Jackson S.D., Hargreaves J.S.J. Metal Oxide Catalysis
Подождите немного. Документ загружается.

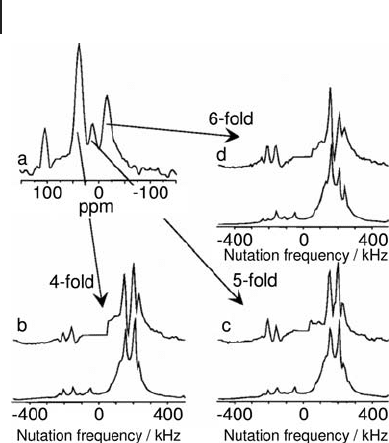
204 5 Solid-State NMR of Oxidation Catalysts
Catalytically relevant applications of this technique are focused around the study
of
27
Al - containing systems [24, 32] . Figure 5.5 shows a single - pulse MAS
27
Al
spectrum and the experimental and simulated off - resonance nutation spectra for
each individual coordination environment in AlPO
4
, a widely used catalyst support
[24] . From these spectra the quadrupolar coupling constants were determined to
be 3.4, 3.1 and 2.7 MHz for 4 - , 5 - and 6 - coordinated Al sites respectively. While
off - resonance nutation NMR lacks the resolution of MQMAS in discriminating
overlapping sites, it has proved itself more reliable in detecting all of the distinct
coordination environments present in a sample [32] .
5.2.1.5 Spin - Echo Mapping
While the techniques described above are widely applied in the study of metal
oxide catalysts, they are not necessary suitable for systems that contain paramag-
netic nuclei. Paramagnetic nuclei, as discussed in Section 5.3.1.2 , are not observ-
able by conventional NMR techniques and furthermore can increase the linewidths,
sometimes to beyond observable limits, of neighboring nuclei. This poses a
problem when studying catalytic metal oxides that contain paramagnetic centers.
Vanadium phosphorus oxide s ( VPO ), for instance, contain paramagnetic V
4+
.
Spin - echo mapping allows the indirect observation of paramagnetic centers
by discerning their effect on neighboring nuclei. The technique was developed
Figure 5.5 (a)
27
Al MAS spectrum of amorphous AlPO
4
.
Resonances are Al(IV) (42 ppm), Al(V) (12 ppm), and Al(VI)
(− 11 ppm). The peak at 105 ppm is due to an Al impurity in the
Si
3
N
4
rotor. For every coordination the experimental (top) and
simulated (bottom trace) off - resonance nutation spectrum is
given: (b) fourfold coordination, (c) fi vefold coordination,
(d) sixfold coordination. Adapted from ref. [24] ; reprinted with
permission from the American Chemical Society.
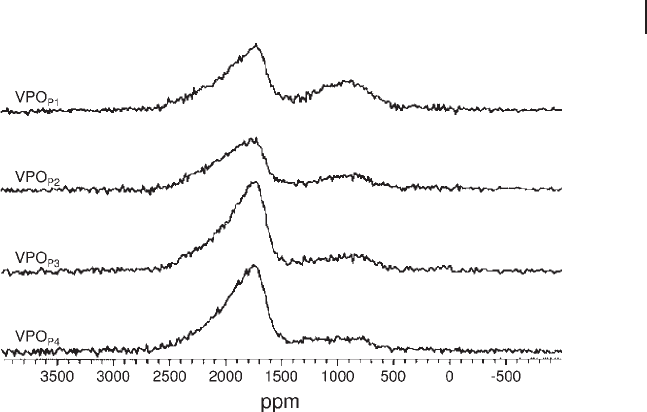
5.2 NMR Techniques 205
through the work of Li and coworkers [33] , and its early application to VPO cata-
lysts has been reviewed by Tuel and coworkers [34] . In such systems, information
on the oxidation state of vanadium can be investigated through observing the
infl uence of vanadium on the
31
P spectrum. For instance,
31
P nuclei bonded to V
5+
nuclei are observed at 0 ppm, while those bonded to V
4+
appear at ∼ 2500 ppm.
