14.10 , were very stable in the reaction
conditions for isobutane partial oxidation up to 633 K and no degradation species
could be detected [146] . Similar cappings have been observed with vanadium
[147] .
Figure 14.10 Example of Te capping a Keggin unit
and helping to stabilize the anion. The Te cation is
fi ve coordinated, involving four oxygens from the
same Keggin unit. (Taken from Ref. [146] ).
14.6 Conclusions and Perspectives in Polyoxometallate Application 587

588 14 Heteropolyoxometallate Catalysts for Partial Oxidation
In this chapter, we have reviewed the acid and redox properties of such materials
which can be controlled and tuned by changing the chemical composition,
whatever the addenda elements or the counter - cations, and the structure of
the POMs.
The porosity of such materials has also been shown to vary depending on their
chemical composition, and some examples have been given above based on “ shape
selectivity ” properties. Moreover new POMs have been discovered (e.g. Figure
14.10 ) for instance the giant wheel - and ball - shaped anions, able to model metal
oxide surfaces or to trap large entities such as metal oxide nanoparticles, metallo-
porphyrins, proteins, enzymes and so on, which opens up tremendous possibili-
ties in many fi elds of application. Spherical clusters such as {Mo
132
} have pores
about 0.5 nm in diameter which is comparable to zeolitic materials such as MFI
or mordenite - type zeolites. However, unlike Si/Al zeolites, the POMs have acid
and redox (M
VI/V
) centers and are electron - rich, which are key parameters to
promote catalytic reactions. The development of functionalized polyoxometallate
[148] and polyoxometallate - based polymers [149] with creation of giant two - dimen-
sional networks has been reported and provides new perspectives in the fi eld of
organic – inorganic frameworks.
The {Mo
154
} clusters are linked to form layered frameworks with nanosized
channels [150] and could be considered as a model for bulk layered materials. The
problem is how to remove cations between the layers, which prevent reactant
access to the sites. These giant clusters can be considered as models for bulk
layered metal oxides, including defects [151] , as observed on large surfaces. The
effect of such defects on catalytic properties, mainly for oxidation reactions, is quite
important although still only partly known. For instance, local defects such as
anionic or cationic vacancies leading to different coordination spheres of the
surface O
2 −
anion in MgO have been characterized by DFT calculation, photolu-
minescence, IR and
1
H NMR [152] and have been shown to infl uence their basic
properties as illustrated by the 2 - methyl - but - 3 - yn - 2 - ol (MBOH) decomposition
reaction, Such a reaction has been shown [153] to give 3 - methyl - but - 3 - en - 1 - yne
(Mbyne) and 3 - methyl - but - 2 - enal (Prenal) on acid sites, 3 - hydroxy - 3 - methyl - buta-
none (HMB) and 3 - methyl - but - 3ene - 2 - one (MIPK) on amphoteric sites and acetone
and acetylene on basic sites. In a study of alkaline earth oxides involving Mg, Ca
and Sr, insertion of Nd
3+
cations and the use of different preparation and activation
procedures (sol – gel against co - precipitation) have been shown in both cases by
detailed XRD analysis to lead to local defects, which have been demonstrated to
be of major importance for the propane oxidation reaction mechanism (radical -
type) and selectivity to propene and ethene [154] .
Great effort is now devoted to the design of functionalized POM catalysts to aid
the development of green catalytic processes. Many examples have been presented
in this chapter using either oxygen (air) or hydrogen peroxide as green oxidants
for the up - grading of many organic chemicals to valuable compounds.
