Iwamoto M., Kwon Y.-S., Lee T. (Eds.) Nanoscale Interface for Organic Electronics
Подождите немного. Документ загружается.

Charge Transport and Electric Conduction in Viologen SAMs 179
In addition, in the viologen derivatives that were self-assembled to
an Au substrate for 24 h under the concentration of 1 mM, the self-
assembled monolayers were verified using STM. The STM measurement
was performed at room temperature, and the scanning in the
measurement was processed by a constant current mode. The HOPG
surface was observed before scanning the sample surface in order to
verify the normal state of the probe. The reason that the HOPG was
selected in this process is that it is possible to observe the surface as an
atomic level of resolution not only for a high vacuum condition but also
in air. The normal state of the probe was verified by investigating the
surface of well-arranged graphite single crystal using a 10 nm × 10 nm
scan size. For verifying the fact that the STM is accurately operated
while it has an atomic level of resolution, this study observed the
surfaces of the highly-oriented pyrolytic graphite (HOPG), which has a
crystallized surface, and Si wafers. Because the HOPG is stable in air
where carbon atoms form certain lattices, it has been largely used to
calibrate x and y axes for measuring STM images.
2.2.2. Cyclic Voltammetry Test and Charge Transport Measurement
This study performed a cyclic voltammetry test using the QCM self-
assembled by using viologen in which the self-assembly was not
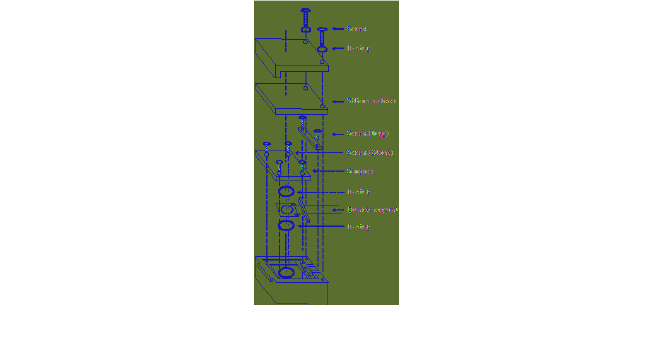
Fig. 3. Liquid cell assembly of quartz crystal for liquid analysis.
N.-S. Lee et al. 180
implemented by chemical adsorption but washing physically precipitated
molecules using a solvent.
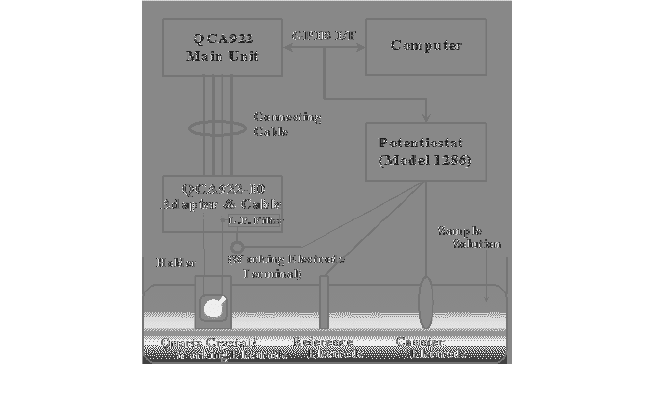
The cyclic voltammetry test was applied using the Potentiostat
(PerkinElmer, USA) and QCA 922 (Seiko EG&G, Japan), and the results
of the test was processed using a computer connected by using GPIB.
The QCM in which viologen was self-assembled to a 0.196 cm
2
Au
electrode was used as a working electrode in all tests. In addition, for
configuring a cell with three electrodes, a 1.5 × 4.5 cm
2
Pt plate and an
Ag/AgCl (MW-4130, BAS) plate were used as counter and reference
electrodes, respectively. Then, the oxidation and reduction reactions
were measured using this three electrodes cell. The test equipments and
measuring process are presented in Fig. 4.
This study performed the cyclic voltammetry test in three different
electrolytes, such as 0.1 M NaClO
4
, Na
2
SO
4
, and Na
3
PO
4
, in order to
verify the influence of the negative ion on the oxidation and reduction of
the viologen in which the voltage ranges were configured by 0 to -1 V.
All cyclic voltammetry tests were repeated by 10 times, and the
electrolyte used in these tests was 18.3 MΩ ultra-pure water, which was
applied after fully purifying it in Ar gas for 30 min.