Figure 5.6 shows
31
P spin - echo mapping spectra of four distinct VPO catalyst
precursors VPO
P1
, VPO
P2
, VPO
P3
and VPO
P4
prepared by two different methods
[35] . VPO
P1
and VPO
P2
are prepared from orthophosphoric acid and correspond
to samples before and after water refl ux. VPO
P3
and VPO
P4
correspond to materials
synthesized from pyrophosphoric acid, again before and after water refl ux.
Two different V environments are observed, V
4+
in the VOHPO
4
· 0.5H
2
O structure
with a signal at 1725 – 1770 ppm and V
4+
– V
5+
dimers with a signal at around
900 ppm. The results show that the dimers formed by the latter route have a higher
stability in water. In addition to characterizing vanadium sites, spin - echo mapping
has also been used to provide similar information on paramagnetic cobalt
centers [36] .
Applied to VPO catalysts, spin - echo mapping not only provides information on
the oxidation state of different vanadium nuclei, but can also differentiate different
phases with the same oxidation state. The development of a NMR technique to
probe such materials has been extremely valuable, as their often poorly crystalline
nature prevents characterization through X - ray diffraction. Additionally, variable -
temperature spin - echo mapping has been shown capable of determining magnetic
characteristics of materials, such as their Weiss temperature [34, 37] .
Figure 5.6
31
P NMR spin - echo mapping spectra of VPO
P1
,
VPO
P2
, VPO
P3
and VPO
P4
catalyst precursors. V
4+
species
appear at 1725 – 1770 ppm and V
4+
– V
5+
dimers at ∼ 900 ppm.
Adapted from ref. [35] ; reprinted with permission from the
Royal Society of Chemistry.

206 5 Solid-State NMR of Oxidation Catalysts
5.2.2
Surface Structure of Catalysts
While understanding the bulk structure of a catalyst is of obvious importance it
is often desirable to acquire information selectively about the nature of the catalyst
surface. Such information may include the nature of any acid sites present, or
differences in the coordination of atoms at the surface as compared to those in
the bulk. NMR is ideally suited to such studies owing to its inherent sensitivity to
the local environment of the nuclei under observation.
5.2.2.1 Double - Resonance Techniques
It is often the case that nuclei present at the surface of catalysts are also present
in the bulk, such as oxygen atoms in metal oxides. Therefore, a number of innova-
tive techniques have been developed in order to selectively probe surface species.
Frequently this entails the use of double - resonance methods, involving, for
example, polarization transfer between two nuclei.
Cross Polarization Cross - polarization ( CP ) NMR, normally combined with MAS
(CP - MAS NMR), provides selective information about nuclei at the surface of a
material. This technique is used to enhance the signal of surface nuclei (often the
metal nuclei, for example
71
Ga or
27
Al in the case of metal oxides such as Ga
2
O
3
or Al
2
O
3
) by magnetization transfer from the nuclei of a species present in, or
adsorbed onto, the surface. These nuclei are most commonly
1
H from surface
hydroxyls or adsorbed probe molecules, although examples of transferring mag-
netization from the metal nuclei in order to study the surface oxygen species also
exist [38] . In order to establish magnetization transfer, the rf pulses applied on the
two frequency channels must fulfi ll the Hartmann – Hahn condition [39] . The
surface - selective nature of this experiment arises from the fact that the cross -
polarization rate is dependent upon the magnitude of the dipolar interaction
between the two nuclei involved and hence on their internuclear distance. In cases
where the nucleus of interest is quadrupolar, for instance in
1
H -
27
Al CP - MAS
NMR, the central 1/2 – 1/2 transition is spin - locked and a modifi ed Hartman – Hahn
condition applies [39] .
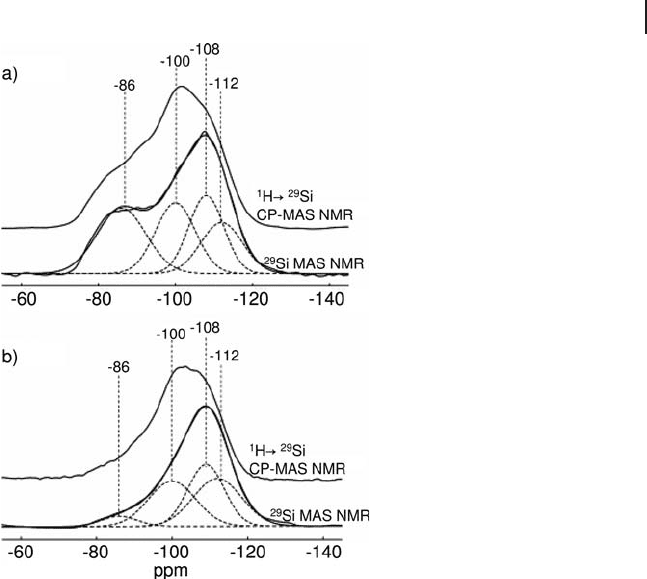
Figure 5.7 shows both
29
Si MAS NMR and
1
H →
29
Si CP - MAS NMR spectra of
(a) a silica - alumina support (ASA) and (b) a NiMo/ASA catalyst [40] . As can be
seen, the CP experiment selectively enhances the signals at − 100 and − 86 ppm.
