Jackson S.D., Hargreaves J.S.J. Metal Oxide Catalysis
Подождите немного. Документ загружается.

16.3 Synthesis 629
material in the presence of cetyltrimethylammonium chloride and ammonia
provided higher surface areas. Moreover, surface area and thermal stability were
found to depend on digestion time. In their study, digestion at 110 ° C for 100 hours
provided high thermal stability and the highest surface area of 370 m
2
g
− 1
; the same
sample when calcined at 700 ° C possessed a surface area of 160 m
2
g
− 1
, which was
the best value obtained to date. Zirconia samples that were digested more than
100 hours had high thermal stability but no increases in surface area were observed.
The same samples that were dried by applying supercritical techniques did not
yield better results.
As previously stated, a common method for producing crystalline nanoparticle
oxides is the co - precipitation of metal cations as carbonates, dicarbonates or oxa-
lates, followed by their subsequent calcination and decomposition. Unfortunately,
the calcination invariably leads to agglomeration or, at high temperatures, aggrega-
tion and sintering. As an example, CeO
2
nanopowders have been prepared by cal-
cining the product of the precipitation between Ce(NO
3
)
3
and (NH
4
)
2
CO
3
, resulting
in crystalline 6 nm particles of CeO
2
at calcination temperatures as low as 300 ° C
[106] . NiO with 10 – 15 nm particles has been similarly prepared by precipitating
aqueous Ni
2+
solutions with (NH
4
)
2
CO
3
and calcining the products at 400 ° C [107] .
In some rare instances, crystalline oxides can be precipitated from aqueous solu-
tion, eliminating the need for a calcination step and greatly reducing the risk of
agglomeration. For example, TiO
2
can be prepared by precipitation using aqueous
TiCl
3
with NH
3
(aq) under ambient conditions. The products were 50 – 60 nm aggre-
gates and stabilized with poly(methyl methacrylate) [108] .
The direct co - precipitation of complex ternary oxides is somewhat uncommon,
but is nonetheless possible, particularly when the product assumes a very thermo-
dynamically favorable structure such as spinel. In such cases, the precipitation
reactions are normally carried out at elevated temperatures (50 – 100 ° C). Condensa-
tion of the two hydroxide intermediates into oxides and the induction of co -
Table 16.2 Survey from the literature of ZrO
2
prepared using precipitation methods.
Starting
material
Ppt agent Surfactant
a)
Conditions Surface area
(m
2
g
− 1
)
Ref.
ZrOC1
2
N H
3
(aq) none calcined 450 ° C 247 [97]
ZrOC1
2
N H
3
(aq) C
12
TACl calcined 450 ° C 274 [97]
ZrOC1
2
N H
3
(aq) C
14
TACl calcined 450 ° C 300 [97]
ZrOC1
2
N H
3
(aq) C
16
TACl calcined 450 ° C 312 [97]
ZrOC1
2
N H
3
(aq) C
18
TACl calcined 450 ° C 313 [97]
ZrOC1
2
NaOH P
123
calcined 450 ° C 103 [98]
ZrOC1
2
NaOH P
123
calcined 600 ° C 51 [98]
Zr(NO
3
)
4
TEA CTAB calcined 500 ° C – [99]
ZrOC1
2
N H
3
(aq) CTAB calcined 600 ° C 168 [100]
ZrOC1
2
N H
3
(aq) CTAB calcined 800 ° C 105 [100]
a C
12
TACl, dodecyltrimethylammonium chloride; P
123
, poly(ethylene oxide)-b-poly(propylene
oxide)-b-poly(ethylene oxide) triblock copolymer; CTAB, cetyltrimethylammonium bromide.
630 16 Properties, Synthesis and Applications of Highly Dispersed Metal Oxide Catalysts
precipitation were carried out in the same reaction vessel. For instance, Pr
3+
- doped
ceria has been precipitated to yield monodispersed 13 nm particles by aging
aqueous solutions of Ce(NO
3
)
3
and PrCl
3
at 100 ° C in the presence of a hexamethy-
lenetetramine stabilizer [109] . In such a case, the stabilizer indirectly serves as the
precipitating agent by raising the pH suffi ciently to induce precipitation of the
metal hydroxides. Likewise, MnFe
2
O
4
was prepared from aqueous Mn
2+
and Fe
2+
at temperatures up to 100 ° C to yield 5 – 25 nm particles [110] .
