Iozzo Renato V. Proteoglycan Protocols
Подождите немного. Документ загружается.

340 Sandy
3. Proteins are transferred onto nitrocellulose membrane in transfer buffer (25 mM Tris base,
192 mM glycine, 20% (v/v) methanol, pH 8.4) at 100 V for 1 h at 4°C.
4. The membrane is blocked by incubation for at least 10 min at room temperature in
200 mM Tris-HCl, 1.37 M NaCl, 0.1% (v/v) Tween-20, 1% (w/v) dry nonfat milk,
pH 7.6, and then incubated for between 1 h and 16 h at 4°C in 200 mM Tris-HCl, 1.37 M
NaCl , 0.1% (v/v) Tween-20, 5% (w/v) dry nonfat milk, pH 7.6, containing the primary
antibody at a dilution that is generally about 1/3000 .
5. The membrane is washed (3 × 2 min) in 200 mM Tris-HCl, 1.37 M NaCl, 0.1% (v/v)
Tween-20, pH 7.6, and then incubated for about 1 h at room temperature in 200 mM
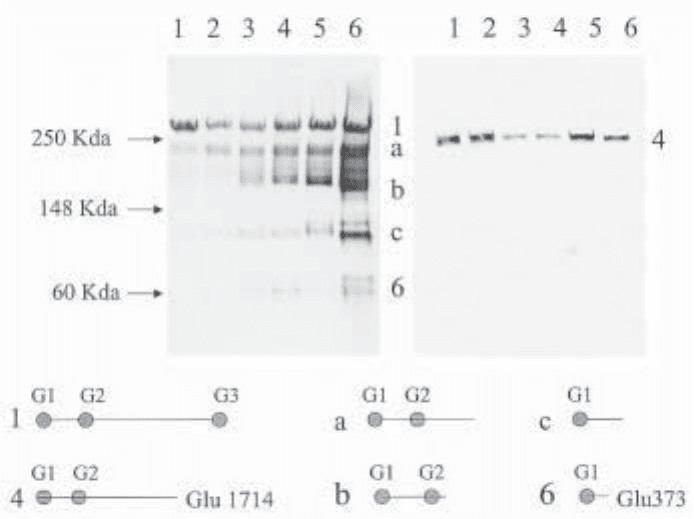
Fig. 1. Western analysis of aggrecan species isolated by guanidine extraction from normal
human articular cartilages of different ages. Lanes 1–6 in both panels show analysis of human
aggrecan from fetal, 2 mo, 1-y, 5-y, 15-y and 68-yr samples.The left panel is probed with a
general aggrecan antiserum (anti-G1-2) raised against bovine aggrecan G1 domain.The right
panel shows the same samples probed with the neo-epitope antiserum (anti-KEEE) to the
ADAMTS-generated C-terminal neoepitope at Glu1714. The structure of the six peptides (1, a–c,
4, and 6) is shown diagrammatically, although the C-terminals of some major human aggrecan
fragments (peptides a, b, and c) have yet to be identified. The figure clearly illustrates the well-
established age-dependent C-terminal truncation of aggrecan in articular cartilage. In addition
peptide 4, which migrates between peptide 1 and peptide a, is of very low relative abundance
and is not detected readily with the anti-G1 antiserum. This illustrates the extreme sensitivity
of the anti-KEEE (anti-peptide) antiserum relative to the anti-G1 antiserum for aggrecan. It
should be noted that, in general, aggrecan peptides migrate with molecular sizes that are about
twice the size predicted from the peptide size.
Proteoglycan Fragments 341
Tris-HCl, 1.37 M NaCl, 0.1% (v/v) Tween-20, 5% (w/v) dry nonfat milk (Bio-Rad),
pH 7.6, containing the secondary antibody at about 1/3000.
6. The membrane is washed (3 × 10 min) in 200 mM Tris-HCl, 1.37 M NaCl, 0.1% (v/v)
Tween-20, pH 7.6, and developed with Amersham or Pierce chemiluminescence reagents
as follows: mix 5 mL each of distilled water, reagent 1, and reagent 2 from the ECL kit.
Submerge the membrane in mix for about 1 min, protein side up. Remove the membrane
with tweezers and allow excess liquid to drip off. Wrap the membrane in clear laboratory
wrap and expose on Hyperfilm ECL.
7. Multiple exposures (for example, 5 s,30 s,1 min, 5 min) should be developed to optimize
signal intensity and separation for major and minor species.
