Iozzo Renato V. Proteoglycan Protocols
Подождите немного. Документ загружается.

128 Plaas et al.
34. Ludwigs, U., Elgavish, A., Esko, J. D., Meezan, E.. and Roden, L. (1987) Reaction of
unsaturated uronic acid residues with mercuric salts. Biochem. J. 45, 795–804.
35. Farndale, R. W., Buttle, D. J.. and Barrett, A. J. (1986) Improved quantitation and dis-
crimination of sulfated glycosaminoglycans by use of dimethylmethylene blue. Biochim.
Biophys. Acta 883, 173–177.
36. Hamai, A., Hashimoto, N., Mochizuki, H., Kato, F., Makiguchi, Y., Horie, K., and Suzuki,
S. (1997) Two distinct chondroitin sulfate ABC lyases. J. Biol. Chem. 272, 9123–9130.
37. Huckerby, T. N., Lauder, R. M., and Nieduszynski, I. A. (1998) Structure determination for
octasaccharides derived from the carbohydrate-protein linkage region of chondroitin sulfate
chains in the proteoglycan aggrecan from bovine articular cartilage. Eur. J. Biochem. 258,
669–676 .
38. West, L. A., Roughley, P., Nelson, F. R., and Plaas, A. H. (1999) Sulfation heterogeneity in
the trisaccharide (GalNAcSbeta1,4GlcAbeta1,3GalNAcS) isolated from the non-reducing
terminal of human aggrecan chondroitin sulfate. Biochem. J. 342, 223–229
39. Aguiar, J. A. and Michelacci, Y. M. (1999) Preparation and purification of Flavobac-
terium heparinum chondroitinases AC and B by hydrophobic interaction chromatography.
Braz. J. Med. Biol. Res. 32, 45–50.

Glycan Sequencing of HS and Heparin Saccharides 129
129
From:
Methods in Molecular Biology, Vol. 171: Proteoglycan Protocols
Edited by: R. V. Iozzo © Humana Press Inc., Totowa, NJ
13
Integral Glycan Sequencing
of Heparan Sulfate and Heparin Saccharides
Jeremy E. Turnbull
1. Introduction
The functions of heparan sulfate (HS) are determined by specific saccharide motifs
within HS chains. These sequences confer selective protein-binding properties and
the ability to modulate protein bioactivities (1,2). HS chains consist of an alternating
disaccharide repeat of glucosamine (GlcN; N-acetylated or N-sulfated) and uronic
acid (glucuronic [GlcA] or iduronic acid [IdoA]). The initial biosynthetic product
containing N-acetylglucosamine (GlcNAc) and GlcA is modified by N-sulfation of
the GlcN, ester (O)-sulfation (at positions 3 and 6 on the GlcN and at position 2 on the
uronic acids) and by epimerization of GlcA to IdoA. The extent of these modifications
is incomplete, and their degree and distribution varies in HS between different cell
types. In HS chains, N- and O-sulfated sugars are predominantly clustered in
sequences of up to 8 disaccharides separated by N-acetyl-rich regions with a low
sulfate content (3).
Sequence analysis of HS saccharides has presented a daunting analytical problem
and until very recently sequence information had been obtained for only relatively
short saccharides from HS and heparin. Gel chromatography and high-performance
liquid chromatography (HPLC) methods have been employed to obtain information
on disaccharide composition (3,4). Other methods such as nuclear magnetic resonance
(NMR) spectroscopy and mass spectroscopy (5–9) have provided direct sequence
information, but are difficult for even moderately sized oligosaccharides and in
the case of NMR requires large amounts of material (micro-moles). However, the
scene has changed rapidly in the last few years with the availability of recombinant
exolytic lysosomal enzymes. These exoglycosidases and exosulphatases remove spe-
cific sulfate groups or monosaccharide residues from the nonreducing end (NRE) of
saccharides (10). These can be used in combination with PAGE separations to derive
direct information (based on band shifts) on the structures present at the nonreducing
end of GAG saccharides (11; see Fig. 1 for an example).