Chinnasamy and coworkers have reported an extensive series of experiments
for the preparation of spinel - structured CoFe
2
O
4
[111] . The sizes of the products
were determined by the infl uence of reaction temperature, reactant concentration
and reactant addition rate. In each case, aqueous solutions of Fe
3+
and Co
2+
were
precipitated with dilute NaOH. The results were predominantly in line with expec-
tations based on the considerations outlined in reaction temperature, reactant
concentration and reactant addition rate. Increasing the temperature from 70 ° C
to 98 ° C increased the average particle size from 14 to 18 nm. Increasing the NaOH
concentration from 0.73 to 1.13 M increased particle size from 16 to 19 nm. NaOH
concentrations of 1.5 M or greater resulted in the formation of a secondary FeOOH
phase and slowing the NaOH addition rate appeared to broaden the particle size
distribution. Li and coworkers also prepared 12 nm CoFe
2
O
4
by a similar route but
stabilized the product by acidifi cation with dilute nitric acid [112] .
A summary of oxides precipitated from aqueous solutions, including the rele-
vant reaction conditions, is given in Table 16.3 [113 – 119] .
16.3.2.2 Sonochemical Co - precipitation
The principle of sonochemistry is breaking the chemical bond with the application
of high - power ultrasound waves, usually between 10 and 20 MHz. The physical
Table 16.3 Summary of reactions for the precipitation of oxides from aqueous solution.
Oxide Starting
material
Ppt agent Stabilizer Conditions Product size
(nm)
Ref.
Fe
2
O
3
FeCl
2
N H
3
(aq) H
+
calcined 550 ° C 53.5 [113]
TiO
2
Ti(SO
4
)
2
N H
3
(aq) H
+
calcined 550 ° C 9.2 [113]
Al
2
O
3
Al(NO
3
)
3
N H
3
(aq) H
+
calcined 550 ° C 13.2 [113]
CeO
2
Ce(NO
3
)
3
N H
3
(aq) none calcined 650 ° C 12 – 15 [114]
TiO
2
TiCl
3
N a
2
O
2
NaCl calcined 700 ° C 30 [115]
VO
2
N H
4
VO
3
N
2
H
4
· H
2
O none calcined 300 ° C 35 [116]
Cr
2
O
3
K
2
Cr
2
O
7
N
2
H
4
· H
2
O none calcined 500 ° C 30 [116]
γ - Mn
2
O
3
KMnO
4
N
2
H
4
· H
2
O none – 8 [116]
Fe
3
O
4
FeCl
2
N H
3
(aq) H
+
N
2
atm 8 – 50 [117]
NiO NiCl
2
N H
3
(aq) CTAB annealed 500 ° C 22 – 28 [118]
ZnO ZnCl
2
N H
3
(aq) CTAB annealed 500 ° C 40 – 60 [118]
SnO
2
SnCl
4
N H
3
(aq) CTAB annealed 500 ° C 11 – 18 [118]
Sb
2
O
3
SbCl
3
NaOH PVA annealed 350 ° C 10 – 80 [119]

16.3 Synthesis 631
phenomenon responsible for the sonochemical process is acoustic cavitation.
According to published theories for the formation of nanoparticles by sonochem-
istry, the main events that occur during the preparation are creation, growth and
collapse of the solvent bubbles that are formed in the liquid. These bubbles are in
the nanometer size range. Solute vapors diffuse into the solvent bubble and when
the bubble reaches a certain size, its collapse takes place. During the collapse very
high temperatures of 5000 – 25 000 K [127] are obtained, which is enough to break
chemical bonds in the solute. The collapse of the bubble takes place in less than
a nanosecond [128, 129] hence a high cooling rate (1011 K s
− 1
) is also obtained. This
high cooling rate hinders the organization and crystallization of the products.
Since the breaking of bonds in the precursor occurs in the gas phase, amorphous
nanoparticles are obtained. Though the reason for the formation of amorphous
products is well understood, the formation of nanostructures is not. One possible
explanation is that in each collapsing bubble a few nucleation centers are formed
and while the fast kinetics does not stop the growth of nuclei that growth is limited
by the collapse. Another possibility is that the precursor is a non - volatile com-
pound and the reaction occurs in a 200 nm ring surrounding the collapsing bubble
[130] . In the latter case, the sonochemical reaction occurs in a liquid phase and
the products could be either amorphous or crystalline depending on the tempera-
ture in the ring region of the bubble. Suslick has estimated the temperature of the
ring region as 1900 ° C. The sonochemical method has been found useful in many
areas of material science, from the preparation of amorphous products [131, 132]
through the insertion of nanomaterials into mesoporous materials [102, 103] to
the deposition of nanoparticles on ceramic and polymeric surfaces [104, 105] .
