Jackson S.D., Hargreaves J.S.J. Metal Oxide Catalysis
Подождите немного. Документ загружается.

15.4 Chromia 599
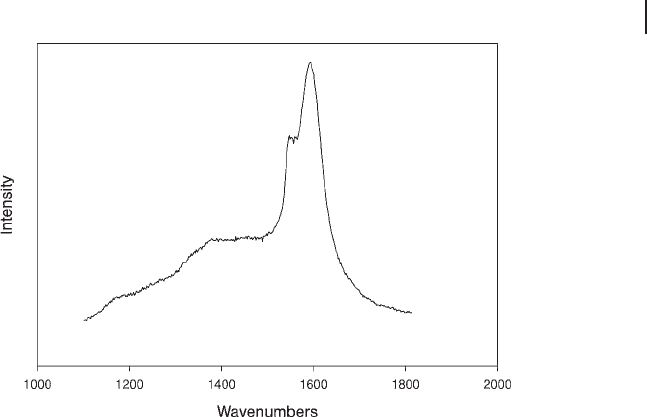
or pre - graphitic paracrystals [32] The shoulder at 1550 cm
− 1
is characteristic of
conjugated olefi ns or polyenes.
Confi rmation of this interpretation of the Raman spectrum came from analysis
of yellow oil produced during extended use of a catalyst. The analysis revealed the
presence of pyrene (C
16
H
10
), fl uoranthene (C
16
H
10
), benzanthracene (C
18
H
12
) and
perylene (C
20
H
12
) among other compounds. The alumina support principally pro-
duced perylene in the absence of chromia but with chromia a wider range of
polynuclear aromatics were detected [26] .
Studies using isotopically labeled propane [30] have shown that the route to
the coke deposits during propane dehydrogenation goes through a C
1
species,
probably CH(a). However, before these species convert to coke there is a period
when they are labile and can be hydrogenated off the surface as methane [26, 30] .
This was shown using isotopically labeled propane [26] and by using a mixed
continuous/pulse reaction system where a chromia/alumina catalyst was reduced
and subjected to a continuous fl ow of propane for 10 min at 873 K followed by two
pulses of propane in helium [20] . With both pulses the H : C ratio was ∼ 3.3 much
higher than the inlet 2.7; also the mass balance was > 100%. However the propene :
propane ratio was what would have been expected from a continuous fl ow experi-
ment after 10 min. Clearly carbonaceous material retained by the catalyst was
being desorbed into the pulse as methane. Therefore under continuous fl ow condi-
tions there is a reservoir of C
1
fragments on the surface of the catalyst that is con-
tinually in fl ux between removal as methane and consolidation on the surface to
generate higher molecular weight species. A study by Krause and coworkers [33]
showed that if the chromia is not pre - reduced the reduction of chromates by
propane may proceed through the formation of isopropoxide species, which react
Figure 15.2 UV Raman spectrum of coke deposited on 1%
Cr/Al
2
O
3
catalyst used for propane dehydrogenation.
600 15 Alkane Dehydrogenation over Vanadium and Chromium Oxides
further fi rst to adsorbed acetone and then to formates and acetates. They also
found that dehydrogenation of propane resulted in sequential formation of ali-
phatic, unsaturated/aromatic and graphite - like hydrocarbon deposits, which deac-
tivated the catalyst.
The situation with butane is similar but with signifi cant differences. The forma-
tion of coke on the surface is very similar to that found with propane; however,
Edussuriya and coworkers (unpublished results) found that although the majority
of the catalyst deactivation was due to polynuclear aromatics or other forms of
coke strongly adsorbed reaction intermediates were also detected. These species
can be desorbed at room temperature by treating with a low concentration O
2
in
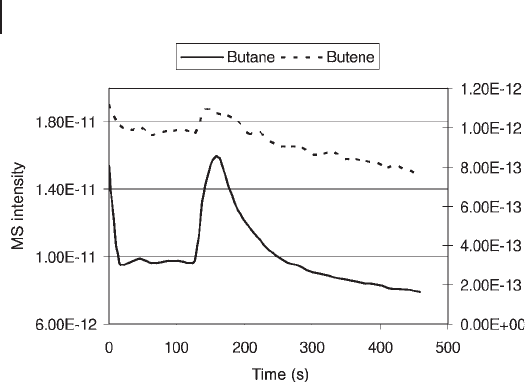
Ar mix. Figure 15.3 shows the desorption of butane. The catalyst had been catalyz-
ing the dehydrogenation reaction for 2 h at 873 K when the gas stream was switched
from butane to argon. Online mass spectrometry indicated that all hydrocarbon
components were rapidly swept out of the reactor at 873 K and no other gases apart
from the argon carrier were detected during the cool - down period. However, when
2% O
2
/Ar was passed over the sample at room temperature, butane, butenes and
butadiene were evolved. This desorption process can be viewed as an oxidative
displacement, resulting in recombination of reaction intermediates (hydrogen and
alkyl, alkenyl and alkadienyl) from the surface of reduced CrO
x
species. Removal
of these species did not regenerate the catalyst. The polynuclear aromatics were
only removed when the catalyst was subjected to a full regeneration involving
high - temperature oxidation. Only after this treatment did the catalyst regain its
activity.