130 Turnbull
Integral glycan sequencing (IGS), a PAGE-based method using the exoenzymes,
was recently developed as the first strategy for rapid and direct sequencing of
heparan sulfate and heparin saccharides (11). Its introduction has been quickly fol-
lowed by a variety of similar approaches using other separation methods including
HPLC and MALDI mass spectrometry (12–14). In IGS, an oligosaccharide (previ-
ously obtained from the polysaccharide by partial chemical or enzymatic degrada-
tion and purification) is labeled at the reducing terminal with a fluorescent tag. This
is subjected to partial nitrous acid degradation to give a ladder of evenly numbered
oligosaccharides (di-, tetra-, hexa-, etc.), each bearing a fluorescent tag at its reduc-
ing-end terminus. Portions of this material are then treated with a variety of highly-
specific exolytic lysosomal enzymes (exosulfatases and exoglycosidases), which act
only at the nonreducing end of each saccharide if it is a suitable substrate. The vari-
ous digests are then separated on a high-density polyacrylamide gel and the posi-
tions of the fragments are detected by excitation of the fluorescent tag with an
ultraviolet (UV) transilluminator. Band shifts due to the different treatments permit
the sequence to be read directly from the banding pattern (see Fig. 1 for an example).
This novel strategy allows direct readout sequencing of a saccharide in a single set
of adjacent gel tracks in a manner analogous to DNA sequencing (11). IGS provides
for the first time a rapid approach for sequencing HS saccharides, and it has proved
invaluable in recent structure–function studies (15). It should be noted that this meth-
odology is designed for sequencing purified saccharides, not whole HS prepara-
tions. Clearly, a critical factor in all sequencing methods is the availability of
sufficiently pure oligosaccharide starting material. HS and heparin saccharides can
be prepared following selective scission by enzymic (or chemical) reagents and iso-
lation by methods such as affinity chromatography (4). Final purification usually
requires the use of strong anion-exchange HPLC (15; see Chapter 14).
2. Materials
1. 2-aminobenzoic acid (2-AA; Fluka Chemicals).
2. Formamide.
3. Sodium cyanoborohydride (>98% purity).
4. Distilled water.
5. Oven or heating block at 37°C.
6. Desalting column (Sephadex G-25; e.g., HiTrap
TM
desalting columns, Pharmacia).
7. Centrifugal evaporator.
8. 200 mM HCl.
9. 20 mM sodium nitrite. 1.38 mg/mL in distilled water; prepare fresh.
10. 200mM sodium acetate, pH 6.0: 27.2 g/L sodium acetate trihydrate, pH to 6.0 using
acetic acid.
11. Enzyme buffer: 0.2 M Na acetate, pH 4.5. Make 0.2 M sodium acetate (27.2g/L sodium
acetate trihydrate) and 0.2 M acetic acid (11.6 mL/L) and mix in a ratio of 45 mL to 55 mL
respectively.
12. Enzyme stock solutions (typically at concentrations of 500 mU/mL, where 1U = 1 µmol
substrate hydrolyzed per minute). Available from Glyko (Novato, CA).
13. Vortex tube mixer.
14. Microcentrifuge.
Glycan Sequencing of HS and Heparin Saccharides 131
15. Acrylamide stock solution (T50%/C5%). Caution: acrylamide is neurotoxic. Wear gloves
(and a face mask when handling powdered forms). It is convenient to use pre-mixed
acrylamide-bis, such as Sigma A-2917. Add 43 mL of distilled water to the 100 mL bottle
containing the premixed chemicals and dissolve using a small stirrer bar (~2 h). Final
volume should be ~80 mL. Store the stock solution at 4°C. Note that it is usually necessary
to warm gently to redissolve the acrylamide after storage.
16. Resolving gel buffer stock solution: 2 M Tris-HCl, pH 8.8. 242.2 g/L Tris base, pH to 8.8
with HCl.