Sonochemical methods for the preparation of nanoparticles were pioneered by
Suslick in 1991 [127] . If a reaction is carried out in the presence of oxygen by
similar methods, the product formed will be an oxide. NiFe
2
O
4
has been synthe-
sized by sonicating the mixture of Fe(CO)
5
and Ni(CO)
4
in decalin under 1 – 1.5 atm
pressure of oxygen [133] . ZrO
2
was synthesized using Zr(NO)
3
and NH
3
(aq) in
aqueous phase under ultrasonic irradiation, followed by calcination at 300 – 1200 ° C
to improve the crystallinity or, for temperatures of 800 ° C and above, to induce
the tetragonal to monoclinic phase transformation [100, 134] . Nanocrystalline
La
1 - x
Sr
x
MnO
3
was prepared in a similar manner [135] . The method has also been
successfully applied to synthesize Ni(OH)
2
and Co(OH)
2
nanoparticles [136] and
iron(III) oxide [137] . Polycrystalline CeO
2
nanorods 5 – 10 nm in diameter and 50 –
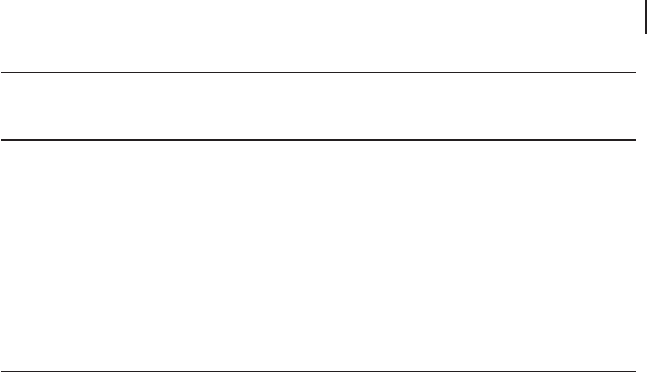
150 nm in length were synthesized via ultrasonication using polyethylene glycol
(PEG) as a structure - directing agent at room temperature. The content of PEG,
the molecular weight of PEG and the sonication time were confi rmed to be the
crucial factors determining the formation of one - dimensional CeO
2
nanorods. A
possible ultrasonic formation mechanism is suggested in Figure 16.9 [138] .
16.3.2.3 Microwave - Assisted Co - precipitation
The microwave processing of nanoparticles results in rapid heating of the reaction
mixtures, particularly those containing water. As a consequence, the precipitation
632 16 Properties, Synthesis and Applications of Highly Dispersed Metal Oxide Catalysts
of particles from such solutions tends to be rapid and nearly instantaneous,
leading to very small particle sizes and narrow size distributions within the prod-
ucts. The method offers the additional benefi t of requiring very short reaction
times and has similarly been used for the synthesis of Ag and Au nanoparticles
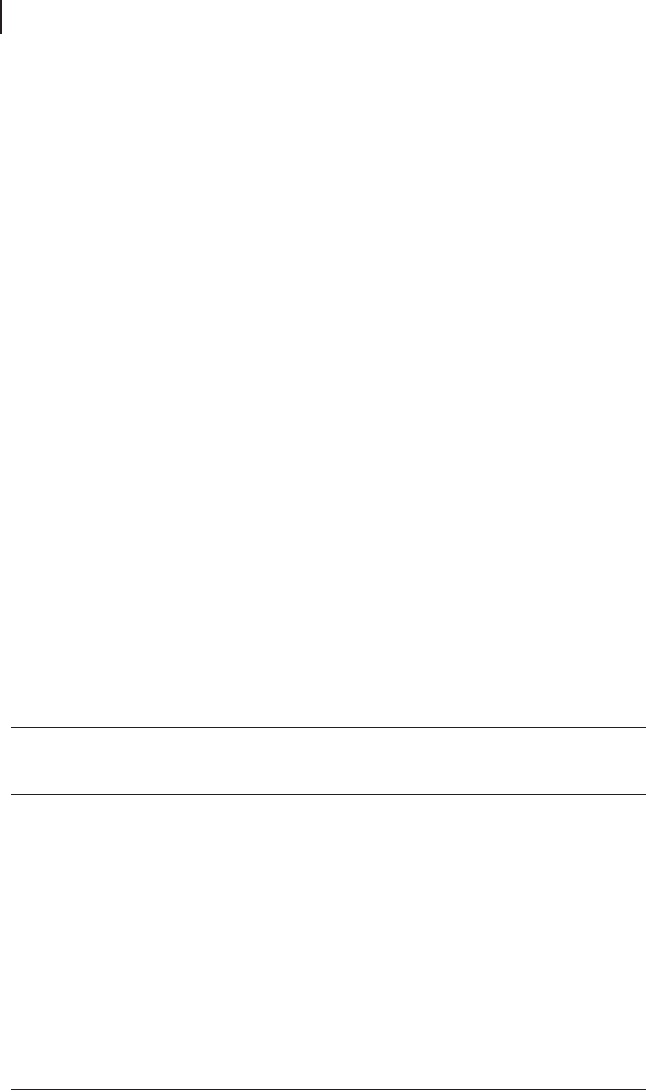
and transition metal oxides. Tu and coworkers [139] adapted a continuous fl ow
reactor which is depicted in Figure 16.10 . For example, two nano - structured
cerium(IV) oxide powders were synthesized by different synthetic routes: two
samples were obtained by precipitation from a basic solution of cerium nitrate and
treated at 523 and 923 K, respectively. A broad particle size distribution is observed
for CeO
2
obtained by precipitation (8.0 – 15.0 nm). Smaller particles (sizes around
3.3 – 4.0 nm) with a narrow particle size distribution characterize the ceria obtained
by microwave irradiation. Uniform α - Fe
2
O
3
nanoparticles were prepared by forced
hydrolysis of ferric salts under microwave irradiation. Gedanken ’ s group has also
published extensively on various oxides and chalcogenides prepared by microwave -
assisted irradiation [120 – 122, 140] .
Figure 16.9 Possible formation mechanism of CeO
2
nanorods (from [139] ).