Figure 15.3 Desorption of butane and butene at room
temperature in a fl ow of 2% O
2
/Ar from a 6% chromia/
alumina catalyst after butane dehydrogenation at 873 K.

15.5
Vanadia
Although vanadia catalysts have not been used commercially there is a signifi cant
literature concerning the dehydrogenation reaction over supported vanadia
systems. The structure of supported vanadia catalysts under redox reaction condi-
tions is directly related to the catalytic performance. Vanadia catalysts are usually
reduced to some extent during redox reactions, and the reduced vanadia species
have been proposed as the active sites [20, 34, 35] . Therefore, information on the
valence state and molecular structure of the reduced vanadia catalysts is of great
interest. A number of techniques have been applied to investigate the reduction
of supported vanadia catalysts, such as TPR [36 – 38] , X - ray photoelectron spectros-
copy (XPS) [35] , electron spin resonance ( ESR ) [39] , UV - Vis DRS [40 – 45] , X - ray
absorption fi ne structure spectroscopy ( XAFS ) [46] and Raman spectroscopy [37,
47 – 50] . Most of these techniques give information only on the oxidation state of
vanadia species. Although Raman spectroscopy is a powerful tool for character-
ization of the molecular structure of supported vanadia [34, 42, 51] , it has been
very diffi cult to detect reduced supported vanadia species with conventional
(visible) Raman measurements [37, 47 – 52] . A widely accepted explanation for this
phenomenon is that the Raman cross - section of reduced vanadium oxide species
is very small or near zero [53] . Thus, it remains challenging to use Raman spec-
troscopy for obtaining information on the molecular state of reduced vanadia
species.
It is notable that most Raman studies of supported VO
x
catalysts were carried
out using a single excitation wavelength in the visible region (488, 514 or 532 nm)
[34, 46, 47, 54] . However, several recent investigations on supported transition
metal oxides [55 – 58] , including vanadium oxides [55, 56] under ambient condi-
tions, using both UV and visible wavelength Raman excitations suggest that more
complete and sometimes new structural information of supported metal oxides
can be achieved by using multiple excitation wavelengths. The reason lies in the
strong electronic absorptions in the UV and visible wavelength regions exhibited
by most transition metal oxides, which make it possible to measure resonance -
enhanced Raman spectra. Under circumstances where supported VO
x
species are
present in a distribution of cluster sizes or coordination geometries, it is likely that
these species also possess a corresponding distribution of electronic absorption
wavelengths. Excitation of Raman spectra within the absorption region will produce
resonance - enhanced spectra from the subset of VO
x
species with absorptions at
the excitation wavelength. By measuring the Raman spectra at several wavelengths,
more information can be obtained about the various VO
x
species in the distribu-
tion. Moreover, when UV excitation is employed, even Raman spectra from sup-
ported VO
x
at low loadings ( < 1 wt%) on oxides having strong fl uorescence are
possible because of the avoidance of fl uorescence and enhanced sensitivity [59,
60] . In addition, the decreased self - absorption effects in the UV region indicated
by in situ UV - Vis DRS studies of reduced VO
x
and CrO
x
[40 – 44, 61, 62] suggests
15.5 Vanadia 601
602 15 Alkane Dehydrogenation over Vanadium and Chromium Oxides
that UV Raman spectroscopy may be capable of detecting reduced supported metal
oxides.
