Jackson S.D., Hargreaves J.S.J. Metal Oxide Catalysis
Подождите немного. Документ загружается.

214 5 Solid-State NMR of Oxidation Catalysts
NMR techniques as a detailed knowledge of all the NMR parameters, such as
chemical shielding anisotropy and quadrupole tensor parameters, as well as their
distributions, is necessary to draw detailed conclusions on the local environment
and long - range order of V atoms. However, the SATRAS analysis of
51
V MAS
spectra coupled with the results of
51
V 3Q – MAS and 5Q – MAS studies (Figure 5.15 )
can provide this information and demonstrate the formation of a V
−
P
−
Ti com-
pound, in contrast to previous hypotheses for such systems.
51
V MQMAS studies are at present relatively rare. However, MQMAS techniques
have been applied in studies of other nuclei such as
27
Al and
95
Mo (see Section
5.3.2 ). The success of these studies suggests that
51
V MQMAS NMR may play a
role in future investigations of vanadium oxide catalysts.
It is relatively straightforward to characterize V
5+
- containing systems by tech-
niques such as MAS and MQMAS NMR. This is not true, however, for materials
in which vanadium is in a lower oxidation state. Only diamagnetic species can be
detected by NMR and, under ambient and reaction conditions, only V
5+
is diamag-
netic. Both V
3+
and V
4+
are paramagnetic and as such are themselves unobservable
and may, under certain structural conditions, prevent the observation of V
5+
. This
is due to the dipole interaction between the magnetic moments of the V
5+
nuclei
and the paramagnetic nuclei, causing the spectral lines to be broadened, often
beyond detection [100] . This may be the result of a direct linkage between the
para - and diamagnetic ions [84] or an interaction based on spatial proximity.
Shubin and coworkers [101] calculated that, in V
2
O
5
, no NMR signal would be
detected from a V
5+
ion within a ∼ 10 Å spherical radius of a V
3+
ion.
This problem is highlighted in the case of an alumina - supported vanadium cata-
lyst employed for the dehydrogenation of n - butane. The hydrocarbon creates a
reducing environment, which results in a reduction in the oxidation state of
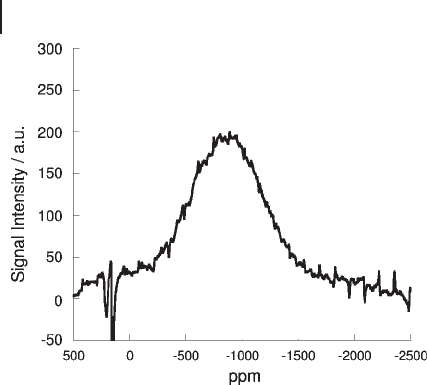
Figure 5.14
51
V MAS NMR of 3.5 wt% vanadium VO
x
/Al
2
O
3
catalyst after calcination at 700 ° C. Spinning rate = 14 kHz.
B
0
= 9.40 T.
vanadium in the catalyst. Figure 5.16 shows
51
V NMR spectra for the fresh catalyst
and for the sample after reaction. In contrast to the fresh catalyst the reacted cata-
lyst shows no observable NMR spectrum. It is not possible to quantify the degree
of reduction of the catalyst samples based on
51
V NMR measurements alone. In
V
2
O
5
a V
3+
content of only 2% has been observed to result in a decrease in signal
intensity from V
5+
of 70% [84] .
The decrease in signal intensity caused by the presence of paramagnetic nuclei
presents a challenge in NMR spectroscopy. However, a variety of NMR techniques
have been developed to overcome this diffi culty. Specifi cally, information concern-
ing the nature, location and oxidation state of vanadium nuclei can be obtained
from the NMR spectra of neighboring nuclei. Examples of such techniques will
be described in the following section, focusing on VPO catalysts.