Fig. 4. Schematic diagram of electrochemical detection sensing system used.
Charge Transport and Electric Conduction in Viologen SAMs 181
2.2.3. Analysis of the Characteristics of Electric Conduction
STM is a microscope that can observe individual atoms in a real space. It
has a simple principle in its operation in which a specific voltage level is
applied to the space between a conductive sample and a metal probe and
represents tunnel current as the space is approached up to 1 nm. The
current corresponds to the change in the distance between two materials
and is very sensitive to the change. The change is represented as an
exponential function. As the change in the distance shows 0.1 nm, the
tunnel current is to be changed by the scale of one order.
16
For producing a video of atoms, the probe is inserted to the surface of
the sample using a piezoelement while the tunnel current is maintained
as a constant level. If the change in voltages that is applied to the
piezoelement is detected and visualized, the surface structure of the
sample can be observed as an atomic scale.
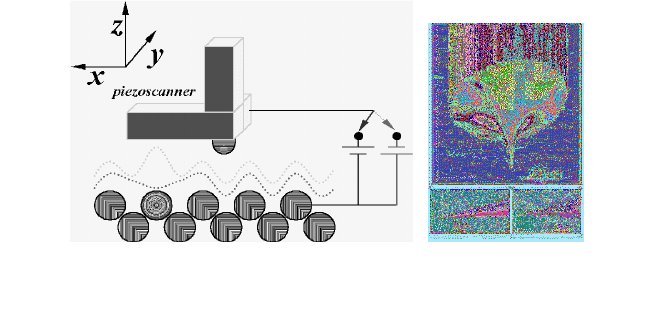
For observing the characteristics of electric conduction in self-
assembled monolayers, the surface image and voltage-current
characteristics were measured using the system by Veeco as shown in
Fig. 5.
Because the tunnel current ranged by 1 ~ 10 nA can be detected and
controlled as a higher level than 1%, the resolution for the vertical
direction is to be determined by about 0.01 nm. Also, in the resolution
within the face, the sharper end of the probe shows more higher
(a) (b)
Fig. 5. Principle of STM system and the image of STM tip.
N.-S. Lee et al. 182
resolution levels than other cases. Based on a model calculation under
the conditions that the radius of curvature and distance in the end of the
metal probe are determined by R and Z, respectively, the resolution
within the face can be determined as Eq. (1):
1/2
[0.2( )] .
R Z+ (1)
In the case of the end of the probe that has one atom, the values of
R and resolution within the face are determined by 0.1 nm and 0.3 nm,
respectively.
3. Analysis of Self-Assembled Monolayers Using STM
STM has a resolution that is able to observe atoms in air. In the case of
the distance between a conductive sample and a metal probe that is
approached by about 0.01 nm while a specific voltage is applied to this
distance, there is a quantum physical tunneling effect that shows some
currents in which electrons pass through an energy barrier by applying a
proper voltage between these two sides even though these two
conductors are separated. If the distance is changed by 0.1 nm, the tunnel
current will be varied by a scale of one order.
The obtaining of the images of atoms or molecules using STM, it is
possible to observe the surface structure of the sample up to the scale of
atom or molecule based on the image processing using the detection of
the change in tunneling currents during the injection through moving the
probe onto the surface of the sample using the piezoelement while the
tunneling current is maintained as a constant level.
This study observed the surface images of the viologen derivatives,
which were self-assembled to the surface of an Au(111) substrate, and
presented a possibility of the application of the derivatives as molecular
elements using STM.
In this study, the Au(111) substrate used as a lower electrode was
fabricated using a thermal evaporation system in which the fabrication
was performed by the thermal evaporation with 100 nm thickness Au
on the prebaked mica at 320°C for 2 h under maintaining the vacuum
of 6.5
× 10
-7
Torr. The viologen derivatives were self-assembled on the
Charge Transport and Electric Conduction in Viologen SAMs 183
fabricated Au(111) substrate at the concentration of 1 mM for 24 h. The
fabricated samples after completing the self-assembly were washed using
ethanol and stored these samples in a desiccator after drying them using
N
2
gas.