Studies on alumina supported catalysts with a range of vanadia weight loadings
revealed [63, 64] that isolated VO
x
species dominated at surface densities below
1 V/nm
2
; polyvanadates coexisted with monovanadates at surface densities between
1.2 – 4.4 V/nm
2
; and V
2
O
5
formed at a surface density higher than 4.4 V/nm
2
. The
vanadia densities of the catalysts were 1.1 V/nm
2
for the sample with the 1%
loading, 3.7 V/nm
2
for the 3.5% loading and 10.4 V/nm
2
for the 8% loading. Only
the XRD pattern for the catalyst with the 8% loading exhibited additional charac-
teristic peaks of crystalline V
2
O
5
at 2 θ values 26.3 and 34.6 [65, 66] . However,
analysis by
51
V NMR revealed V
2
O
5
on the 3.5% vanadium as well as on the 8%
(Gladden and McGregor, unpublished results).
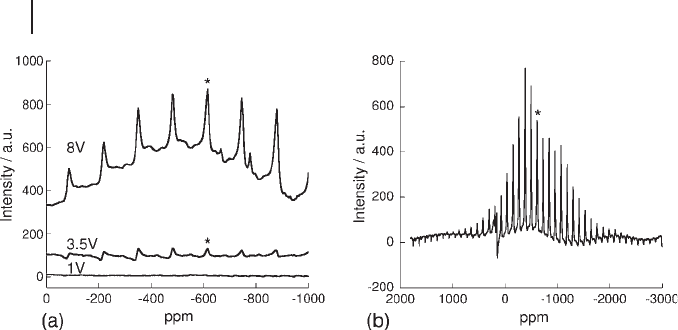
Figure 15.4 a shows simple pulse - acquire
51
V MAS NMR spectra for catalysts
with vanadium loadings of 1, 3.5 and 8 wt%. This simple 1 - D spectroscopy identi-
fi es a
51
V resonance only in the 3.5 and 8 wt% catalysts. The spectra are dominated
by spinning sidebands, with the isotropic chemical shift (denoted by * ) at − 612 ppm
being indicative of V
5+
in a V
2
O
5
environment. The broad background upon which
these peaks are superimposed in the case of the 8 wt% sample is characteristic of
V
5+
in more amorphous environments. The spectra clearly suggest that the popula-
tion of V
2
O
5
- like species in the 8 wt% catalyst is larger than in the 3.5 wt% catalyst,
consistent with the Raman data. Alongside characterizing the
51
V environment,
NMR spectroscopy of the
27
Al species is also of interest. Figure 15.5 a shows a
simple pulse - acquire
27
Al spectrum of an as - prepared 8 wt% catalyst. Two broad
resonances are observed; the peak at ∼ 6 ppm is characteristic of sixfold, octahe-
drally coordinated Al, while Al in four - fold and fi ve - fold coordinated sites contrib-
ute to the broader resonance in the range 25 – 70 ppm. Interpretation of the broader
Figure 15.4 51 - V MAS NMR spectra. (a) As - prepared
catalysts, from top 8, 3.5 and 1 V; (b) 8 V after regeneration at
873 K. * indicates an isotropic resonance. In spectrum (b) the
peaks to negative intensity correspond to Al atoms in the
support.
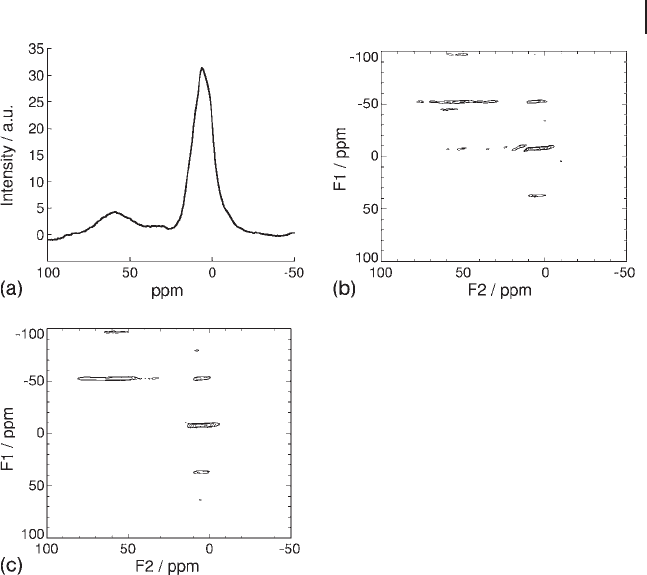
resonance is made signifi cantly easier – although the NMR technique itself is more
challenging – by using multiple quantum NMR spectroscopy. A triple - quantum
spectrum from the 8 wt% catalyst is shown in Figure 15.5 b. The projection of such
spectra in the F1 dimension yields the isotropic peaks, while the anisotropic line-
shapes (corresponding to a standard MAS spectrum) are displayed by the projec-
tion in the F2 dimension. The resolution of the isotropic resonance provided by
the triple - quantum MAS experiment unambiguously confi rms the presence of
three distinct
27
Al environments, assigned to 4 - , 5 - and 6 - coordinate Al.