17. Stacking gel buffer stock solution: 1 M Tris-HCl, pH 6.8. 121.1 g/L Tris base, pH to 6.8
with HCl.
18. Electrophoresis buffer: 25 mM Tris-HC1, 192 mM glycine, pH 8.3. 3 g/L Tris base, 14.4 g/L
glycine, pH to 8.3 if necessary with HCl.
19. 10% ammonium persulfate in water (made fresh or stored at –20°C in aliquots).
20. TEMED.
21. Vertical slab gel electrophoresis system (minigel or standard size).
22. DC power supply unit (to supply up to 500–1000 V and 200 mA).
23. UV tansilluminator (312-nm maximum emission wavelength).
24. Glass UV bandpass filter larger than gel size (type UG-11 or M-UG2).
25. CCD imaging camera fitted with a 450-nm (blue) band -pass filter.
3. Methods
3.1. Derivatization of Saccharides with the Fluorophore 2AA
HS and heparin saccharides can be labeled by reaction of their reducing aldehyde
functional group with a primary amino group (reductive amination). For sulfated
saccharides anthranilic acid (2-aminobenzoic acid; 2-AA; ref. 11) has been found to
be effective for the IGS methodology. 2-AA conjugates typically display an excita-
tion maxima in the range 300–320 nm, which is ideal for visualization with a com-
monly available 312 nm UV source (e.g., transilluminators used for visualizing
ethidium bromide stained DNA). Emission maxima are typically in the range 410–420
nm (bright violet fluorescence). The approach described below allows rapid labeling
and purification of tagged saccharide from free tagging reagent, gives quantitative
recoveries, and the product is free of salts that might interfere with subsequent enzymic
conditions. For saccharides in the size range hexa- to dodecasaccharides, approxi-
mately 2–3 nmol of purified starting material is the minimum required (~2–10 µg).
1. Dry down the purified saccharide (typically 2–20 nmol) in a microcentrifuge tube by
centrifugal evaporation.
2. Dissolve directly in 10–25 µL of formamide containing freshly prepared 400 mM 2-AA
(54.8 mg/mL) and 200 mM reductant (sodium cyanoborohydride; 12.6 mg/mL) and incu-
bate at 37°C for 16–24 h in a heating block or oven. (Caution: the reductant is toxic and
should be handled with care.) The volume used should be sufficient to provide a 500- to
1000-fold molar excess of 2-AA over saccharide (see Note 1).
3. Remove free 2-AA, reductant, and formamide from the labeled saccharides by gel filtra-
tion chromatography (Sephadex G-25 Superfine). Dilute the sample (maximum 250 µL
of reaction mixture) to a total of 1 mL with distilled water (see Note 2).
4. Load onto two 5 mL HiTrap
TM
desalting columns (Pharmacia) connected in series. Alter-
natively, it is possible to use self-packed columns of other dimensions.
132 Turnbull
5. Elute with distilled water at a flow rate of 1 mL/min and collect fractions of 0.5 mL.
Saccharides consisting of four or more monosaccharide units typically elute in the void
volume (approximately fractions 7–12).
6. Pool and concentrate these fractions by centrifugal evaporation or freeze-drying.
3.2. Treatment of Saccharides with Nitrous Acid
Low-pH nitrous acid cleaves only at linkages between N-sulfated glucosamine and
adjacent hexuronic acid residues (16,17; see also Chapter 34). Under controlled con-
ditions nitrous acid cleavage can be used to create a ladder of bands that correspond to
the positions of internal N-sulfated glucosamine residues in the original intact saccha-
ride (11). To achieve this a series of different reaction stop points are pooled to pro-
duce a partial digest with a range of different fragment sizes.
1. Dry down 1–2 nmol of labeled saccharide by centrifugal evaporation.
2. Redissolve in 80 µl of distilled water and chill on ice.
3. Add 10 µl of 200 mM HCl and 10 µl of 20 mM sodium nitrite (both prechilled on ice) and
incubate on ice.