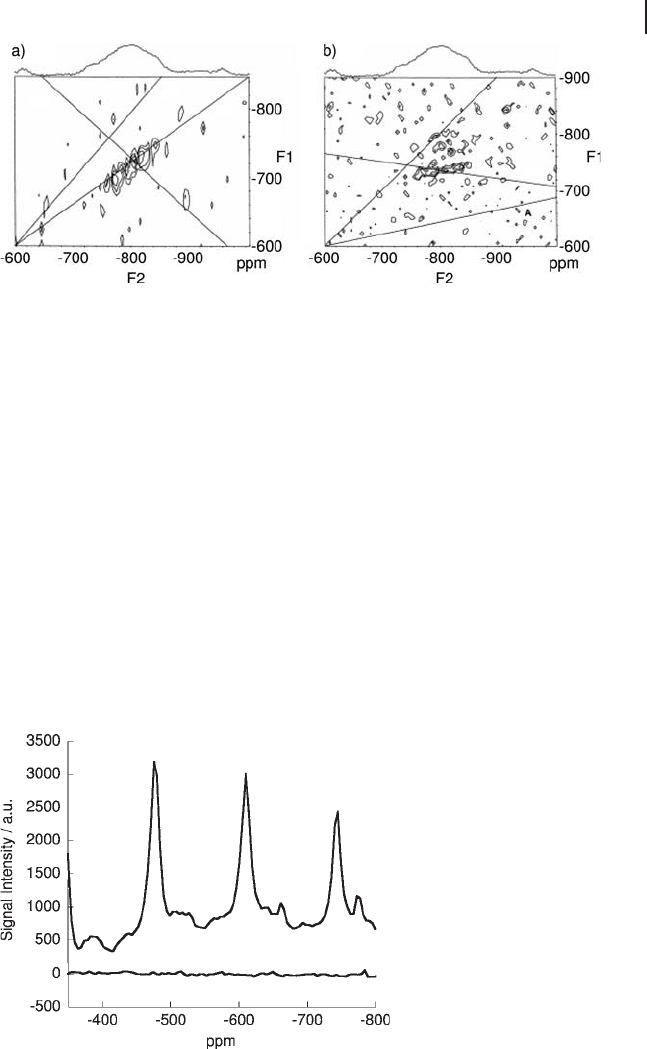
Figure 5.15 (a)
51
V 3Q - MAS and (b) 5Q - MAS spectra of
VO
x
/TiO
2
catalyst containing 10 wt% P
2
O
5
. Adapted from
ref. [27] ; reprinted with permission from Elsevier.
Figure 5.16
51
V MAS NMR of 8 wt% vanadium VO
x
/Al
2
O
3
catalyst prior to reaction (top) and after reaction with butane
at 600 ° C (bottom). Spinning rate = 14 kHz. B
0
= 9.40 T.
5.3 Structure of Bulk Oxides 215

216 5 Solid-State NMR of Oxidation Catalysts
5.3.1.2 VPO Catalysts
VPO catalysts are widely employed for the oxidation of butane to maleic anhydride
[1, 102 – 104] . As such, a large number of studies have focused on developing an
improved understanding of their structure. This task is complicated by the fact
that numerous different VPO phases exist, each with a different structure. In par-
ticular, the relative distribution of V
4+
and V
5+
is crucial in determining catalytic
activity [105] . As these materials contain V
4+
, conventional NMR measurements
on either
51
V or
31
P can result in signals that are very much broadened under the
infl uence of the unpaired electrons in the paramagnetic nuclei.
31
P spin - echo
mapping is, however, ideally suited to the study of such catalysts. This technique
allows paramagnetic nuclei to be indirectly observed through the observation of a
large
31
P spectral region. VPO catalyst systems represent the main area of applica-
tion for this approach. The use of NMR spectroscopy has benefi ts over techniques
such as X - ray diffraction ( XRD ), which cannot be applied to poorly crystalline
materials, and over X - ray photoelectron spectroscopy, which provides information
only on the surface species.
Li and coworkers were the fi rst group to apply a spin - echo mapping technique
to vanadium – phosphorus materials [33] . Studying VOPO
4
treated in n - butane,
both V
4+
and V
5+
phases could be distinguished, and their evolution monitored
over the course of reaction. In this manner it is possible to make inferences as to
the active phase of the catalyst. For instance, when treated with butane or butene,
the spectra of β - VOPO
4
indicate that, as expected, the proportion of V
4+
relative to
V
5+
increases. (VO)
2
P
2
O
7
is, however, relatively unaffected by exposure to such
compounds. This indicates that the reducing hydrocarbons interact with the
former phase but not the latter, implying that it is the β - VOPO
4
that is catalytically
active.