Studies reveal that reduction takes place over a broad range of temperatures and
the onset of reduction follows the weight loading with 8% < 3.5% < 1% but reduc-
tion is complete for each sample by ∼ 873 K. This difference in onset of reduction
may be attributed to different reducibilities of vanadia species, monovanadates,
polyvanadates and V
2
O
5
coexisting on the catalyst ’ s surface. The weight loss at the
reduction step increases with vanadia loading: when these weights are translated
into oxygen loss we see that there is a 1 : 1 relationship between vanadium atoms
and oxygen atom loss (Table 15.1 ). The same trend in mass loss is observed during
Figure 15.5
27
Al NMR spectra. (a)
27
Al MAS NMR spectrum of
as - prepared 8%V/alumina catalyst; (b)
27
Al 3Q MAS NMR
spectrum of as - prepared 8%V/alumina; (c)
27
Al 3Q - MAS NMR
spectrum of 8%V/alumina after regeneration at 873 K.
15.5 Vanadia 603
604 15 Alkane Dehydrogenation over Vanadium and Chromium Oxides
Tapered Element Oscillating Microbalance ( TEOM ) studies of catalyst reduction
(Gladden and McGregor, unpublished results). These studies also yield an average
1 : 1 relationship between vanadium atoms and oxygen atom loss.
This would suggest a change of 2 in the vanadium oxidation state, so if the
vanadium were in a +5 oxidation state before reduction it would be converted to
+3 after reduction. This was confi rmed by in situ UV - Vis DRS. Before reduction
the catalysts were in a V
5+
oxidation state with typical charge transfer bands at
267 nm for a 1% loading, 290 nm for a 3.5% loading and 357 nm for an 8% loading.
This change in position of the charge transfer band is indicative of the increasing
polymeric nature of the vanadia [67] . Over the course of the reduction the spectrum
changes to give spectra typical for a V
3+
state with d – d transitions around 575 nm
and 625 nm for 3.5 %V and 8 %V. However, the spectrum of the 1% sample is
more indicative of V
4+
, with a single band at 585 nm [31] .
51
V NMR spectroscopy
revealed a total loss in signal after reduction, indicating the presence of V(III) or
V(IV) species (Gladden and McGregor, unpublished results).
Using oxygen chemisorption at 293 K and 873 K the average oxidation state of
the 1% VO
x
/alumina was calculated as 3.8, whereas the average oxidation state for
the 3.5% VO
x
/alumina was calculated as 2.6 [31] . Both of these fi gures are in excel-
lent agreement with the conclusions from the UV - Vis DRS.
Bulk re - oxidation data in conjunction with thermogravimetric analysis ( TGA )
and TEOM results indicated that the ease of replacing oxygen that had been
removed during reduction was 8% > 3.5% > 1% [31] , (Gladden and McGregor,
unpublished results). This difference was also seen in the UV - Vis DRS and in
TEOM data. TEOM data provide information on the quantity of oxygen taken up
by the catalysts and on their rate of re - oxidation. The rate of catalyst re - oxidation
was seen to be greater for the 8 wt% catalyst than for materials of lower vanadium
loading (Gladden and McGregor, unpublished results). Once reduced, the 3.5 %V
sample did not regain its original state even after high temperature oxidation;
however, the reduced 8 %V sample did convert to a species that was similar to the
original oxidation state.
Supported VO
x
catalysts show good catalytic performance in both oxidation and
reduction reactions; for example, they are among the most active and selective
simple metal oxides for dehydrogenation and oxidative dehydrogenation of
alkanes. The possibility of using oxide systems other than chromia is being
Table 15.1 Extent of oxygen removal during reduction of vanadia/alumina catalysts.