Figure 16.10 Schematic of a continuous - fl ow microwave
reactor consisting of (a) a liquid column as a pressure
regulator, (b) a metal salt solution container, (c) a microwave
oven cavity, (d) a metal cluster dispersion receiver and (e) a
spiral tube reactor (from [139] ).

16.3 Synthesis 633
16.3.3
Solvothermal Technique
Hydrothermal processes [123 – 126] involve using water at elevated temperatures
and pressures in a closed system, often in the vicinity of its critical point. A more
general term, “ solvothermal, ” refers to a similar reaction in which a non - aqueous
solvent (organic or inorganic) is used. Under solvo(hydro)thermal conditions,
certain properties of the solvent, such as density, viscosity and diffusion coeffi -
cient, change dramatically and the solvent behaves much differently from what is
expected under ambient conditions [126] . Consequently, the solubility, the diffu-
sion process and the chemical reactivity of the reactants (usually solids) are greatly
increased or enhanced, enabling the reaction to take place at a much lower tem-
perature than normal. The method has been widely applied and adopted for crystal
growth of many inorganic materials, such as zeolites, quartz, metal carbonates,
phosphates and other oxides and halides [141 – 148] .
Solvothermal techniques have been extensively developed for the synthesis of
metal oxides [149 – 152] . Unlike many other synthetic techniques, solvothermal
synthesis concerns a much milder and softer chemistry conducted at low tempera-
tures. The mild and soft conditions make it possible to leave polychalcogen build-
ing - blocks intact while they reorganize themselves to form various new structures,
many of which might be promising for applications in catalysis, electronic, mag-
netic, optical and thermoelectronic devices [153 – 155] . They also allow the forma-
tion and isolation of phases that may not be accessible at higher temperatures
because of their metastable nature [156, 157] .
Although some solvothermal processes involve supercritical solvents, most
simply take advantage of the increased solubility and reactivity of metal salts and
complexes at elevated temperatures and pressures without bringing the solvent to
its critical point. The metal complexes are decomposed thermally either by boiling
the contents in an inert atmosphere or by using an autoclave. A suitable capping
agent or stabilizer such as a long - chain amine, thiol, trioctylphosphine oxide
( TOPO ), etc. is added to the reaction contents at a suitable point to hinder the
growth of the particles and hence stabilize them against agglomeration. The sta-
bilizers also help in dissolution of the particles in different solvents. Unlike the
cases of co - precipitation and sol – gel methods, solvothermal processes also allow
substantially reduced reaction temperatures, and the products of solvothermal
reactions are usually crystalline and do not require post - annealing treatments.