The most common application of spin - echo mapping is to identify different
phases and their oxidation states in VPO catalysts [33, 105 – 111] . Tuel has reviewed
much of the early literature in this area [110] . More recently Siegel has applied
31
P
spin - echo mapping, and
31
P MAS NMR, to the study of catalysts prepared from
VPO - NbPO precursors [112] . NMR results show that the chemical shift of
31
P in
the VOHPO
4
· 0.5H
2
O precursor is ∼ 100 ppm higher than the value of ∼ 1625 ppm
observed in the absence of Nb. The physical proximity of Nb to P indicated by this
change suggests that Nb acts as a dopant to the VPO catalyst altering the V
4+
/V
5+
ratio. The new catalyst has both improved catalytic performance and a shorter
activation period than the undoped material.
Spin - echo mapping therefore provides valuable structural information on VPO
catalysts, and can also be used, for example, to determine magnetic characteristics
such as the Weiss temperature of pure phases. Additionally, standard MAS NMR
techniques have successfully been employed to yield important information on
VPO catalysts, in a similar manner as for supported catalysts discussed above
[105, 106, 108, 112, 113] .
5.3.1.3 Other Vanadates
Other vanadate materials are also widely employed as catalysts, notably K - V - O
materials in the oxidation of SO
2
to sulfuric acid. Such catalysts typically contain
vanadium in a 5+ oxidation state. Lapina and coworkers [74] have provided a thor-
ough review of
51
V NMR assignments (both MAS and static) for these materials
and a large number of other vanadates containing V atoms in a range of different
environments. As an example of the role
51
V solid - state NMR can play in the study
of such systems, Lapina and coworkers consider the nature of active complex in
SO
2
oxidation over K - V - O catalysts. Through a comparison of the spectra of cata-
lysts with those of model compounds it is proposed that the active phase contains
vanadium atoms in a square - pyramidal coordination in a K
3
VO
2
SO
4
S
2
O
7
phase
which forms during reaction.
In addition to K - V - O catalysts [114, 115] , other vanadates which have been the
subject of NMR study include V - Mg - O materials [116] , bismuth vanadates [117] ,
LaVO
4
[118] and aluminum orthovanadate (AlVO
4
) [77, 86, 119 – 121] , which as
discussed above also has relevance to VO
x
/Al
2
O
3
catalysts. In a study of AlVO
4
,
Neilsen has applied both
51
V MAS NMR and
51
V and
27
Al MQMAS techniques in
order to elucidate the structural environment of aluminum and vanadium nuclei
[120, 121] . Figure 5.17 shows
27
Al MAS spectra acquired at 21.15 T which indicate
the presence of three distinct Al sites confi rming that AlVO
4
is isostructural with
FeVO
4
, that is, that it contains one pentacoordinated Al site and two AlO
6
octahe-
dra.
51
V 3Q - MAS spectra have also been acquired at 14.1 T. These indicate the
presence of three isolated VO
4
tetrahedra as shown in Figure 5.18 . In addition to
being signifi cant in their own right, studies on AlVO
4
are also valuable for the
identifi cation of such species in VO
x
/Al
2
O
3
catalysts. Figure 5.19 shows a
27
Al MAS
NMR spectrum of 8 wt% vanadium VO
x
/Al
2
O
3
after calcination at 700 ° C. Com-
parison with Figure 5.17 allows the formation of AlVO
4
to be confi rmed.
MQMAS NMR has also proven to be a useful for structural characterization of
other vanadates, such as LaVO
4
[118] and will likely become widely applied in such
studies in the coming years.
5.3.2
Other Metal Oxide Catalysts
All of the above studies have focused on oxides containing vanadium. While vana-
dium catalysts are very widely used, and studied, the use of solid - state NMR is not
Figure 5.17
27
Al MAS NMR spectra of AlVO
4
recorded
at B
0
= 21.15 T (234.5 MHz). Adapted from ref. [120] ;
reprinted with permission from the Royal Society of
Chemistry.