The STM measurement was performed at room temperature using
the UHV-STM (UNISOKU, USM-1200) in which a probe made by
using a Pt-Ir(80:20) alloy was used. Also, the voltages applied to the
space between the STM tip and the sample were ranged from -2.5 V to
+2.5 V. The scanning was applied by a constant current mode at the
tunneling region of ~0.20 nA, and the change in tunneling currents was
verified using STM/STS.
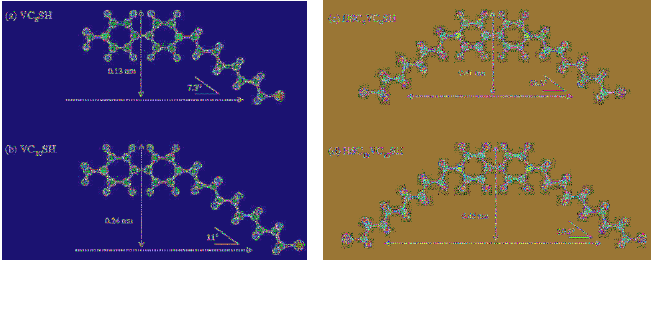
Figures 6(a)-6(d) show the data analyzed by using the ChemDraw
for viologen. In addition, an ellipsometer was used to measure the
thicknesses of viologen molecules, which were self-assembled on the
Au(111) substrate, VC
8
SH, VC
10
SH, HSC
8
VC
8
SH, and HSC
10
VC
10
SH.
In the results of the measurement using the ellipsometer, the heights of
VC
8
SH, VC
10
SH, HSC
8
VC
8
SH, and HSC
10
VC
10
SH were 0.33 nm,
0.74 nm, 0.84 nm, and 0.87 nm, respectively. Although there were some
little differences between these values and the results analyzed by using
STM, the results of these two cases showed similar figures within a
specific error range because the heights obtained by using STM were
determined as the convolution of the electric conductivity and physical
height of samples and that caused some differences in heights.
Fig. 6. Chemical Structure of viologen derivatives. (a) VC
8
SH, (b) VC
10
SH, (c)
HSC
8
VC
8
SH, and (d) HSC
10
VC
10
SH.
N.-S. Lee et al. 184
The STM is able to measure the spectra measurement of tunnel
spectrum (STS). It measures the change in tunnel currents by varying
bias voltages while feedbacks are halted under the application of set-
point bias voltages after moving the probe to the region that is to be
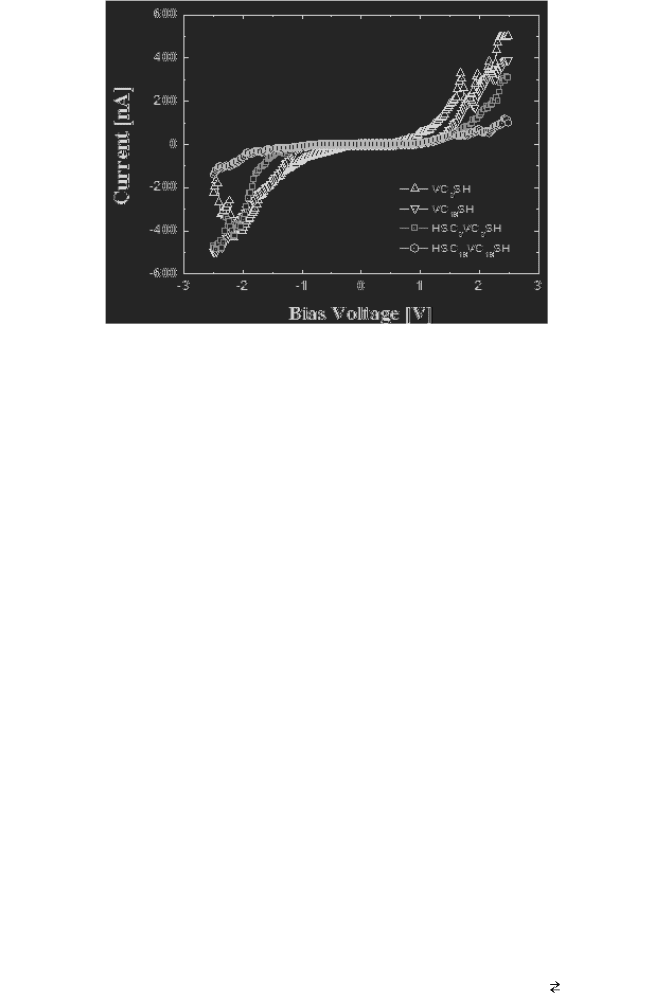
measured. Figure 7 illustrates the I-V characteristics of the viologen
monolayers measured by using STS. The I-V characteristics were
observed by fixing the STM tip at a specific point after measuring four
different viologen monolayers.