The synthesis of nanocrystalline TiO
2
, which is an important photocatalyst for
the decomposition of toxic chemicals, is one of the more thoroughly investigated
solvothermal/hydrothermal reactions. Approaches to this preparation have
involved the decomposition of metal alkoxides, [158] a TOPO - capped autoclave
synthesis of TiO
2
by metathetic reaction [159] and decomposition of a metal N -
nitroso - N - phenyl hydroxylamine complex [160, 161] . In 1988, Oguri and coworkers
reported the preparation of anatase by hydrothermally processing hydrous titania
prepared by the controlled hydrolysis of Ti(OEt)
4
in ethanol [162] . The reaction
conditions leading to monodispersed anatase nanoparticles by this approach

634 16 Properties, Synthesis and Applications of Highly Dispersed Metal Oxide Catalysts
were elucidated by others [163] . Extension of the method to the preparation of
lanthanide - doped titania particles has also been reported [164] . Cheng and cowork-
ers developed a method for preparing nano - scale TiO
2
by hydrothermal synthesis
using an aqueous TiCl
4
solution [165] . They found that acidic conditions favored
rutile while basic conditions favored anatase. It was also found that higher tem-
perature favored the highly dispersed product and that grain size could be con-
trolled by the addition of minerals such as SnCl
4
or NaCl, although the presence
of NH
4
Cl led to agglomeration of particles. The approach was extended, and
revealed that phase purity of the products depends primarily on concentration,
with higher concentrations of TiCl
4
favoring the rutile phase, while particle size
depends primarily on reaction time [166] . Yin and coworkers produced 2 – 10 nm
crystallites of monodispersed, phase - pure anatase by using citric acid to stabilize
the TiO
2
nanoparticles and treating the precursors hydrothermally in the presence
of KCl or NaCl mineralizers [167] .
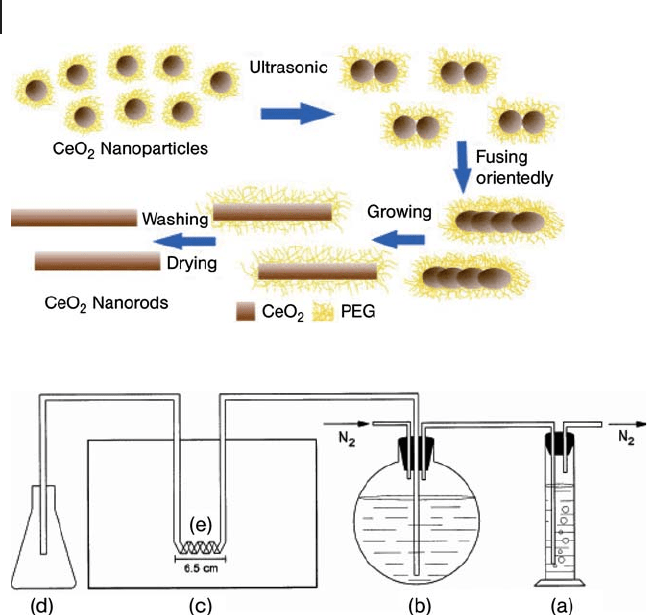
Niederberger and coworkers [150] reported a widely applicable solvothermal
route to nanocrystalline iron, indium, gallium and zinc oxides based on the reac-
tion between the corresponding metal acetylacetonate as metal oxide precursor
and benzylamine as solvent. They proved that with the exception of the iron oxide
system, in which a mixture of the two phases magnetite and maghemite is formed,
only phase - pure materials are obtained, γ - Ga
2
O
3
, zincite ZnO and cubic In
2
O
3
. The
particle sizes lie in the ranges 15 – 20 nm for the iron, 10 – 15 nm for the indium,
2.5 – 3.5 nm for the gallium and around 20 nm for the zinc oxide (Figure 16.11 ).
Moreover, the same group developed mixed nanocrystalline BaTiO
3
, SrTiO
3
and
(Ba,Sr) - TiO
3
. BaTiO
3
nanoparticles are nearly spherical in shape, with diameters
ranging from 4 to 5 nm, while SrTiO
3
particles display less - uniform particle shapes,
the size vaying between 5 and 10 nm [168] .