5.3 Structure of Bulk Oxides 217

218 5 Solid-State NMR of Oxidation Catalysts
Figure 5.18
51
V 3Q - MAS NMR spectrum of AlVO
4
. Spinning
rate = 10.5 kHz. B
0
= 14.1 T. The projection onto the
anisotropic dimension (F2) is a summation. V(1), V(2), and
V(3) indicate the isotropic peaks in this dimension for the
three
51
V sites. Adapted from ref. [121] ; reprinted with
permission from the American Chemical Society.
Figure 5.19
27
Al MAS NMR spectrum of 8 wt% vanadium
VO
x
/Al
2
O
3
calcined at 700 ° C The peak at − 10 ppm
corresponds to AlVO
4
. Spinning rate = 14 kHz. B
0
= 9.40 T.
restricted to such materials. Indeed
95
Mo,
89
Y,
119
Sn,
71
Ga and
43
Ca solid - state NMR
techniques have been applied to the study of metal oxide catalysts. Additionally,
27
Al and
29
Si may be applied not only to alumina and silica supports but also to
mixed - metal oxides.
17
O NMR has also been employed to study the environment
of oxygen atoms in oxide materials [38, 79, 122 – 124] .
Molybdenum oxide catalysts are widely employed in oxidation, hydrodesulfur-
ization and hydrodenitrogenation reactions. The active phase is most commonly
supported on alumina, although mixed - metal oxides are also used. As for sup-
ported vanadium catalysts, both static and MAS NMR techniques were widely
employed in early studies of such systems [125 – 129] . Like
51
V and
27
Al,
95
Mo is a
quadrupolar nucleus (spin = 5/2), with a natural abundance of 15.8%. Its relatively
small quadrupole moment (0.011 × 1 0
− 28
m
2
A) means that problems associated
with line broadening are minimal.
In addition to conventional static and MAS measurements on molybdenum
oxide catalysts, static - echo techniques have also been applied [125, 127, 129] .
Spin - echo spikelet experiments allow the presence of dynamically active surface -
interactive molybdenum oxide species to be resolved. Figure 5.20 compares the
spikelet - echo spectra of MoO
x
/Al
2
O
3
of different loadings before and after calcina-
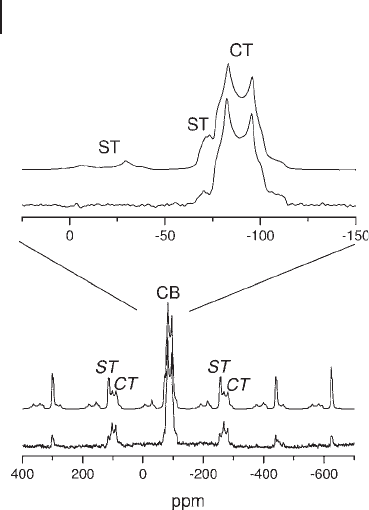
tion. The existence of the broad resonance on which the observed spikelets are
superimposed indicates that the surface is dynamically inhomogeneous. As dis-
cussed in Section 5.2.2.2 the basis of the spikelet - echo experiment is that surface
species with high mobility will have short spin – spin relaxation times ( T
2
) in com-
parison with species immobilized on the surface. The spikelet - echo experiment is
designed to distinguish species on the basis of their T
2
values.
More recently
95
Mo MQMAS spectroscopy has been applied to the study
of molybdates [130] . Figure 5.21 shows the
95
Mo 3Q - MAS spectra of
[(NH
4
)
6
Mo
7
O
24
] · 4H
2
O powder obtained at 19.6 T. This spectrum demonstrated
that the peak observed in the one - pulse MAS spectrum (also shown in Figure 5.21 )
at ∼ 30 ppm was in fact a convolution of signals from two distinct
95
Mo sites.