3.7. Image Capture and Quantitation (Note 7)
1. The film images are captured on an HP ScanJet 3c/T with DeskScan II software (set to
Black and White Photo) and opened for quantitation in NIH Image and/or presentation in
Adobe Photoshop.
2. For quantitation, individual bands are selected and integrated pixel density values
obtained with preset value of pixel aspect ratio (generally about 750). Standardization
with known loadings of core protein or fragment (where available) can be used to establish
the linear detection range for the assay (23).
4. Notes
1. Treatment with Streptomyces hyaluronidase should totally eliminate viscosity due to
hyaluronan before application of samples to DE 52, and the concentration of GAG in the
applied samples should not exceed 100 µg/mL. The DE52 flow-through will contain pro-
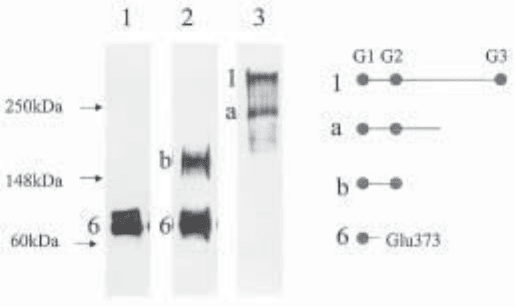
Fig. 2. Western analysis of aggrecan species present in immature pig proximal femoral epi-
physeal cartilage and separated by DE 52 chromatography. A guanidine extract of pig cartilage
was processed by DE 52 chromatography as detailed in steps 3–6 under Subheading 3.1. The
samples shown in lanes 1, 2, and 3 were recovered from the 0.1 M NaCl, 0.2 M NaCl, and 0.8 M
NaCl eluants, respectively, and the blot was probed with the general aggrecan antiserum, anti-G1-2.
The structure of the four peptides (1, a, b, and 6) is shown diagrammatically, although the
precise C-terminal sequence for peptides a and b have not been identified. Peptides b and 6
elute from DE 52 with low-salt buffer because they are not substituted with CS.
342 Sandy
tein that is not GAG-substituted whereas the washes with 0.1, 0.2, 0.8, and 1.5 M NaCl
will contain proteoglycans of different compositions. The bed volume of the DE 52 can
be increased for isolation of larger amounts (capacity is about 500 µg of GAG-substituted
proteoglycan per milliliter of DE 52) but the concentration loaded should not exceed
100 µg/mL. Also, the volume of wash solutions should be increased proportionately for
larger amounts.
2. An alternative approach to isolation of proteoglycans from all tissues described above is
extraction in guanidine-HCl as under Subheading 3.2., followed by ethanol precipitation
as follows: To a portion of the guanidine extract add 3 vol of ice-cold ethanol (sodium
acetate saturated) and let stand at –20°C for 16 h. Centrifuge at 4°C in the Eppendorf
microfuge at maximum speed, remove, and discard the ethanol. Dry the pellet and sus-
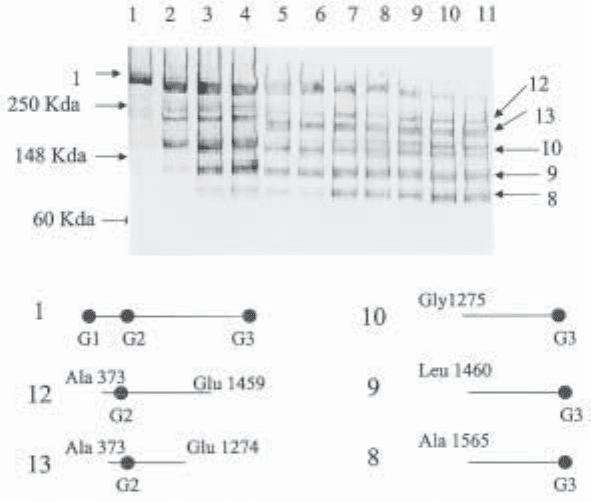
Fig. 3. Western analysis of aggrecan species generated in rat chondrosarcoma cell cultures
treated with IL-1b. Cultures of rat chondrosarcoma cells (each containing about 30 µg of GAG)
were treated with IL-1b and at intervals (0, 24, 42, 48, 66, 72, 93, 98, 110, 114, and 138 h) the
total culture was terminated by addition of buffer and digestion with chondroitinase ABC
(step 5 under Subheading 3.1.) After removal of cells by centrifugation, the supernatants
(1–11, respectively) were taken directly for Western analysis with monoclonal Ab 2-B-6 (reac-
tive with chondroitin-4-sulfate stubs left on the core protein after Chase ABC digestion). The
figure illustrates the time-dependent change in composition of immunoreactive fragments. The
structure of six of the peptides is shown diagrammatically. In each case the N-terminal and
C-terminal of the individual species were established either by N-terminal sequencing of bands
or immunoreactivity with polyclonal antisera to G1 and G3 and neo-epitope antisera to the
terminals at Ala 374 (anti-ARGSV), Glu1459 (anti-KEEE), and Glu1274 (anti-SELE).