Masui and coworkers [169] reported the hydrothermal synthesis of nanocrystal-
line, monodispersed CeO
2
with a very narrow size distribution. They combined
CeCl
3
· 6H
2
O and aqueous ammonia with a citric acid stabilizer and heated the
solution in a sealed Tefl on container at 80 ° C. The CeO
2
nanoparticles exhibited a
3.1 nm average diameter. The ceria particles were subsequently coated with tur-
bostratic boron nitride by combining them with a mixture of boric acid and 2,2 ′ -
iminodiethanol, evaporating the solvent and heating at 800 ° C under fl owing
ammonia. Inoue and coworkers were able to reduce the particle size of hydrother-
mally prepared colloidal CeO
2
to 2 nm using a similar approach by autoclaving a
mixture of cerium metal and 2 - methoxyethanol at 250 ° C [170] . In this case, the
autoclave was purged with nitrogen prior to heating and the colloidal particles
were coagulated after heating with methanol and ammonia. The higher reaction
temperatures resulted in increased particle size.
In many cases, anhydrous metal oxides have been prepared by solvothermal
treatments of sol – gel or micro - emulsion - based precursors. Wu and coworkers
prepared anatase and rutile TiO
2
by a micro - emulsion - mediated method, in which
the micro - emulsion medium was further treated by hydrothermal reaction [171] .
This micro - emulsion - mediated hydrothermal ( MMH ) method could lead to the
formation of crystalline titania powders under much milder reaction conditions.
16.3 Synthesis 635
The micro - emulsion medium was heated to 120 – 200 ° C in a stainless steel auto-
clave. Micro - emulsions acidifi ed with HNO
3
produced monodispersed anatase
nanoparticles while those acidifi ed with HCl produced rutile nanorods. Titanium
dioxide (TiO
2
) nanoparticles prepared this way have been shown to be active
toward the photocatalytic oxidation of phenol [172] .
Metal oxides can also be synthesized by the decomposition of metal – cupferron
complexes, M
x
Cup
x
(Cup = C
6
H
5
N(NO)O
−
) [160] . Seshadri and coworkers were
able to replace amine - based solvents with toluene, and prepared ≈ 10 nm diameter
γ - Fe
2
O
3
and ≈ 7 nm CoFe
2
O
4
by hydrothermal processes [161] . They synthesized
maghemite γ - Fe
2
O
3
nanoparticles from a Fe
III
– cupferron complex and spinel
CoFe
2
O
4
nanoparticles starting from Co
II
– cupferron complex and Fe
III
– cupferron
Figure 16.11 TEM overview images of (a) iron oxide,
(c) indium oxide, (e) gallium oxide and (g) and (h) zinc oxide
nanoparticles (scale bar 100 nm) and their respective selected -
area electron diffraction patterns (b, d, f and i); (i) corresponds
to (g) (from [150] ).
636 16 Properties, Synthesis and Applications of Highly Dispersed Metal Oxide Catalysts
complex. However, the authors found that the presence of at least trace quantities
of a strongly coordinating amine was necessary to act as a capping agent and
prevent aggregation. They were also able to synthesize nanoparticulate ZnFe
2
O
4
by a similar approach [173] .