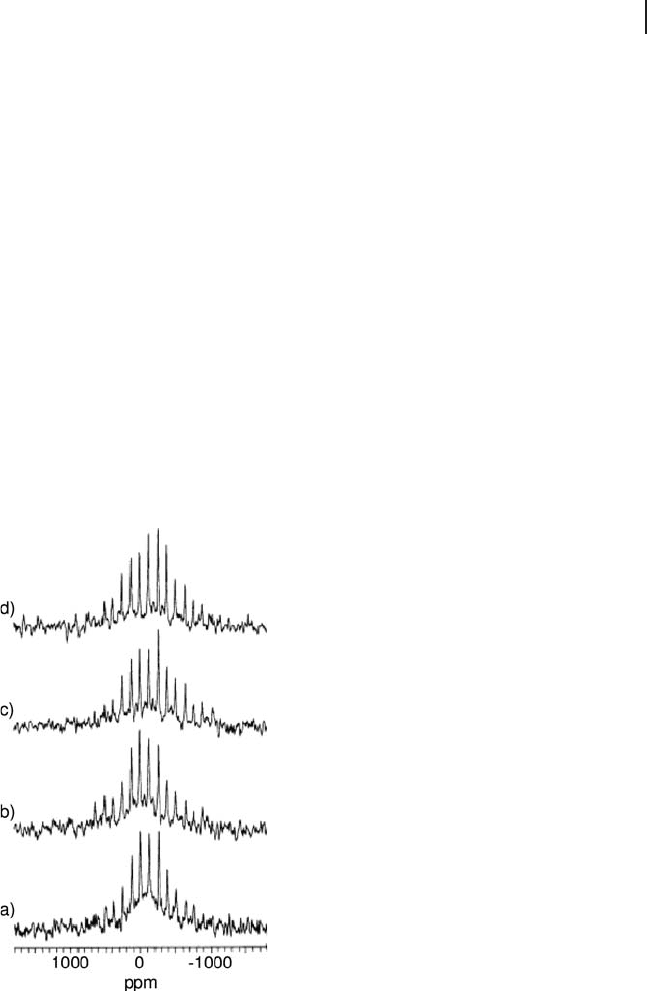
Figure 5.20 Spikelet echo spectra of the central transition
obtained for uncalcined and calcined MoO
x
/Al
2
O
3
catalysts
with 16 wt% and 24 wt% loadings. (a) 16% uncalcined,
(b) 24% uncalcined, (c) 16% calcined, and (d) 24% calcined.
Adapted from ref. [125] ; reprinted with permission from the
American Chemical Society.
5.3 Structure of Bulk Oxides 219
220 5 Solid-State NMR of Oxidation Catalysts
These sites have similar chemical shifts, hence are diffi cult to resolve by MAS
NMR, but have different C
Q η
allowing them to be distinguished by MQMAS
NMR.
95
Mo NMR spectroscopy is becoming increasingly applied to the study of molyb-
denum oxide catalysts [131 – 134] , while a number of more exotic nuclei have also
been the subject of study in catalytically relevant systems. These include
89
Y [135] ,
71
Ga [136] and
43
Ca [137] . Trokiner and coworkers have acquired one - pulse and
spin - echo
43
Ca spectra of calcium peroxide and its precursors [137] . These studies
aimed to gain insight into the mechanism of hydrogen peroxide disproportion-
ation as catalyzed by calcium hydroxide.
43
Ca NMR spectra of CaO, Ca(OH)
2
,
CaO
2
· 8H
2
O and CaO
2
· 2H
2
O
2
have all been acquired, characterizing the species
involved in the generation of singlet oxygen that is key to the reaction mechanism.
The value of the isotropic chemical shift in these compounds decreases through
the series 136 ± 1, 71 ± 3, 2 ± 2, − 40 ± 3 ppm. This change is indicative of progres-
sively increased shielding as the number of peroxo groups in the two fi rst coordi-
nation shells of the Ca
2+
ion increases.
71
Ga and
69
Ga solid - state NMR techniques
are also increasingly widely applied to gallium - containing catalysts [136, 138 – 140] .
Ga NMR, and the challenges involved in acquiring spectra from such systems, is
discussed in detail in Section 5.3.3.2 .
Figure 5.21
95
Mo MAS NMR of [(NH
4
)
6
Mo
7
O
24
] · 4H
2
O. The
lower trace is the one - pulse spectrum obtained at 54 MHz
( B
0
= 19.6 T). The upper trace was generated by SIMPSON.