Proteoglycan Fragments 343
pend it in chondroitinase buffer at 37°C and proceed as from step 5 under Subhead-
ing 3.1. This protocol isolates both protein and proteoglycan and is most successful
with tissues, such as cartilage, that do not contain abundant guanidine-extractable
nonproteoglycan proteins.
3. High-yield purification of proteoglycans and fragments substituted only with KS, such as
fibromodulin and lumican, will require the addition of 0.5% CHAPS as detergent.
Fibromodulin can be purified on MonoQ anion exchanger in buffers containing 6 M urea
and 0.5% CHAPS (25) and lumican from corneas on Q-Sepharose in buffers containing
8 M urea and 0.5% CHAPS (26).
4. Reference (27) gives more detail on the isolation of specific proteoglycans and fragments
from nervous tissues.
5. Clear N-terminal sequencing requires 10–100 pmol of protein, which is about 1–10 µg
for most proteoglycan core species. This is similar in sensitivity to Coomassie staining of
proteins and therefore, if the species of interest can be identified by Coomassie staining,
it should be possible to obtain an N-terminal sequence.
6. Optimal separation and detection of proteoglycan core proteins by Western analysis
requires strict control of the completeness of the deglycosylation steps (steps 5 and 6
under Subheading 3.1.). This can be monitored by measuring the loss of reactivity in the
dimethylmethylene blue assay (28). For all CS/DS- and KS-substituted proteoglycans,
greater than 85% loss of the DMMB reactivity should be achieved on deglycosylation
before Western analysis is attempted.
7. Since the chemiluminescence signal obtained with each species and antibody is highly
dependent on epitope presentation on the nitrocellulose and the reactivity of the anti-
body in use, each species requires independent standardization. Because of the unavail-
ability of standard preparations of many proteoglycan core proteins and fragments,
quantitation by Western analysis is not possible in most cases. Other methods, such as
radioimmunoassays and N-terminal quantitation by chemical means, are needed for
stricter quantitation of these products.
References
1. Iozzo R. V. (1998) Matrix proteoglycans: from molecular design to cellular function. Annu.
Rev. Biochem. 67, 609–652.
2. Tortorella, M. D., Burn, T. C., Pratta, M. A., Abbaszade, I., Hollis, J. M., Liu R., et al.
(1999). Purification and cloning of aggrecanase-1: a member of the ADAMTS family of
proteins. Science 284, 1664–1666.
3. Yamaguchi, Y. (1996) Brevican: a major proteoglycan in adult brain Perspect. Dev.
Neurobiol. 3, 307–317.
4. Carrino, D. A., Sorrell, J. M., and Caplan, A. I. (2000) Age-related changes in the
proteoglycans of human skin. Arch. Biochem. Biophys. 373, 91–92.
5. Sorrell, J. M., Carrino, D. A., Baber, M. A., and Caplan, A. I. (1999) Versican in human
fetal skin development. Anat Embryol (Berl.) 199, 45–56.
6. Kinsella, M. G., Tsoi, C. K., Jarvelainen, H. T., and Wight T. N. (1997) Selective expres-
sion and processing of biglycan during migration of bovine aortic endothelial cells. The
role of endogenous basic fibroblast growth factor. J. Biol. Chem. 272, 318–325.
7. Dhodapkar, M. V., Kelly, T., Theus, A., Athota, A. B., Barlogie, B., and Sanderson, R. D.
(1997) Elevated levels of shed syndecan-1 correlate with tumour mass and decreased
matrix metalloproteinase-9 activity in the serum of patients with multiple myeloma. Br. J.
Haematol. 99, 368–371.