16.3.4
Micro - Emulsion Technique
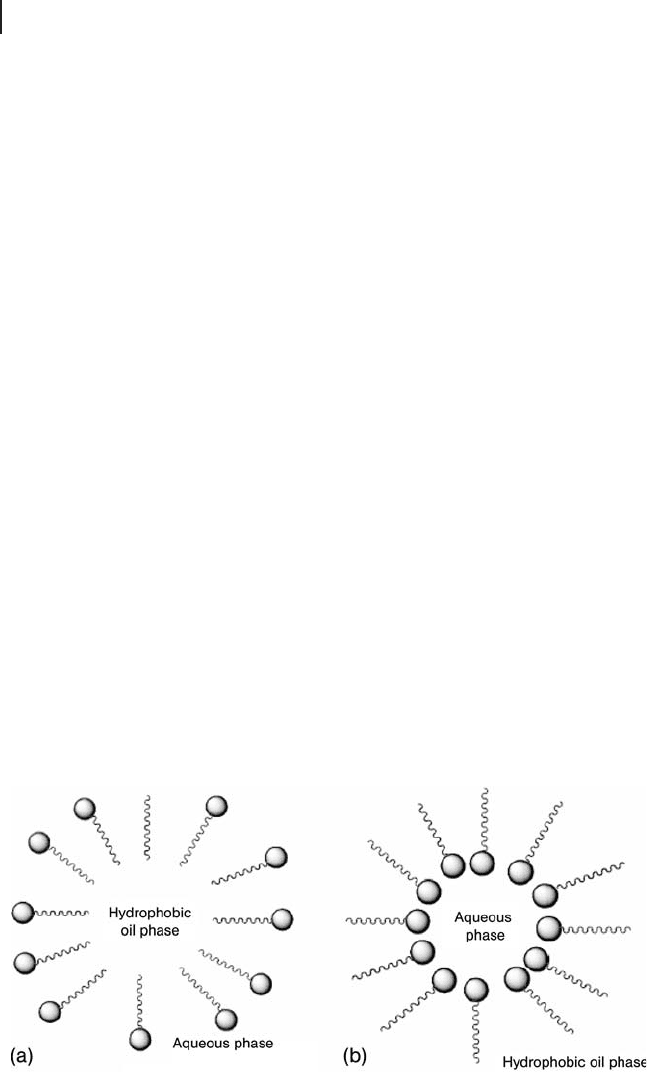
Micro - emulsions or micelles (including reverse micelles) represent an approach
based on the formation of micro/nano reaction vessels for the preparation of
nanoparticles, and has received considerable interest in recent years [174 – 180] . A
literature survey indicates that ultra - fi ne nanoparticles in the size range 2 – 50 nm
can be easily prepared by this method. This technique uses an inorganic phase in
water - in - oil micro - emulsions, which are isotropic liquid media with nano - sized
water droplets dispersed in a continuous oil phase. In general, micro - emulsions
consist of, at least, a ternary mixture of water, a surfactant (or a mixture of surface -
active agents) and oil. The dispersion of the aqueous phase is shown in Figure
16.12 [68] . The classical examples of emulsifi ers are sodium dodecyl sulfate ( SDC )
and aerosol bis(2 - ethylhexyl) sulfosuccinate (AOT). The surfactant (emulsifi er)
molecule stabilizes the water droplet because they have polar head groups and
non - polar organic tails. The organic (hydrophobic) portion faces towards the oil
phase and the polar (hydrophilic) group towards water. In diluted water (or oil)
solutions, the emulsifi er dissolves and exists as a monomer, but when its concen-
tration exceeds a certain limit called the critical micelle concentration ( CMC ), the
molecules of emulsifi er associate spontaneously to form aggregates called micelles.
These micro - water droplets then form nano - reactors for the formation of nanopar-
ticles. The nanoparticles formed usually have monodisperse properties. One
method of formation consists of mixing two micro - emulsions or macro - emulsions
and aqueous solutions carrying the appropriate reactants in order to obtain the
Figure 16.12 Schematic representation of (a) a micelle and
(b) an inverse micelle (from [68] ).

16.3 Synthesis 637
desired particles. The interchange of the reactants takes place during the collision
of the water droplets in the micro - emulsions. The interchange of the reactant is
very fast so that, for the most commonly used micro - emulsions, it occurs simply
during the mixing process. The reduction, nucleation and growth occur inside the
droplets, which controls the fi nal particle size. The chemical reaction within the
droplet is very fast, so the rate determining step will be the initial communication
step of the micro - droplets with different droplets. The rate of communication
has been defi ned by a second - order communication - controlled rate constant and
represents the fastest possible rate constant for the system. The reactant concentra-
tion has a major infl uence on the reaction rate. The rate of both nucleation and
growth are determined by the probabilities of the collisions between several atoms,
between one atom and a nucleus and between two or more nuclei. Once a nucleus
forms with the minimum number of atoms, the growth process starts. For the
formation of monodisperse particles, all of the nuclei must form at the same time
and grow simultaneously and with the same rate.
The preparation of metal oxide nanoparticles within micelles involves forming
two micro - emulsions, one with the metal salt of interest and the other with the
reducing or oxide - containing agent and mixing them together. When the two dif-
ferent reactants mix, the interchange of the reactants takes place through the
collision of water micro - droplets. The reaction (reduction, nucleation and growth)
takes place inside the droplet, which controls the fi nal size of the particles. The
interchange of nuclei between two micro - droplets does not take place owing to the
special restrictions from the emulsifi er. Once the particle inside the droplets
attains its full size, the surfactant molecules attach to the metal surface, thus sta-
bilizing and preventing further growth.