Adapted from ref. [130] ; reprinted with permission from the
American Chemical Society.

5.3.3
Metal Oxide Supports
Metal oxides are widely used as catalyst supports. For instance, α - Al
2
O
3
is employed
as a support for catalysts in the partial oxidation of ethylene to ethylene oxide,
because a non - reactive material is essential for such applications [141] . However,
aluminas are also important catalysts in their own right. Transition aluminas
are known to catalyze the isomerization of alkenes, the dehydration of alcohols,
H/D exchange reactions and C
−
H bond activation [142] . Consequently, the devel-
opment of an understanding of both their bulk and their surface structure has
been a key goal in catalysis, with solid - state NMR being widely employed to this
end.
In addition to aluminas, other oxides such as SiO
2
, SnO
2
and Ga
2
O
3
are also
employed as catalyst supports. As these materials also contain NMR - active nuclei,
such as
119
Sn or
71
Ga, it is also possible to apply solid - state NMR to their charac-
terization. In general the study of such oxides is not as well developed as that of
27
Al NMR. However, recent technical and theoretical advances have led to an
increasing interest in these materials.
5.3.3.1 Aluminum Oxides
Solid - state
27
Al NMR has been applied to the study of aluminas and catalytic mate-
rials since the 1950s [143] . In 1960 O ’ Reilly reported the use of
27
Al NMR spec-
troscopy to identify different structural environments – tetrahedral and octahedral
Al atoms – in α - and γ - Al
2
O
3
[144] . The characterization of the structural environ-
ment of Al atoms remains the main application of
27
Al NMR in catalysis. Al atoms
in alumina can occupy different geometries, principally octahedral (AlO
6
), penta-
hedral (AlO
5
) and tetrahedral (AlO
4
). In MAS NMR these species show signals at
distinct frequencies corresponding to − 10 to 20 ppm (AlO
6
), 30 – 40 ppm (AlO
5
) and
50 – 85 ppm (AlO
4
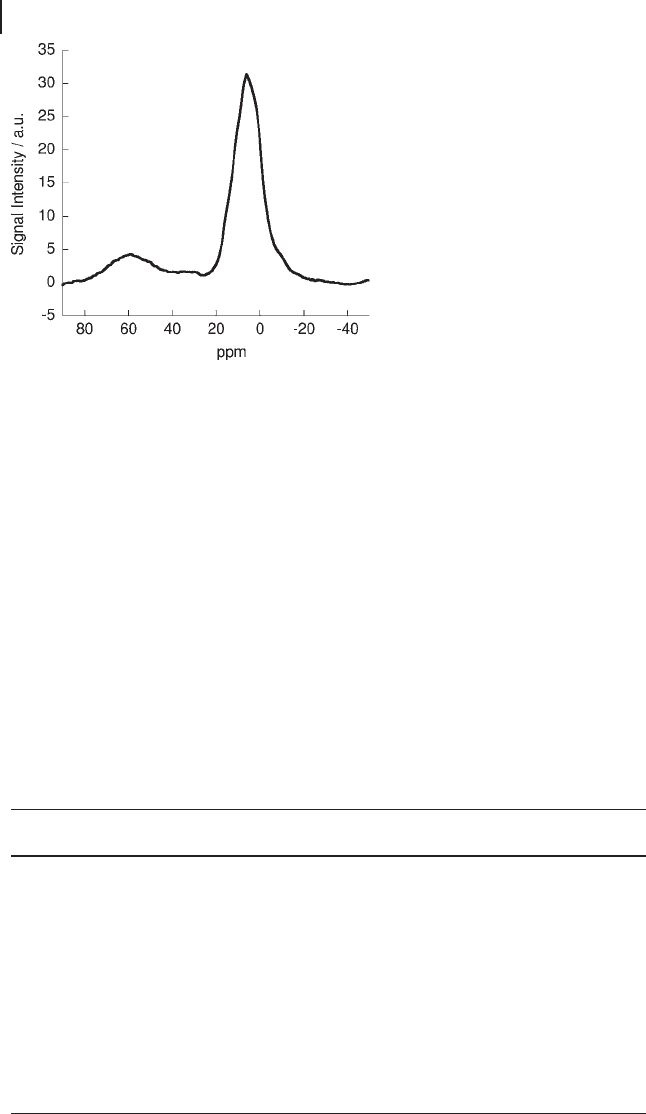
) [145, 146] . A
27
Al MAS NMR spectrum (acquired at 9.40 T with
a spinning rate of 14 kHz) of a VO
x
/Al
2
O
3
catalyst, with a vanadium loading of
8 wt%, showing alumina containing aluminum in all three environments, is
shown in Figure 5.22 . The proximity of these peaks to one another can make reso-
lution of individual peaks quite complex. Better resolution can be obtained through
other NMR techniques such as MQMAS NMR; however, the approach often
employed is to computationally simulate the experimental spectrum, optimizing
various NMR parameters such as chemical shift, chemical shift anisotropy, asym-
metry parameter and line broadening. Various packages are available for such
work including SIMPSON, a general purpose solid - state NMR simulation soft-
ware, developed by Bak and coworkers [147] . SIMPSON has previously been
employed to simulate
27
Al NMR spectra by Goldbourt and Madhu [148] . This
approach has been successfully employed by a number of workers on a variety of
systems [149 – 151] .