4. At a series of individual time points (typically 0.5, 1, 2, and 3 h), remove an aliquot and
stop the reaction by raising the pH to approximately 5.0 by the addition of 1/5 volume of
200 mM sodium acetate buffer, pH 6.0 (see Note 3).
5. Pool the set of aliquots and either use directly for enzyme digests or desalt as described
under Subheading 3.1.
3.3. Treatment of Saccharides with Exoenzymes
The basic approach for treatment of HS/heparin samples with exoenzymes is
described below. Details of the specificities of the exoenzymes are given in Table 1.
Although these enzymes have differing optimal pH and buffer conditions, in general
it is possible to use them under the single set of conditions given here, simplifying
multiple enzyme treatments (see Note 4).
1. Dissolve the sample (typically 10–200 pmol of saccharide) in 10 µL of H
2
O in a
microcentrifuge tube.
2. Add 5 µL of exoenzyme buffer, 1 µL of 0.5 mg/mL bovine serum albumin, 2 µL of
appropriate exoenzyme (0.2–0.5 mU) and distilled water to bring the final volume to 20 µL.
3. Mix the contents well on a vortex mixer, and centrifuge briefly to ensure that the reactants
are at the tip of the tube.
4. Incubate the samples at 37°C for 16 h in a heating block or oven.
3.4. PAGE Separation of Saccharides
Polyacrylamide gel electrophoresis (PAGE) is a high-resolution technique for the sepa-
ration of HS and heparin saccharides of variable sulfate content and disposition. It provides
a level of resolution for oligosaccharides larger than tetrasaccharides that is superior to gel
filtration or anion-exchange HPLC (18,19). It is possible to obtain improved resolution
using gradient gels. However, these are more difficult to prepare and use routinely and in
most cases adequate resolution can be obtained with isocratic gels (see Note 5).
Oligosaccharide mapping by PAGE is a rapid and reproducible method for the simulta-
neous comparison of multiple samples. It thus provides a simple but powerful approach for
separating the saccharide products generated in the sequencing process.
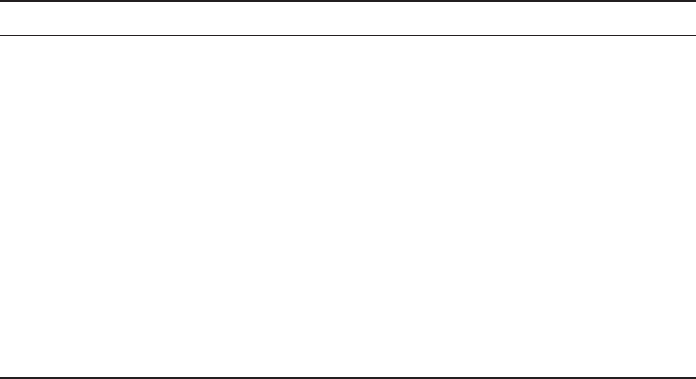
Glycan Sequencing of HS and Heparin Saccharides 133
3.4.1. Preparing the PAGE Gel
1. Assemble the gel unit (consisting of glass plates and spacers, etc.).
2. Prepare and degas the resolving gel acrylamide solution without ammonium persulfate or
TEMED. To make a 30% acrylamide gel solution for a 16 cm ×12cm × 0.75 mm gel, 16
mL are required. Mix 9.6 mL of T50%/C5% acrylamide stock with 3 mL of 2 M Tris pH
8.8 and 3.4 mL of distilled water.
3. Add 10% ammonium persulfate (30 µl) and TEMED (10 µL) to the gel solution, mix
well, and immediately pour into the gel unit.
4. Overlay the unpolymerized gel with resolving gel buffer (375 mM Tris-HCl, pH 8.8,
diluted from the 2 M stock solution) or water-saturated butanol. Polymerization
should occur within ~30–60 min. The gel can then be used immediately or stored at
4°C for 1–2 wk.
3.4.2. Electrophoresis
1. Immediately before electrophoresis, rinse the resolving gel surface with stacking gel
buffer (0.125 M Tris-HC1 buffer, pH 6.8, diluted from the 1 M stock solution).