344 Sandy
8. Kato, M., Wang, H., Kainulainen, V., Fitzgerald, M. L., Ledbetter, S., Ornitz, D. M.,
and Bernfield, M. (1998) Physiological degradation converts the soluble syndecan-1
ectodomain from an inhibitor to a potent activator of FGF-2. Nat. Med. 4, 691–697.
9. Philip, A., Hannah, R., and O’Connor-McCourt, M. (1999) Ectodomain cleavage and
shedding of the type III transforming growth factor-beta receptor in lung membranes
effect of temperature, ligand binding and membrane solubilization. Eur. J. Biochem.
261, 618–628.
10. Nishiyama, A., Lin, X. H., and Stallcup, W. B. (1995) Generation of truncated forms of
the NG2 proteoglycan by cell surface proteolysis. Mol. Biol. Cell. 12, 1819–1832.
11. Alliel, P. M., Perin, J. P., Jolles, P., and Bonnet, F. J. (1993) Testican, a multidomain
testicular proteoglycan resembling modulators of cell social behaviour. Eur. J. Biochem.
214, 347–350.
12. Eriksen, G. V., Carlstedt, I., Morgelin, M., Uldbjerg, N., and Malmstrom, A (1999) Isolation
and characterization of proteoglycans from human follicular fluid. Biochem. J. 340, 613–620.
13. Delpech, B., Girard, N., Olivier, A., Maingonnat, C., van Driessche, G., van Beeumen, J.,
Bertrand, P., Duval, C., Delpech, A., and Bourguignon, J. (1997) The origin of
hyaluronectin in human tumors. Int. J. Cancer 72, 942–948.
14. Perides, G., Asher, R. A., Lark, M. W., Lane, W. S., Robinson, R. A., and Bignami, A.
(1995) Glial hyaluronate-binding protein: a product of metalloproteinase digestion of
versican? Biochem. J. 312, 377–384.
15. Rauch, U., Karthikeyan, L., Maurel, P., Margolis, R. U., and Margolis, R. K. (1992) Clon-
ing and primary structure of neurocan, a developmentally regulated, aggregating chon-
droitin sulfate proteoglycan of brain. J. Biol. Chem. 267, 19,536–19,546.
16. Matsui, F., Nishizuka, M., Yasuda, Y., Aono, S., Watanabe, E. and Oohira, A. (1998)
Occurrence of a N-terminal proteolytic fragment of neurocan, not a C-terminal half, in a
perineuronal net in the adult rat cerebrum. Brain Res. 790, 45–51.
17. Yamada, H., Watanabe, K., Shimonaka, M., Yamasaki, M., and Yamaguchi, Y. (1995)
cDNA cloning and the identification of an aggrecanase-like cleavage site in rat brevican.
Biochem. Biophys. Res. Commun., 216, 957–963.
18. Sandy, J. D., Neame, P. J., Boynton, R. E., and Flannery, C. R. (1991) Catabolism of
aggrecan in cartilage explants. Identification of a major cleavage site within the inter-
globular domain. J. Biol. Chem. 266, 8683–8685.
19. Arner, E. C., Pratta, M. A., Trzaskos, J. M., Decicco, C. P., and Tortorella, M. D. (1999)
Generation and characterization of aggrecanase. A soluble, cartilage-derived aggrecan-
degrading activity. J. Biol. Chem. 274, 6594–6601.
20. Abbaszade, I., Liu, R. Q., Yang, F., Rosenfeld, S. A., Ross, O. H., Link, J. R., et al. (1999)
Cloning and characterization of ADAMTS11, an aggrecanase from the ADAMTS family.
J. Biol. Chem. 274, 23,443–23,450.
21. Sandy, J. D., Plaas, A. H., and Koob, T. J. (1995) Pathways of aggrecan processing in joint
tissues. Implications for disease mechanism and monitoring. Acta Orthop. Scand. Suppl.
266, 26–32.
22. Sandy, J. D., Flannery, C. R., Neame, P. J., and Lohmander, L. S. (1992) The structure of
aggrecan fragments in human synovial fluid. Evidence for the involvement in osteoarthri-
tis of a novel proteinase which cleaves the Glu 373-Ala 374 bond of the interglobular
domain. J. Clin. Invest. 89, 1512–1516.
23. Sandy, J. D., Gamett, D., Thompson, V., and Verscharen, C. (1998) Chondrocyte-medi-
ated catabolism of aggrecan: aggrecanase-dependent cleavage induced by interleukin-1 or
retinoic acid can be inhibited by glucosamine. Biochem. J. 335, 59–66.