These signals correspond to silicon atoms coupled to
1
H nuclei of OH groups.
Comparing the spectra of the supported catalyst with that of the pure support, a
strong decrease in intensity is observed at − 86 ppm and a slight decrease at
− 100 ppm. This indicates that Mo and Ni species interact with the silanol groups
and are adsorbed more easily on the geminal silanols present on the support.
Other Double Resonance Techniques Despite the fact that dipolar couplings add
to line broadening and are removed through techniques such as MAS, it is often
desirable to re - introduce them. This is because dipolar couplings contain informa-
5.2 NMR Techniques 207
tion about the environment of the nuclei, including interatomic distances. DOR
experiments are therefore often used to determine dipolar couplings between dif-
ferent nuclei. Examples of such techniques include Rotational Echo Double Reso-
nance ( REDOR ) [41, 42] , Rotational Echo Adiabatic Passage Double Resonance
( REAPDOR ) [43, 44] , Spin - Echo Double Resonance ( SEDOR ) [45] , Transferred -
Echo Double Resonance ( TEDOR ) [41, 46] and Transfer of Population in Double
Resonance ( TRAPDOR ) [47, 48] . TRAPDOR was developed by Grey and coworkers
[47, 48] and exploits the heteronuclear dipole coupling between quadrupolar and
spin - 1/2 nuclei, thus revealing connectivity and spatial interactions. It facilitates
the recoupling of quadrupole spins in Zeeman states other than ± 1/2. DOR experi-
ments have been employed by a number of workers in order to determine the
location of Br ø nsted acid sites on catalyst surfaces. For instance, Yang and cowork-
ers have used TRAPDOR to study the acid sites on a sulfated aluminum oxide
catalyst [49] . Figure 5.8 shows
1
H spin - echo spectra of (a) γ - Al
2
O
3
, (b) SO
3
/ γ - Al
2
O
3
,
(c) SO
3
/ γ - Al
2
O
3
(under TRAPDOR conditions) and (d) difference spectrum of (b)
and (c). The
1
H source is surface hydroxyl groups. Under TRAPDOR conditions
the
1
H signals at 0.3, 2.4 and 4.3 ppm are signifi cantly suppressed, while the
intensity of the 11.2 ppm signal remains almost unchanged. This indicates that
Figure 5.7
29
Si MAS NMR and 1H →
29
Si CP/MAS NMR
spectra of (a) a silica - alumina support (ASA) and (b) NiMo/
ASA catalyst. Adapted from ref. [40] ; reprinted with permission
from Elsevier.
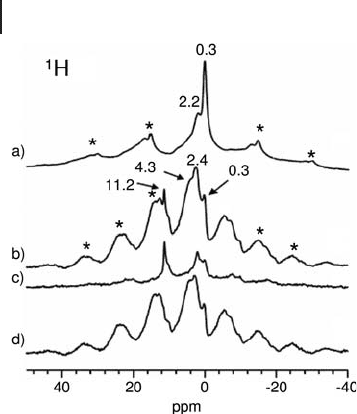
208 5 Solid-State NMR of Oxidation Catalysts
the
1
H species giving rise to the peak at 11.2 ppm are not in close proximity to an
Al nucleus and may instead be bound to a sulfur atom.
5.2.2.2 Spin - Echo Techniques
As discussed above, one of the key requirements in obtaining high - resolution
NMR spectra in the solid state is to overcome the various line - broadening mecha-
nisms that exist. Inhomogeneous broadening occurs because of the variation of
the macroscopic magnetic fi eld over the sample, during the NMR experiment. This
may be caused by susceptibility effects or by imperfections in the instrumentation.