Catalyst (V/nm
2
) V atoms in 1 g of
catalyst ( × 10
20
)
O atoms removed from
1 g of catalyst ( × 10
20
)
V : O ratio
1.1 1.18 1.13 1.0 : 1
3.7 4.13 4.14 1.0 : 1
10.4 9.45 7.90 1.2 : 1

explored in an effort to make the alkane – alkene conversion process more selec-
tive and stable. Supported VO
x
catalysts have been extensively studied for the oxi-
dative dehydrogenation of light alkanes [40, 54, 68 – 72] . They have also been
explored in the dehydrogenation of light alkanes including propane and butane
[35, 39, 73, 74] and have shown promise to be both active and selective for the
dehydrogenation reactions. However deactivation of VO
x
catalysts by coke species
is also observed with time - on - stream in butane dehydrogenation reactions. Owing
to the limited number of studies of supported VO
x
for alkane dehydrogenation,
the mechanism of deactivation/coke deposition and also the structure of sup-
ported VO
x
species under dehydrogenation conditions are unclear. Conventional
visible Raman spectroscopy usually cannot provide meaningful spectra for a cata-
lytic system that produces carbonaceous deposits because of strong fl uorescence
interference. These two issues were addressed in an investigation of butane dehy-
drogenation reaction on V/ θ - Al
2
O
3
catalysts with various surface VO
x
densities
(0.03 – 14.2 V/nm
2
) via in situ UV Raman spectroscopy [75] .
V/ θ - Al
2
O
3
catalysts with surface VO
x
density from 0.03 to 14.2 V/nm
2
were tested
for butane dehydrogenation under low partial pressures of butane at 873 K, and
the activity and selectivity data on these different catalysts were compared [75] . In
essence, the initial dehydrogenation activity increases as a function of surface VO
x
density. However, the activity decreases signifi cantly with prolonged reaction time
for samples with VO
x
density higher than 1.2 V/nm
2
, apparently due to the forma-
tion of surface coke deposits and blocking of surface sites during the reaction.
Interestingly, the dehydrogenation activity remains nearly unchanged on the cata-
lysts with surface VO
x
density no higher than 1.2 V/nm
2
. However, at higher
butane pressures, although the behavior of the 1.2 V/nm
2
sample is unchanged,
catalysts with higher V/nm
2
densities show enhanced activity (Table 15.2 ).
In the in situ UV Raman study [75] , V/ θ - Al
2
O
3
samples (0.03 V, 1.2 V, 4.4 V and
14.2 V) that contain surface VO
x
species with different structures were treated in
dilute butane fl ow at different temperatures and examined by Raman spectra
measurements at each temperature. Taking the 1.2 V sample as an example, the
Raman spectra collected during butane dehydrogenation at different temperatures
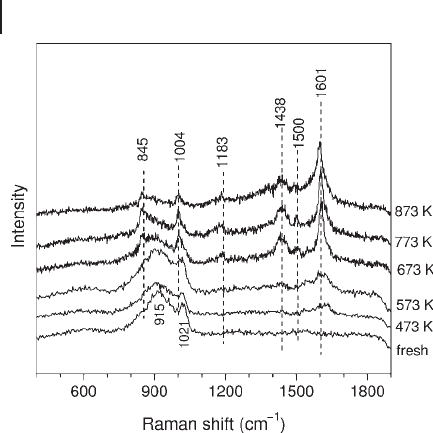
are shown in Figure 15.6 .
Table 15.2 Activities and turnover frequencies for butane dehydrogenation
at 1 bar pressure and 873 K with time - on - stream.
Time - on - stream
(min)
Rate ( µ mole g
− 1
s
− 1
) Turnover frequency (s
− 1
)
1.1 V/nm
2
3.7 V/nm
2
10.4 V/nm
2
1.1 V/nm
2
3.7 V/nm
2
10.4 V/nm
2
15 1.1 20.3 5.8 0.051 0.269 0.037
30 1.0 5.8 3.7 0.047 0.077 0.023
45 1.0 4.7 2.9 0.044 0.063 0.018
60 1.0 4.2 2.5 0.044 0.056 0.016
105 0.9 3.5 0.041 0.046 –
15.5 Vanadia 605
606 15 Alkane Dehydrogenation over Vanadium and Chromium Oxides
At temperatures below 673 K, a weak Raman band at 1620 cm
− 1
, assigned to C
=
C
stretching in polyalkenes, is observed together with the two bands at 1021 and
915 cm
− 1
due to the V
=
O and V
–
O
–
Al modes of surface VO
x
species, respectively.