Proteoglycan Fragments 345
24. Sandy, J. D. and Lark, M. W. (1998) Proteolytic degradation of normal and osteoarthritic
cartilage matrix, in Osteoarthritis (Brandt, K. D., Doherty, M., Lohmander, S. L., eds.),
Oxford University Press, pp. 84–93.
25. Plaas, A. H., Neame, P. J., Nivens, C. M., and Reiss, L. (1990) Identification of the keratan
sulfate attachment sites on bovine fibromodulin. J. Biol. Chem. 265, 20,634–20,640.
26. Midura, R. J., Hascall, V. C., MacCallum, D. K., Meyer, R. F., Thonar, E. J., Hassell, J.
R., Smith, C. F., and Klintworth, G. K. (1990) Proteoglycan biosynthesis by human
corneas from patients with types 1 and 2 macular corneal dystrophy. J Biol Chem. 265,
15,947–15,955.
27. Margolis, R. U. and Margolis, R. K. (1994) Aggrecan-versican-neurocan family
proteoglycans. Meth. Enzymol. 245, 105–126.
28. Farndale, R. W., Buttle, D. J., and Barrett, A. J. (1986) Improved quantitation and
discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue.
Biochem. Biophys. Acta 883, 173–177.

Heparan Sulfate Degradation 347
347
From:
Methods in Molecular Biology, Vol. 171: Proteoglycan Protocols
Edited by: R. V. Iozzo © Humana Press Inc., Totowa, NJ
34
Degradation of Heparan Sulfate by Nitrous Acid
H. Edward Conrad
1. Introduction
All glycosaminoglycans contain an amino sugar in every second position in their
linear chains. In most cases these amino sugars are N-acetylated, e.g., N-acetyl-
D-galactosamine (GalNAc) in chondroitin sulfates and dermatan sulfates, or N-acetyl-
D-glucosamine (GlcNAc) in heparins, heparan sulfates, and hyaluronic acids. Heparins
and heparan sulfates contain only glucosamine residues, and, in a high percentage of
these, the N-acetyl substituents are replaced by N-sulfate groups. The N-sulfated
glucosamines (GlcNSO
3
), found only in heparin-like glycosaminoglycans, are sites
that are unique in their susceptibility to facile cleavage by nitrous acid at room tem-
perature and at low pH (~1.5). Thus, when heparins or heparan sulfates are treated
with nitrous acid, they are specifically cleaved into fragments with ranges of molecu-
lar weights that depend on the distributions of the GlcNSO
3
residues in the chain.
Since N-acetylated amino sugars are not affected by the nitrous acid treatment, only
the heparin-like structures are cleaved when they are present in mixtures containing
other glycosaminoglycans.
Interestingly, glycosaminoglycans containing amino sugars that have unsubstituted
amino groups can be cleaved specifically with nitrous acid at a higher pH (~4), again
at room temperature. Under these conditions, the GlcNSO
3
residues do not react.
Since a small number of N-unsubstituted glucosamine residues (GlcN) occur in
heparan sulfates (1), cleavage occurs at these positions.
N-acetylated amino sugars do not react with nitrous acid under any conditions.
However, by hydrazinolysis, it is possible to remove N-acetyl groups from N-acety-
lated amino sugars under conditions that do not remove N-sulfate groups or otherwise
alter the glycosaminoglycan structures (2). Thus, following hydrazinolysis, nitrous
acid (at pH 4) cleaves heparins and heparan sulfates specifically at the glucosamine
residues that were originally N-acetylated, yielding fragments with ranges of molecular
weights that depend on the distributions of the GlcNAc residues in the chains. In a
348 Conrad
mixture of glycosaminoglycans, the hydrazinolysis/nitrous acid procedure yields the
variously sized fragments from the heparin-like glycosaminoglycans, but converts all
other glycosaminoglycans completely to disaccharides (since all other glycosami-
noglycans contain N-acetylated amino sugars at every second residue).
The cleavage of the glycosidic bonds of the amino sugars with nitrous acid is an
elimination reaction not a hydrolysis reaction. In all cases, the reaction yields frag-
ments in which the amino sugar at the site of cleavage is converted to a reducing
terminal anhydrosugar—in the case of D-glucosamine, 2,5-anhydro-D-mannose is
formed; in the case of D-galactosamine, 2,5-anhydro-D-talose is formed. In order
to stabilize the products, it is helpful to reduce the aldehyde groups of these
anhydrosugars to alditols using NaBH
4
. The nitrous acid, hydrazinolysis, and NaBH
4
reactions have been discussed in detail elsewhere (3).