Spin - echo methods reverse the inhomogeneous part of the signal decay through
the application of a second rf pulse during the experiment. A spectrum is then
generated through the Fourier transformation of the echo - train, yielding informa-
tion on, for example, coupling constants. Such processes are discussed in detail
in a number of texts [50, 51] .
Spikelet - Echo The spikelet - echo experiment involves the digitization of an entire
train of spin - echoes [52] . The spectra produced by this method consist a series of
sharp “ spikelets ” (see Figure 5.9 ) which reveal the presence of more than one
species through inconsistencies in the spikelet spacings and linewidths, as these
are dependent upon the spin – spin relaxation time, T
2
, and hence species sensitive.
Figure 5.9 shows a
17
O MAS NMR spikelet spectrum of a partially metamict zircon
(ZrSiO
4
) as measured by Ashbrook and Farnan [53] . Two peaks are observed in
the spectrum. The peak at ∼ 150 ppm is typical of oxygen in a non - bridging environ-
Figure 5.8
1
H spin - echo spectra of (a) γ - Al
2
O
3
(without Al
irradiation), (b) SO
3
/ γ - Al
2
O
3
(without Al irradiation), (c) SO
3
/ γ -
Al
2
O
3
(with Al irradiation), (d) difference spectrum of (b) and
(c). Asterisks denote spinning sidebands. Adapted from
ref. [49] ; reprinted with permission from the Royal Society
of Chemistry.
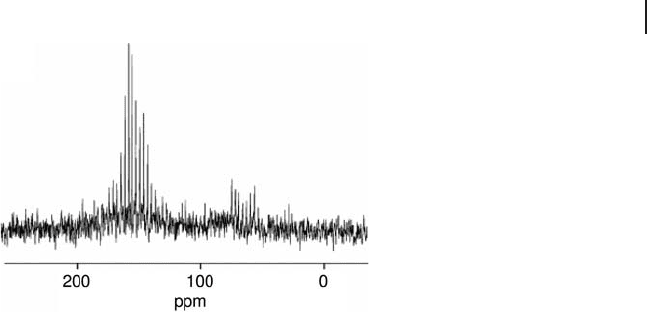
ment such as those found in the isolated SiO
4
tetrahedra, while that at ∼ 65 ppm
is typical of oxygen species that bridge SiO
4
tetrahedral units.
5.2.2.3 CRAMPS
Combined Rotation and Multiple Pulse Spectroscopy ( CRAMPS ) is a technique in
which the dipolar interaction is averaged through a multiple - pulse sequence [54,
55] . The simultaneous spinning around the magic angle, as in MAS NMR, aver-
ages the chemical shift anisotropy. Under appropriate conditions, CRAMP spectra
can be of greater resolution than MAS NMR spectra. While CRAMPS is not exclu-
sively a surface - sensitive technique, the majority of catalytic applications have
focused on the study of adsorbed species, and the information on surface structure
that can be extracted from their spectra.
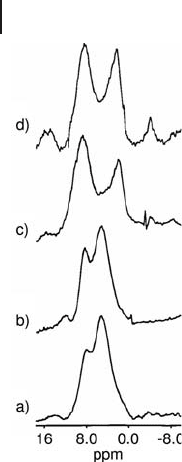
Fitzgerald and coworkers have employed CRAMPS to identify and monitor the
1
H species of surface Al
−
OH groups and physisorbed water associated with a high
surface area alumina [56] . Figure 5.10 shows the
1
H CRAMPS spectra of four
alumina samples dehydrated under a variety of conditions. The data show that
heating at 25 ° C and 110 ° C at ambient pressure results in the partial removal of
physisorbed water (4.0 ppm). The other peaks in the spectra correspond to octahe-
dral AlOH (3.0 ppm) and Al
2
OH (8.2 ppm) sites.
5.3
Structure of Bulk Oxides
Metal oxide - based materials are widely employed as catalysts for a wide number
of applications, particularly in processes such as dehydrogenation and oxidation,
where redox chemistry is important. The structure of metal oxides facilitates these
reactions through the transfer of oxygen, or the removal of hydrogen. In order to
fully understand the structural dependence of these processes, and hence to refi ne
existing catalysts and catalytic processes and to develop new active materials, it is
Figure 5.9
17
O spikelet - echo MAS NMR spectra of a partially
metamict ZrSiO
4
. Adapted from ref. [53] ; reprinted with
permission from Elsevier.