Reverse micelles are used to prepare nanoparticles by using an aqueous solution
of reactive precursors that can be converted to insoluble nanoparticles. Nanoparti-
cle synthesis inside the micelles can be achieved by a variety of methods, including
hydrolysis of reactive precursors, such as alkoxides, and precipitation reactions of
metal salts [181, 182] . Solvent removal and subsequent calcination leads to the fi nal
product. A variety of surfactants can be used in these processes such as pentadeca-
oxyethylene nonylphenylether (TNP - 35) [182] , decaoxyethylene nonylphenyl ether
(TNT - 10) [182] , poly(oxyethylene)
5
nonylphenylether (NP5) [183] and many others
that are commercially available. Several parameters, such as the concentration of
the reactive precursor in the micelle and the weight percentage of the aqueous
phase in the micro - emulsion, affect the properties, including particle size, particle
size distribution, agglomerate size and phases of the fi nal oxide powders. There
are several advantages to using this method, including the preparation of very small
particles and the ability to control the particle size. Disadvantages include low pro-
duction yields and the need to use large amounts of solvents and surfactants.
This method has been successfully applied for the synthesis of metals, metal
oxides, alloys and core – shell nanoparticles. The synthesis of metal oxides from
reverse micelles is similar in most aspects to their synthesis in aqueous phase by
a precipitation process. For example, precipitation of hydroxides is obtained
by addition of a base such as NH
3
(aq) or NaOH to a reverse micelle solution

638 16 Properties, Synthesis and Applications of Highly Dispersed Metal Oxide Catalysts
containing aqueous metal ions at the micellar cores. This method has been also
used to synthesize mixed metal iron oxides [162] ,
MFeOHexcess MFeOHO
O
22
24 2
2
2
++−
++
()
⎯→⎯⎯ + ↑
∆,
x
where M = Fe, Mn or Co. The resultant particles obtained are of the order of 10 –
20 nm in diameter. The cation distribution in the case of spinels depends on the
temperature used in the reaction [184] . If the transition metal cation is unstable
or insoluble in aqueous media, nanoparticles of those metals can be prepared by
hydrolysis of suitable precursors; for example, TiO
2
has been prepared from tet-
raisopropyl titanate [185] in the absence of water. The hydrolysis occurs when a
second water - containing solution of reverse micelles is added to the solution of
the fi rst micelle containing the metal precursor. Adjusting the concentration of
reactants can change the fi nal crystal form, that is between amorphous and crystal-
line. The reaction can be described as follows,
Ti O H O TiO Pr-OH
ii
Pr
()
+→+
()
4
22
24
Micro - emulsions have been also employed to prepare precursors that decompose
during calcination, resulting in desired mixed metal oxides. A series of mixed -
metal ferrites have been prepared by precipitating the metal precursor in a H
2
O -
AOT - isooctane system and calcining the products at 300 – 600 ° C [186] . A
superconductor material YBa
2
Cu
3
O
7 - δ
with 10 nm particles have been prepared by
combining a micellar solution of Y
3+
, Ba
2+
and Cu
2+
prepared in an Igepal CO -
430 – cyclohexene system with a second micellar solution containing oxalic acid in
the aqueous cores (Note: Igepal CO - 430 is a surfactant with chemical name “ Nonyl
phenol 4 mole ethoxylate ” ). The precipitate was subsequently calcined to 800 ° C to
remove oxalic precursors [187] . Other metals and metal oxides that are prepared
by a similar method include tungsten and tungsten oxide nanoparticles [188] and
high - surface - area Al
2
O
3
[189] .
16.3.5
Combustion Methods
The combustion synthesis technique consists of bringing a saturated aqueous
solution of the desired metal salts and a suitable organic fuel to the boil, until the
mixture ignites a self - sustaining and rather fast combustion reaction, resulting in
a dry, usually crystalline, fi ne oxide powder. By simple calcination, the metal
nitrates can, of course, be decomposed into melt oxides upon heating to or above
the phase transformation temperature.
Flame processes have been widely used to synthesize nanosize powders of oxide
materials. Chemical precursors are vaporized and then oxidized in a combustion
process using a fuel/oxidant mixture such as propane/oxygen or methane/air
[190] . They combine the rapid thermal decomposition of a precursor/carrier gas
stream in a reduced pressure environment with thermophoretically driven deposi-