2. Prepare and degas the stacking gel solution (for 5 ml, mix 0.5 mL of T50%/C5%
acrylamide stock with 0.6 mL of 1 M Tris pH 6.8 and 3.9 mL of distilled water).
Table 1
Exoenzymes for Sequencing Heparan Sulfate and Heparin
Enzyme
a
Substrate specificity
b
Sulfatases
Iduronate-2-sulfatase IdoA(2S)
Glucosamine-6-sulfatase GlcNAc(6S), GlcNSO
3
(6S)
Sulphamidase (glucosamine N-sulfatase) GlcNSO
3
Glucuronate-2-sulfatase GlcA(2S)
Glucosamine-3-sulphatase GlcNSO
3
(3S)
Glycosidases
Iduronidase IdoA
Glucuronidase GlcA
α-N-Acetylglucosaminidase GlcNAc
Bacterial exoenzymes
∆-4,5-Glycuronate-2-sulfatase ∆-UA(2S)
∆-4,5-Glycuronidase ∆-UA
a
Enzyme availability: Glucuronidase is widely available commercially as purified enzyme.
Recombinant iduronate-2-sulphatase, iduronidase, glucosamine-6-sulphatase, sulfamidase, and
α-N-acetylglucosaminidase are available from Glyko (Novato, CA). Glucuronate-2-sulfatase and
glucosamine-3-sulfatase have only been purified from cell and tissue sources to date. The bacterial
exoenzymes are available from Grampian Enzymes, Nisthouse, Harray, Orkney, Scotland; e-mail
grampenz@aol.com.
b
The specificities are shown as the nonreducing terminal group recognised by the enzymes. Sulfatases
remove only the sulfate group, whereas the glycosidases cleave the whole nonsulfated monosaccharide.
134 Turnbull
3. Add 10% ammonium persulfate (10 µL) and TEMED (5 µL). Immediately pour on to the
top of the resolving gel and insert the well-forming comb.
4. After polymerization (~15 min), remove the comb and rinse the wells thoroughly with
electrophoresis buffer.
5. Place the gel unit into the electrophoresis tank and fill the buffer chambers with electro-
phoresis buffer.
6. Load the oligosaccharide samples (5–20 µL depending on well capacity, containing ~10%
(v/v) glycerol or sucrose in 125 mM Tris-HCl, pH 6.8, carefully into the wells with a
microsyringe. Marker samples containing bromophenol blue and phenol red should also
be loaded into separate tracks.
7. Run the samples into the stacking gel at 150–200 V (typically, 20–30 mA) for 30–60 min,
followed by electrophoresis at 300–400 V (typically 20–30 mA and decreasing during
run) for approximately 5–8 h (for a 16 cm gel). Heat generated during the run should be
dissipated using a heat exchanger with circulating tap water, or by running the gel in a
cold room or in a refrigerator.
8. Electrophoresis should be terminated before the Phenol red marker dye is about 5 cm
from the bottom of the gel. (At this point, disaccharides should be 3–4 cm from the bot-
tom of the gel.)
Fig. 1. Principles of integral glycan sequencing and an example. (A) Fluorescence detection
of different amounts of a 2AA-tagged heparin tetrasaccharide run on a 33% minigel. (B)
Exosequencing of a 2AA-tagged heparin tetrasaccharide with lysosomal enzymes and separation
of the products on a 33% minigel (15 pmol per track). Band shifts following the exoenzyme
treatments shown reveal the structure of the nonreducing end disaccharide unit (track 1,
untreated). I2Sase, iduronate-2-sulfatase; Idase, iduronidase; G6Sase, glucosamine-6-sulfa-
tase; Nsase, sulfamidase. (C) Schematic representation of IGS of a hexasaccharide (pHNO2,
partial nitrous acid treatment). (D) Actual example of IGS performed on a purified heparin
hexasaccharide, corresponding to the scheme in (C), using the combinations of pHNO
2
and
exoenzyme treatments indicated (track 1, untreated, 25 pmol; other tracks correspond to ~200
pmol/per track of starting sample for pHNO
2
digest). The hexasaccharide (purified from bovine
lung heparin) has the putative structure IdoA(2S)-GlcNSO
3
(6S)-IdoA(2S)-GlcNSO
3
(6S)-
IdoA(2S)-AMannR(6S). Electrophoresis was performed on a 16 cm 35% gel. Copyright ©