4. Cyclic Voltammetry and Charge Transport
4.1. Oxidation and Reduction According to Changes in Electrolytes
The previous results reported the relationship between the cyclic
voltammetry curve and the peak current versus injection speed in a 0.1 M
NaClO
4
solution for VC
8
SH. The peaks in oxidation and reduction were
presented at -0.45 V and -0.51 V, respectively, in which the currents in
the oxidation and reduction showed the same levels. Also, it can be
seen that the oxidation and reduction of the positive radicals, which
were caused by the one electron reduction presented by V
2+
V
+
, were
Fig. 7. I-V characteristic curves of viologen derivatives (VC
8
SH, VC
10
SH, HSC
8
VC
8
SH,
and HSC
10
VC
10
SH).
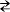
Charge Transport and Electric Conduction in Viologen SAMs 185
reversibly generated as it is expected that the peak currents of the
oxidation and reduction showed the same levels, and the relationship
between the peak current and the injection speed was determined as a
linear way.
17
Although the oxidation and reduction peaks were generated
at a certain constant voltage level regardless of the type of positive ion
due to the reaction of V
2+
V
+
in viologen monolayers, the scales of
peak currents decreased by the order of SO
4
2-
, ClO
4
-
, and PO
4
3-
. It is
considered that there are some differences in the amount of transported
charges caused by the limited molar conductivity according to the
mobility in each ion.
4.2. Characteristics of Interfacial Charge Transport Caused by the
Change in Mass
The previous results reported the change in the resonance frequencies
of QCM measured in the cyclic voltammetry test of VC
8
SH and
HSC
8
VC
8
SH. As results, the change in resonance frequencies occurred
by oxidation and reduction reactions simultaneously and that can be
presented as a change in mass using the Sauerbrey equation. According
to the reduction reaction in two stages, the change in resonance
frequencies increased by two stages, and the decrease in resonance
frequencies occurred by two stages according to the oxidation. It shows a
process that reassociates the ClO
4
-
ion, which is associated to the N
+
ion
that is an electrochemical active place of the viologen monolayers, is
separated by its reduction according to the oxidation. The entire changes
in the resonance frequencies of VC
8
SH and HSC
8
VC
8
SH were 18.1 Hz
and 8.4 Hz, respectively, and that can be converted by the change in
masses as 19.36 ng and 8.98 ng, respectively. The numbers of associated
and dissociated ions obtained from the mass of ClO
4
-
ion for VC
8
SH and
HSC
8
VC
8
SH were 2.33 × 10
13
and 1.08 × 10
13
. It can be considered that
the results were affected by the electrical double layer that formed the
confronted layers of charges, which were located at both the electrode
and solution in which the Au electrode where the viologen monolayers
were unself-assembled represented electricity at the boundary between
the metal surface and the solution, because the viologen monolayers
were not self-assembled on the surface of the Au electrode of the QCM.
18

N.-S. Lee et al. 186
In the case of the 0.1 M Na
2
SO
4
electrolyte solution, the changes in
the resonance frequencies were 19.3 Hz and 9.5 Hz and that can be
converted by the change in masses as 20.65 ng and 19.0 ng. Also, the
numbers of associated and dissociated Cl
-
ions obtained from the change
in masses were 2.49
× 10
13
and 1.22 × 10
13
, respectively. In the case of
the 0.1 M Na
3
PO
4
electrolyte solution, the changes in the resonance
frequencies were 16.0 Hz and 7.1 Hz and that can be converted by
the change in masses as 17.16 ng and 7.60 ng. Also, the numbers of
associated and dissociated Cl
-
ions obtained from the change in masses
were 2.06
× 10
13
, 9.1 × 10
12
, respectively.
5. Electric Conductive Characteristics of Viologen Derivatives
In general, the charge insertion at the interface between metal and
organic materials can be explained by the thermionic emission
(dominated in a low bias level) and Fowler-Nordheim tunneling
(dominated in a high bias level). That is, as a strong electric field is
applied to the interface between metals and conductors, the barrier is
decreased due to the Schottky effect and that shows a decrease in the
width, which is forward to the same electric field in a Fermi level. Then,
tunneling occurs at this position.