The change in the structure of aluminas through the dehydration sequence from
either gibbsite or boehmite to, ultimately, α - Al
2
O
3
, is perhaps where
27
Al NMR
spectroscopy has been most frequently applied. The results of a selection these
5.3 Structure of Bulk Oxides 221
222 5 Solid-State NMR of Oxidation Catalysts
studies, in terms of the distribution of aluminum coordinations are summarized
in Table 5.2 .
Solid - state NMR has a number of advantages over other techniques for charac-
terizing the structure of alumina. For instance, if the phase is determined by XRD
it is possible that crystallites of other phases, too small to be detected by XRD, are
also present. The infl uence of such small crystallites will, however, be apparent in
the ratio of aluminum in different coordination environments. Furthermore,
examples of alumina phases being incorrectly assigned because of a reliance on
X - ray diffractograms are present in the literature. Pecharroman and coworkers
[155] studied an alumina previously assigned as δ - Al
2
O
3
through inspection of
XRD data. Through a combination of infrared measurements and
27
Al MAS NMR
Figure 5.22
27
Al MAS of 8 wt% vanadium VO
x
/Al
2
O
3
. Spinning rate = 14 kHz. B
0
= 9.40 T.
Table 5.2 The distribution of Al( VI ) (octahedral), Al( IV )
(tetrahedral) and Al ( V ) (pentacoordinate) aluminum in
alumina polymorphs and precursors as determined by
literature
27
Al NMR studies.
% Al(VI) % Al(IV) % Al(V) References
Gibbsite 100
0 0
[146] , [152] , [153] , [154] , [155] , [225]
Boehmite 100
0 0
[146]
ρ
55
25 20 [152] , [150] , [154]
η 7 1 ± 1 21 ± 1
0
[152] , [154]
χ
73
20
7
[150] , [154]
κ
75
25
0
[156]
γ 7 2 ± 4 29.5 ± 4 1.5 ± 4
[24] , [151] , [152] , [153] , [154] , [155] , [226]
γ ′
69
31
0
[153]
δ
57
43
0
[152]
θ 57.5 ± 4.5 42.5 ± 4.5
0
[152] , [154]
α
100
0 0
[146] , [151] , [155] , [226]
data revealing the distribution of Al sites, the sample was shown to in fact be a
mixed - phase γ / θ / α - alumina.
Additionally, Paglia and coworkers [158] have recently identifi ed a new alumina
phase, denoted γ ′ - Al
2
O
3
through the use of
27
Al MAS NMR. γ ′ - Al
2
O
3
occurs in the
dehydration sequence of boehmite after γ - Al
2
O
3
where δ - Al
2
O
3
might be expected.
θ - Al
2
O
3
and α - Al
2
O
3
formed as normal at higher temperatures.