5.3 Structure of Bulk Oxides 209
210 5 Solid-State NMR of Oxidation Catalysts
necessary to understand the structure of the oxides themselves and the active sites
within them. Solid - state NMR techniques have proven to be an invaluable tool in
this area. Such techniques and their applications are outlined in the coming
sections.
5.3.1
Vanadium Oxide Catalysts
Vanadium catalysts are among the most signifi cant metal oxide catalysts. For
instance, considering only supported catalysts, between 1967 and 2000, 28% of all
published papers were concerned with vanadium oxide - based materials [57] . This
represents a greater fraction than for any other metal or metal oxide. One reason
for this is the wide range of applications in which vanadium oxide catalysts may
be employed, examples of which are outlined below.
5.3.1.1 Supported Vanadium Oxide Catalysts
Supported vanadium catalysts, whereby vanadium oxide is dispersed on a support
such as alumina or titania are of particular importance in, for instance, the oxida-
tive dehydrogenation of alkanes [58 – 64] . Such materials have attracted consider-
able interest in the direct dehydrogenation of butane, where a key driver is to
identify the relationship between catalytic activity and structural properties [5, 6,
65 – 68] . In the pure (solid) metal oxides the coordination of vanadium is well
defi ned. However, this is not necessarily true in the case of supported catalysts.
Vanadium may be present on the support surface as: isolated vanadium ions;
dimeric or polymeric species; one - and two - dimensional chains of vanadium ions;
Figure 5.10
1
H CRAMP spectra (360 MHz) of Al
2
O
3
· 2.05H
2
O
materials prepared following various dehydration procedures:
(a) desiccation over Drierite at ambient pressure (725 Torr)
for 72 h, (b) heating at 110 ° C (in ambient pressure) for 5 h,
(c) heating at 110 ° C (in ambient pressure) for 5 h, followed by
evacuation at 6.5 mTorr for 24 h, and (d) heating at 110 ° C
under vacuum (8 mTorr) for 24 h. Adapted from ref. [56] ;
reprinted with permission from the American Chemical Society.
amorphous or crystalline oxides such as V
2
O
5
; or as a mixed metal oxide phase
with the support, such as AlVO
4
in the case of an alumina support [69] . Examples
of these are shown in Figure 5.11 . A
51
V MAS NMR spectrum of pure V
2
O
5
is
shown in Figure 5.2 .
The complex nature of vanadium species in catalytic materials means that no
single technique is ideally suited to their study.
51
V NMR techniques can, however,
provide information on the local environment of vanadium nuclei, including the
geometry and coordination number of V
5+
species, the number of non - equivalent
Figure 5.11 Possible molecular confi gurations for supported
vanadium oxides (S represents the support): (a) isolated
vanadium oxide species; (b) dimeric vanadium oxide species;
(c) two - dimensional vanadium oxide chains; (d) V
2
O
5
crystals.
Adapted from ref. [69] ; reprinted with permission from Elsevier.