19
If the energy of the electron that
is approached to the interface within metal shows the distribution
of Fermi-Dirac, the tunnel current, I, can be obtained using Eqs. (2)
and (3):
2
exp
x
I V
V
−
=
(2)
1/ 2 3/2
8 (2 )
3
m
x
qh
π
Φ
= (3)
where m is electron effective mass, and h is Plank constant. The I-V
relationship in Eqs. (2) and (3) shows that it is possible to obtain a line,
which has a negative value as log(I/V
2
) -1/V. Figure 8 shows the
Fowler-Nordheim plot obtained from the I-V characteristics shown in
Fig. 7 for each viologen molecule, VC
8
SH, VC
10
SH, HSC
8
VC
8
SH, and
Charge Transport and Electric Conduction in Viologen SAMs 187
HSC
10
VC
10
SH. A linear relation that has a negative gradient in a high
voltage region determined by ~1 V was obtained. Then, it is verified that
the Fowler-Nordheim tunneling dominated the conduction mechanism in
a high electric field region for the viologen molecules, VC
8
SH, VC
10
SH,
HSC
8
VC
8
SH, and HSC
10
VC
10
SH.
20,21
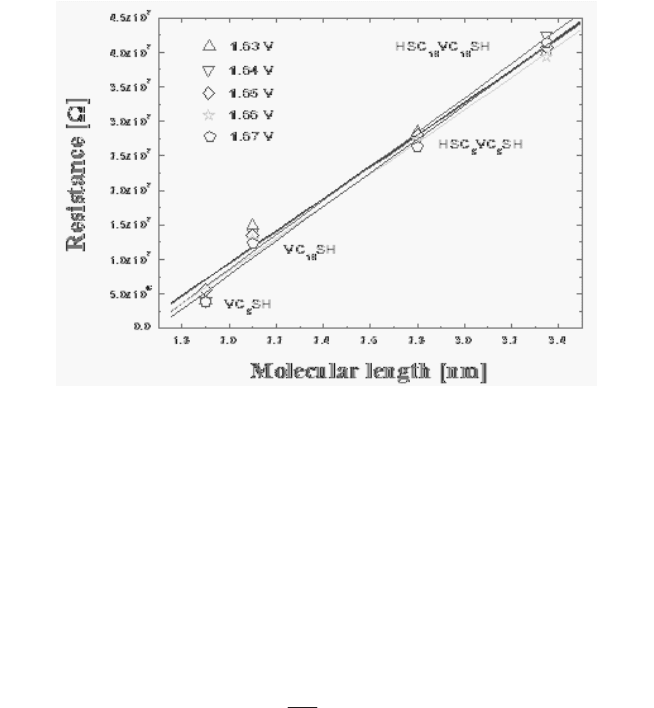
Figure 9 shows the resistance values for the lengths of molecules at
1.63 ~ 1.67 V positive bias regions in the viologen derivatives measured
in self-assembled monolayers. In the results, it was considered that the
resistance values increased according to the increase in the lengths of
molecules and that caused decreases in tunneling currents.
(a) (b)
(c) (d)
Fig. 8. Fowler-Nordheim tunneling plot. (a) VC
8
SH, (b) VC
10
SH, (c) HSC
8
VC
8
SH, and
(d) HSC
10
VC
10
SH, respectively.
N.-S. Lee et al. 188
In addition, based on the measured I-V and resistance characteristics,
the barrier height (φ) determined by damping coefficient (β) was
calculated using Eqs. (4) and (5)
22
:
0
exp( )
R R d
β
=
(4)
1/2
4
(2 ) .
m
h
π
β
= Φ (5)
Figure 10 illustrates the differential conductance (dI/dV-V) for each
molecule in VC
8
SH, VC
10
SH, HSC
8
VC
8
SH, and HSC
10
VC
10
SH. In
the characteristics of dI/dV-V, there is symmetric or asymmetric
characteristic according to the lengths of the molecules of viologen
derivatives, i.e., the scale of the conductivity in molecules, because
the dI/dV-V represents such symmetric or asymmetric characteristic
according to the lengths of viologen molecules and positions of the
STM tip.
23
Fig. 9. Resistance versus molecular length plots of the viologen derivatives.