After butane dehydrogenation at 673 and 773 K, an intense band at 1601 cm
− 1
due
to polyaromatic hydrocarbons develops. Simultaneously, bands at 1500, 1438,
1183, 1004 and 845 cm
− 1
are also observed. Meanwhile, Raman bands at 1021 and
915 cm
− 1
from surface VO
x
species are no longer observable, owing to a combina-
tion of the reduction of VO
x
species [43, 45, 47, 65, 71] and strong optical absorp-
tion by surface coke species. The band at 1500 cm
− 1
is usually assigned to conjugated
polyalkenes or cyclopentadienyl species [76, 77] . The band at 1438 cm
− 1
is due to
the bending mode of CH
3
/CH
2
or C
–
H in aromatic rings. C
–
H bending in aro-
matics usually appears near 1180 cm
− 1
. The two sharp bands at 1004 and 845 cm
− 1
are rarely reported for coke species, and thus one might ascribe them to surface
VO
x
species. However, the absence of an isotope shift on an
18
O - exchanged V/ θ -
Al
2
O
3
sample confi rms that these bands are due to surface coke deposits. As the
dehydrogenation temperature is increased to 873 K, the intensity of the Raman
bands below 1500 cm
− 1
decreases signifi cantly. Raman spectra obtained after the
reaction of butane with pre - reduced 1.2 %V (not shown) are very similar to those
on pre - oxidized 1.2 %V. This suggests that the initial valence state of surface VO
x
does not affect the nature of coke species and most likely the VO
x
species is at
least partially reduced under butane dehydrogenation conditions. This conclusion
can also be inferred from the similar activity/selectivity results from butane dehy-
drogenation tests on either oxidized or pre - reduced 1.2 %V and also the similar
UV - Visible spectra from V/ θ - Al
2
O
3
treated in either hydrogen or butane.
Figure 15.6 UV Raman spectra of butane dehydrogenation
over oxidized 1.2 V at different temperatures.
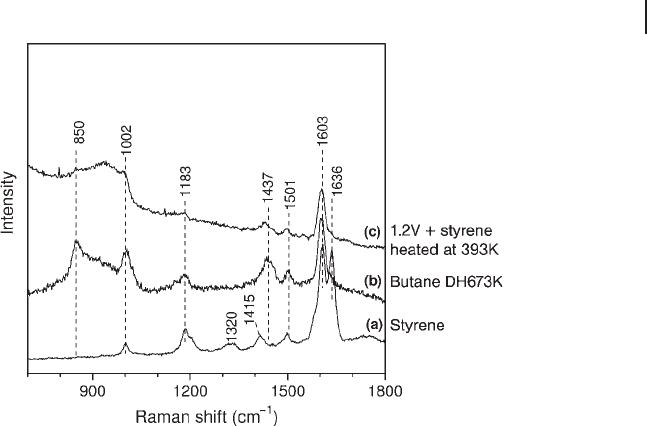
The set of Raman bands near 850, 1002, 1183, 1437, 1501 and 1603 cm
− 1
is also
observed to grow simultaneously on all other V/ θ - Al
2
O
3
samples when treated in
butane at high temperatures. To aid the assignment of these bands, the adsorption
and reaction of different C
4
olefi ns were studied on the V/ θ - Al
2
O
3
samples via
Raman spectroscopy. It turned out that they all form similar coke species, indicat-
ing that they are precursors to coke species in butane dehydrogenation on V/ θ -
Al
2
O
3
catalysts. It is interesting to fi nd out that 1,3 - butadiene adsorption at room
temperature results in similar spectra to that from butane dehydrogenation on
1.2 V at 673 K. Considering the low temperature (298 K), a possible chemical
change for 1,3 - butadiene is its cyclization to form styrene on the surface. A com-
parison of the spectrum from styrene with that after butane dehydrogenation at
673 K on 1.2 %V (Figure 15.7 ) reveals a very similar set of bands. Also included is
the spectrum from a mixture of 1.2 %V with styrene heated at 393 K (Figure
15.7 c).