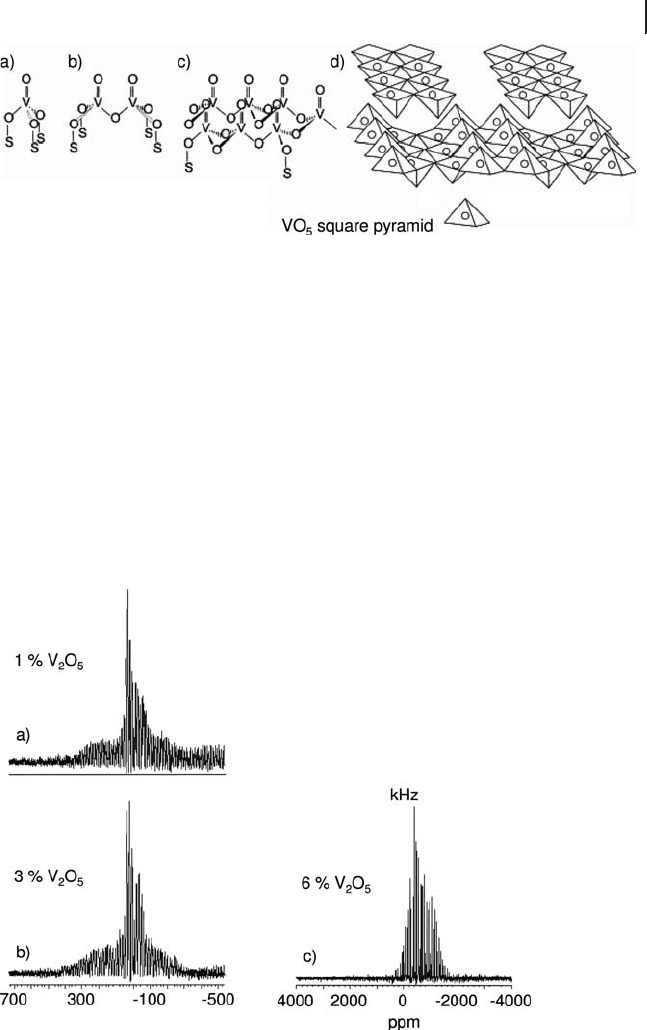
Figure 5.12
51
V MAS NMR spectra of three different VO
x
/TiO
2
catalysts. Vanadia loadings are: (a) 1 wt% V
2
O
5
(b) 3 wt% V
2
O
5
and (c) 6 wt% V
2
O
5
. Adapted from ref. [89] ; reprinted with
permission from the American Chemical Society.
5.3 Structure of Bulk Oxides 211

212 5 Solid-State NMR of Oxidation Catalysts
V
5+
sites, the nature of atoms in the fi rst coordination sphere and the distortion
of this sphere. The quadrupolar (spin = 7/2)
51
V isotope has a natural abundance
of 99.76%, and a relative NMR intensity, with respect to
1
H, of 0.38. These char-
acteristics make vanadium in the 5+ oxidation state an attractive nucleus to study
by NMR techniques.
Much of the pioneering work in this area was conducted by Mastikhin and
coworkers employing both static and MAS techniques [27, 70 – 87] . These studies
probed the development of different vanadate sites on the catalyst surface as a
function of vanadium loading. For instance, for Al
2
O
3
- supported vanadium it was
observed that at low loadings ( ∼ 1 wt% V) isolated VO
4
tetrahedra were present as
indicated by a species with a chemical shift of − 750 ppm [73] . On increasing the
catalyst loading, a second species, assigned to polymeric vanadate species in which
vanadium exists in a distorted octahedral environment ( − 350 ppm), is evident in
the spectra. At higher loadings, evidence of vanadium in a V
2
O
5
- like environment
is observed. V
2
O
5
has an isotropic chemical shift of − 612 ppm. When high loadings
are coupled with high calcination temperature the formation of AlVO
4
is observed.
The spectrum of AlVO
4
is discussed in Section 5.3.1.3 . In addition to Al
2
O
3
sup-
ports [70, 71, 73, 74, 77, 79, 86] this group have also studied vanadium supported
on SiO
2
[70, 74, 79] , SnO
2
[72, 74, 75, 79, 86] , TiO
2
[74, 79, 81, 86, 87] , ZrO
2
[74] ,
Sb
2
O
5
[75] , AlPO
4
[74, 79, 82] , MgO [79] and a number of promoted or mixed - oxide
supports [27, 74, 76, 78 – 80, 82, 85 – 87] .
Similar studies on other supported vanadium oxide catalysts have been carried
out by other workers with similar results [88 – 93] . Among the materials studied
are VO
x
/Ga
2
O
3
and VO
x
/MgAl
2
O
4
catalysts for the oxidative dehydrogenation of
propane [94, 95] and VO
x
/TiO
2
catalysts for the selective catalytic reduction of NO
x
.
Figure 5.12 shows
51
V MAS NMR spectra of three VO
x
/TiO
2
catalysts differentiated
only by their loading [89] . The changes occurring in the spectra as a function of
loading are indicative of the development of new surface vanadate species, with
domains of V
2
O
5
dominating at 6 wt% V
2
O
5
. A feature of these studies is that
crystalline V
2
O
5
is typically only observed in
51
V NMR spectra of supported catalysts
above the theoretical monolayer coverage. This is the loading at which the support
surface is entirely covered by a two - dimensional vanadate network. The value at
which this occurs varies with the nature of the support surface and the geometry
of the vanadate species present. For γ - Al
2
O
3
, TiO
2
and ZrO
2
monolayer coverage
is typically achieved at vanadium loadings of ∼ 3.5 to 7 wt% [57] .