There are a number of technical considerations to take into account when
dealing with
27
Al MAS NMR. Notably, the spinning speed, that is the rate at which
the sample is spun about the magic angle, has a signifi cant infl uence on
27
Al MAS
NMR spectra. This is most apparent when considering the peak corresponding to
AlO
5
at ∼ 30 ppm. For instance, Meinhold and coworkers observed that the intensity
of this peak increased as the spinning speed was raised from 8 kHz to 15 kHz [152] .
This is evident when comparing the results of different workers investigating
the same alumina polymorphs. In studies of AlO
5
containing ρ - and χ - aluminas,
Meinhold and coworkers [152] and Kunath - Fandrei and coworkers [150] report
AlO
6
: AlO
5
: AlO
4
ratios of 55 : 20 : 25 and 73 : 7 : 20 for ρ - and χ - Al
2
O
3
respectively.
MacKenzie and coworkers [154] , however, report ratios of 69 : 9 : 22 and 79.5 : 0.5 : 20.
Meinhold and coworkers employed spinning rates up to 15 kHz and Kunath -
Fandrei and coworkers used 14 kHz but MacKenzie and coworkers employed a
rate of only 12 kHz thereby explaining the discrepancy between the datasets. While
the AlO
5
peak is most affected by this phenomenon the AlO
6
and AlO
4
peaks are
also slightly affected, with AlO
4
showing less change than AlO
6
[152] . An additional
effect of spinning speed is on the NMR linewidths obtained. For instance, high
spinning speeds decrease the linewidth of the octahedral peak [152] .
In addition to conventional MAS NMR, MQMAS NMR has also been employed
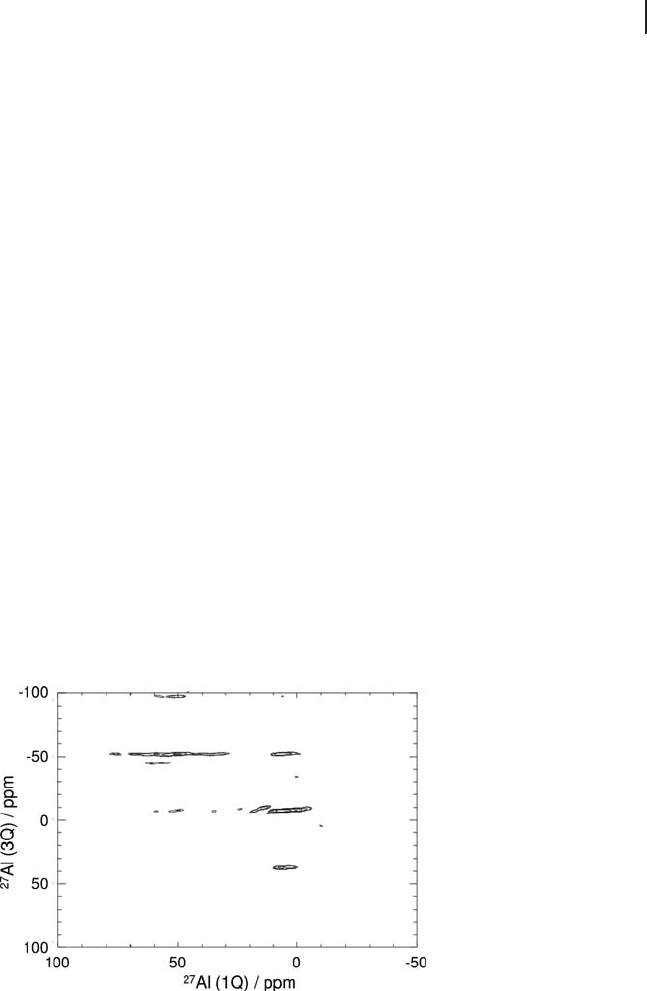
in the study of aluminas [24, 156, 159 – 162] . Figure 5.23 shows a MQMAS spec-
Figure 5.23
27
Al MQMAS spectrum of 8 wt% vanadium
VO
x
/Al
2
O
3
. In contrast with the
27
Al MAS spectrum (Figure
5.22), different Al coordination environments are more readily
distinguishable. Spinning rate = 10 kHz. B
0
= 9.40 T. Shifted -
echo spectra were acquired using the pulse sequence of
Brown and Wimperis [163] .
5.3 Structure of Bulk Oxides 223