Styrene is known to polymerize easily upon heating. Comparison of spectrum
c to that reported for polystyrene [78] confi rms the identity of spectrum c: the set
of Raman bands near 850, 1002, 1183, 1437, 1501 and 1603 cm
− 1
is due to polysty-
rene. Thus, it appears that polystyrene is a key intermediate in the coke formation
process during butane dehydrogenation on the 1.2 V catalyst. Further Raman
spectroscopy study of styrene on V/ θ - Al
2
O
3
samples heated to 873 K showed that
the characteristic Raman bands of polystyrene disappear, owing to its decomposi-
tion. This is consistent with catalytic studies of polystyrene degradation over solid
acid catalysts [79] . An interesting new observation is that although the Raman
Figure 15.7 Comparison of Raman spectra from (a) styrene,
(b) butane dehydrogenation over 1.2 V at 673 K and (c) a
mixture of 1.2 V and styrene heated at 393 K.
15.5 Vanadia 607

608 15 Alkane Dehydrogenation over Vanadium and Chromium Oxides
spectrum seems to indicate that the catalyst surface is free of coke species after
polystyrene decomposition, the follow - up TPO (Temperature Programmed Oxida-
tion) shows the evolution of a considerable amount of CO
2
. This suggests that the
absence of typical Raman bands (1000 – 1650 cm
− 1
range) due to coke species does
not necessarily mean the catalyst is coke free. Investigation of the catalytic surface
with multi - wavelength Raman spectroscopy should be helpful in determining the
chemical nature of this surface carbon. Microanalysis of a used 1.2 V catalyst for
carbon and hydrogen gave a C : H value of 0.9 as per polystyrene monomer in
agreement with the Raman spectra [31] .
One important general characteristic of coke is its topology, which can be
assessed by the intensity ratio of the band at around 1600 cm
− 1
(G band) to the
band at around 1400 cm
− 1
(D band) [76, 80] . UV Raman spectra from a series of
polyaromatic compounds [76, 81] show that the intensity in the spectral range
1600 – 1650 cm
− 1
is signifi cantly higher than that in the region 1300 to 1450 cm
− 1
for coke species with a 2 - D, sheet - like topology. By contrast, the intensity is more
nearly equal in these two spectral regions for coke species with chain - like topolo-
gies. The topology of coke species formed from butane dehydrogenation on the
various V/ θ - Al
2
O
3
catalysts at 873 K is compared by plotting the intensity ratio of
I
G
to I
D
as a function of surface VO
x
density. It is shown that the coke species are
more 2 - D, sheet - like from butane dehydrogenation on V/ θ - Al
2
O
3
with high surface
VO
x
density ( > 1.2 V/nm
2
) while more 1 - D - like on V/ θ - Al
2
O
3
with lower surface
VO
x
density ( ≤ 1.2 V/nm
2
). The 2 - D coke species can, presumably, reorganize into
pre - graphitic entities that have been thought to be the kind of coke that causes the
deactivation of dehydrogenation catalysts [33, 82, 83] . A study of coke deposited
on a 1 wt% V/Al
2
O
3
catalyst, in which the deactivation was studied as a function
of time - on - stream ( TOS ) using
13
C CP MAS ( cross - polarization magic angle spin-
ning ) NMR reveals a signifi cant increase in the aromatic peak at 130 ppm up to
3 h, then a gradual decrease in this peak [84] . The initial increase in the peak
intensity was explained by an initial coke build - up, whereas the decrease in inten-
sity was due to formation of very slowly relaxing, NMR “ invisible ” , highly polyaro-
matic coke, which formed under prolonged exposure to the reaction conditions.
This interpretation is consistent with the constant decrease in T
2
of adsorbed
pentane observed, which also suggests an increasing aromaticity of coke with
increasing TOS. Further, a monotonic decrease in pentane self - diffusion coeffi -
cient as a function of TOS was observed; these data are consistent with restriction
in molecular motion resulting from pore blockage due to coke deposition as deac-
tivation proceeds. However, in another study it was found that the majority of the
catalyst deactivation in the early stages of reaction was not due to polynuclear
aromatics or other forms of coke but to strongly adsorbed reaction intermediates
[31] . These species can be desorbed at room temperature by treating with a low
concentration O
2
in Ar mix. Figure 15.8 shows desorption of butane from a series
of VO
x
/alumina catalysts.
The catalyst had been running under butane for 2 h at 873 K when the gas stream
was switched from butane to argon. Online mass spectrometry indicated that all
hydrocarbon components were rapidly swept out of the reactor at 873 K and no