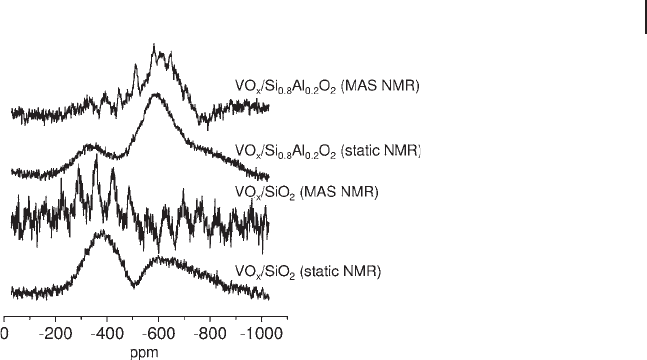
The nature of vanadium species on different supports can vary signifi cantly. For
instance, the polymeric vanadate species shown in Figure 5.11 and observed on
alumina supports, does not form on silica - supported catalysts [62, 96] . Sasikala
and coworkers have added metal ion modifi ers in an attempt to increase the dis-
persion of vanadate species in VO
x
/SiO
2
catalysts and thereby improve catalytic
activity [97] . Figure 5.13 shows that, unlike that for VO
x
/SiO
2
, the
51
V MAS NMR
spectrum of aluminum - modifi ed VO
x
/SiO
2
exhibits a side band pattern similar to
that of bulk - like V
2
O
5
superimposed over a broad peak characteristic of tetrahedral
V
5+
species. From these results it can be inferred that the dispersion of vanadia is
indeed better on the modifi ed support.
A knowledge of the nature of the vanadium species on the catalyst surface allows
links to be made between catalyst structure and catalytic action [98] . Steinfeldt and
coworkers have investigated the effect of both catalyst loading and calcination
temperature for VO
x
/Al
2
O
3
catalysts [99] . The effect of loading is similar to that
seen by other workers and discussed above. The effect of calcination temperature
is dependent upon loading. At high (up to 700 ° C) calcination temperatures AlVO
4
forms, but only at loadings of greater than 4.5 wt% V. At lower loadings the disper-
sion of more crystalline vanadium into polymeric, tetrahedrally coordinated vana-
dium species is observed. The link between speciation and activity was established
by testing the catalysts for the oxidative dehydrogenation of propane ( ODP ). Cor-
relating NMR and activity measurements it was concluded that isolated and polym-
erized tetrahedrally coordinated V
5+
species are active in propane dehydrogenation
while AlVO
4
and bulk - like V
2
O
5
have only a low catalytic activity. In a similar study
we have investigated the direct dehydrogenation of butane over VO
x
/Al
2
O
3
cata-
lysts. Calcination of a catalyst with a vanadium loading of 3.5 wt% at 973 K resulted
in reduction in the intensity of the V
2
O
5
peak in the MAS NMR spectrum of 71%.
Simultaneously a broad peak, assigned to more dispersed vanadium in tetrahedral
environments, appears (Figure 5.14 ). Similarly to Steinfeldt ’ s observation, when
the catalyst loading was increased to 8 wt% V the formation of AlVO
4
was observed
after calcination at 700 ° C. Catalytic studies on the same catalysts have related
dehydrogenation activity to the presence of polymeric vanadate species [5] .
Recent developments have allowed for an improved understanding of the local
environment of vanadium in supported catalysts. Lapina and coworkers [27] have
studied phosphorus - modifi ed VO
x
/TiO
2
catalysts by SATRAS and MQMAS tech-
niques. A key question in such materials is what infl uence the phosphorus has
on the structure of the catalyst. This is not directly answerable by conventional
Figure 5.13
51
V static and MAS NMR spectra of V
2
O
5
,
VO
x
/SiO
2
and VO
x
supported on aluminum - modifi ed SiO
2
(Si
0.8
Al
0.2
O
2
). Adapted from ref. [97] .
5.3 Structure of Bulk Oxides 213