2. Materials
2.1. Chemicals
1. Heparin.
2. Heparan sulfate.
3. 0.1 M NaOH.
4. 0.2 M Na
2
CO
3
.
5. 1 M Na
2
CO
3
.
6. Reacti-Vials (Pierce Chemical Co., Rockford, IL).
7. 0.5 M H
2
SO
4
.
8. 0.5 M Ba(NO
2
)
2
(A. D. Mackay, Darien, CT).
9. 5.5 M NaNO
2
.
10. Water bath.
11. Hydrazine, anhydrous.
12. Hydrazine SO
4
.
13. 3 M H
2
SO
4
.
2.2. Reference Standards
Heparin and heparan sulfate can be obtained from Sigma Chemical Co. Stock solu-
tions of these glycosaminoglycans contain 20 mg/mL water and are stored frozen.
2.3. pH 1.5 Nitrous Acid Reagent
Solutions of 0.5 M H
2
SO
4
and 0.5 M Ba(NO
2
)
2
(114 mg/mL) are prepared and
cooled separately to 0°C in an ice bath. A mixture containing 1 mL of each solution
(0.5 mmol of each reagent) is prepared at 0°C, and the mixture is centrifuged in a
clinical centrifuge to pellet the BaSO
4
precipitate. The supernatant is drawn off with a
Pasteur pipet, and stored on ice. This reagent should be prepared when needed and
used immediately (see Note 1).
2.4. pH 4.0 Nitrous Acid Reagent
pH 4 Nitrous acid is generated by adding 5 mL of 5.5 M NaNO
2
to 2 mL of 0.5 M
H
2
SO
4
. This reagent should be prepared when needed and used immediately. When
glycosaminoglycans are hydrolyzed in 0.5 M H
2
SO
4
, the pH 4 nitrous acid is gener-
ated in situ by adding 5 µL of the 5.5 M NaNO
2
solution to 2 µL of the hydrolysate
(see Notes 2 and 3).
Heparan Sulfate Degradation 349
2.5. Hydrazine Reagent
Hydrazine sulfate (100 mg) is dissolved in 3 mL of distilled water. Anhydrous
hydrazine (7 mL) is added to this solution to give a solution containing 1% hydrazine
sulfate in 30% aqueous hydrazine. Hydrazine is a toxic and corrosive reagent and
should be handled accordingly.
2.6. Sodium Borohydride Reagent
1. 0.5 M NaBH
4
in 0.1 M NaOH.
3. Methods
3.1. Cleavage of N-Sulfated Glycosaminoglycans with Nitrous Acid
at pH 1.5
1. Cool a 5-mL aliquot of a solution of heparin or heparan sulfate (20 mg/mL of water) to
0°C and add 20 mL of the cold pH 1.5 nitrous acid reagent.
2. Let the mixture warm to room temperature; deamination is complete within 10 min
following nitrous acid addition.
3. Adjust the pH of the deaminated product to 8.5 with 1 M Na
2
CO
3
.
4. Reduce the sample with NaBH
4
as described under Subheading 3.3.
5. The reduced sample may be separated into its individual components by gel filtration to
separate di-, tetra-, hexasaccharides, etc., and then by ion-exchange chromatography,
high-pressure liquid chromatography, or capillary electrophoresis to separate the
oligosaccharides according to charge (2,4–14). Since the nitrous acid cleavage products
are complex mixtures of oligosaccharides, multiple separation steps are required to obtain
individual components in a pure form.
3.2. Hydrazinolysis of N-Acetylated Glycosaminoglycans
and Cleavage with pH 4 Nitrous Acid
1. Place 15 µL of a solution of heparin or heparan sulfate (20 mg/mL) in a 100-µL Reacti-
Vial and dry the sample in a stream of air.
2. Redissolve the dried sample in 20 µL of hydrazine reagent.
3. Cap the vial and place it in a 100°C water bath for 4 h.
4. Cool the sample, dry it in a stream of air; and lyophilize the partially dried sample to
remove as much of the hydrazine as possible.
5. Due to residual hydrazine SO
4
and hydrazine, the pH of this solution is actually alkaline
(pH 8–10). Add 5–10 µL of 3 M H
2
SO
4
to the sample to bring the pH to 4.0, as measured
with pH paper.
6. Dry the pH-adjusted sample in a stream of air.
7. Add 20 µL of the pH 4 nitrous acid reagent to the dried sample.
8. After 15 min, the cleavage reaction is complete.
9. Adjust the pH of the reaction mixture to 8.5
10. Reduce the cleavage products with NaBH
4
as described below and separate the individual
components by gel filtration and ion-exchange chromatography (above).
3.3. Reduction of Cleavage Products with NaBH
4
1. Treat the pH 8.5 solutions of the nitrous acid-cleaved products with 10 mL of the sodium
borohydride reagent and incubate at room temperature for 15 min. Since H
2
gas is evolved
in this reaction, all NaBH
4
reductions should be carried out in a fume hood.
350 Conrad
2. Destroy excess NaBH
4
by addition of ~5 mL of 3 M H
2
SO
4
to give a slightly acidic
solution. Evaporate the sample to dryness. Redissolve the sample in water and again
evaporate the solution to dryness to remove as much H
2
as possible.
3. Redissolve the sample in water for separation of the individual components.
4. Notes
1. Once the nitrous acid is prepared, there is a series of complex reactions of the resulting
oxides of nitrogen. Within a short time, the active species of “nitrous acid” undergoes
changes that result in the loss of the capacity of the reagent to cleave the glycosidic bonds
of the amino sugars [for a discussion, see ref. 15)]. Consequently, the nitrous acid
reagents must be used within a few minutes after their preparation. This is of less concern
for the pH 4 reagent than for the pH 1.5 reagent, because the pH 4 reagent is more highly
concentrated in nitrite.
2. The pH of these reactions is important for maintaining the selectivity of the cleavage.
Although there is good selectivity for N-unsubstituted GlcN’s and N-sulfated GlcN’s at
pH 4 and 1.5, respectively, the glycosidic bonds of both types of GlcN residues are cleaved
at a pH between these values. Thus, it is desirable to let the reactions at the respective pH
proceed only for 10–15 min. Also, when samples are derived from buffered solutions, it
is necessary to check the pH with pH paper before addition of the nitrous acid reagent. In
fact, it is desirable to dialyze the glycosaminoglycan solution to remove all salts before
beginning the cleavage step. An important reason for the predialysis is that NaBH
4
is
catalytically destroyed by oxyanions, such as PO
4
3
(16).
3. Although the nitrous acid reactions cleave the bonds of β-linked amino sugars virtually
stoichiometrically, the reaction of nitrous acid with α-linked amino sugars takes two path-
ways. In both cases treatment with nitrous acid leads to the loss of the amino group and
the formation of a carbonium ion at carbon 2. In the most prominent further conversion,
the α-glycosidic bond is cleaved with the formation of the reducing terminal anhydrosugar
as described above. However, a significant proportion (~10%) of the carbonium ion un-
dergoes a reaction in which the ring is contracted to a furanose ring without glycosidic
bond cleavage. This “ring contraction reaction” is described elsewhere (3).
References
1. van den Born, J., Gunnarsson, K., Bakker, M. A. H., Kjellén, L., Kusche-Gullberg, M.,
Maccarana, M., Berden, J. H. M., and Lindahl, U. (1995) Presence of N-unsubstituted
glucosamine units in native heparan sulfate revealed by a monoclonal antibody. J. Biol.
Chem. 270, 31,303–31,309.
2. Guo, Y. and Conrad, H. E. (1989) The disaccharide composition of heparins and heparan
sulfates. Anal. Biochem. 176, 96–104.
3. Conrad, H. E. (1998) Heparin-Binding Proteins, Academic Press, San Diego, CA.
4. Ampofo, S. A., Wang, H. M., and Linhardt, R. J. (1991) Disaccharide compositional analy-
sis of heparin and heparan sulfate using capillary zone electrophoresis. Anal. Biochem. 199,
249–255.
5. Bienkowski, M. J. (1984) Structure and metabolism of heparin and heparan sulfate, Ph.D.
dissertation, University of Illinois, Urbana, IL.
6. Delaney, S. R., Leger, M., and Conrad, H. E. (1980) Quantitation of the sulfated dis-
accharides of heparin by high performance liquid chromatography. Anal. Biochem.
106, 253–261.