1999 National Academy of Sciences, USA. From ref. (11).
Glycan Sequencing of HS and Heparin Saccharides 135
3.5. Imaging the Gels
Effective gel imaging requires a CCD camera that can detect faint fluorescent band-
ing patterns by capturing multiple frames. Systems commonly used for detection of
ethidium bromide stained DNA can usually be adapted with appropriate filters as
described in Note 6.
1. Place a UV filter (UG-1, UG-11, or MUG-2) onto the transilluminator, and fit a 450-nm
blue filter onto the camera lens.
2. Remove the gel carefully from the glass plates after completion of the run and place on
the UV transilluminator surface wetted with electrophoresis buffer. Also wet the upper
surface of the gel to prevent gel drying and curling.
3. Switch on transilluminator and capture image using CCD camera. Exposure times are
typically 1–5 s depending on the amount of labeled saccharide (see Note 7).
3.6. Interpreting the Data
The sequence of saccharides subjected to IGS can be read directly from the banding
pattern by interpreting the band shifts due to removal of specific sulfate or sugar
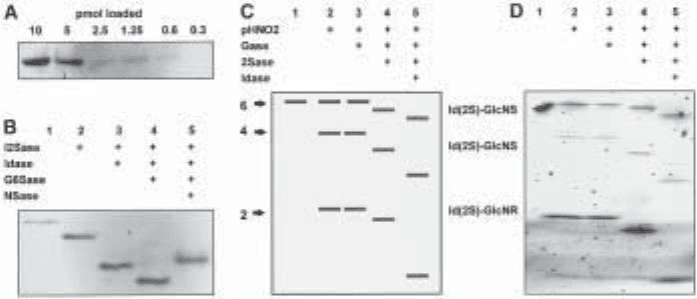
Fig. 2. IGS of a heparin hexasaccharide of known structure. Α heparin hexasaccharide with
the structure DHexA(2S)-GlcNSO
3
(6S)-IdoA-GlcNAc (6S)-GlcA-GlcNSO
3
(6S), was 2AA-
tagged and subjected to sequencing on 16cm 33% gel. (A) IGS of hexasaccharide using the
combinations of pHNO
2
and exoenzyme treatments indicated (track 1, untreated, 20 pmol;
other tracks correspond to ~90 pmol/per track of starting sample for pHNO
2
digest). NAG,
N-acetylglucosaminidase. (B) Determining the sequence of the nonreducing disaccharide unit
of the hexasaccharide using the I2Sase, G6Sase, and mercuric acetate (MA) treatments shown
(~20 pmol/track; track 1, untreated). Copyright © 1999 National Academy of Sciences, USA.
From ref. (11).
136 Turnbull
moieties. Figure 1 shows an actual example and a schematic representation. First,
bands generated by the partial nitrous acid treatment indicate the positions of N-sul-
fated glucosamine residues in the original saccharide (see Fig. 1C, track 2). Lack of a
band at a particular position indicates the presence of an N-acetylated glucosamine
residue (an example of this is shown in Fig. 2). Such saccharides can be sequenced
with the additional use of the exoenzyme N-acetylglucosaminidase, which removes
this residue and allows further sequencing of an otherwise “blocked” fragment. Fol-
lowing the nitrous acid treatment, the “ladder” of bands is then subjected to various
exoenzyme digestions. The presence of specific sulfate or sugar residues can be
deduced from the band shifts that occur (see Fig. 1C, tracks 3–5). Figure 3 shows an
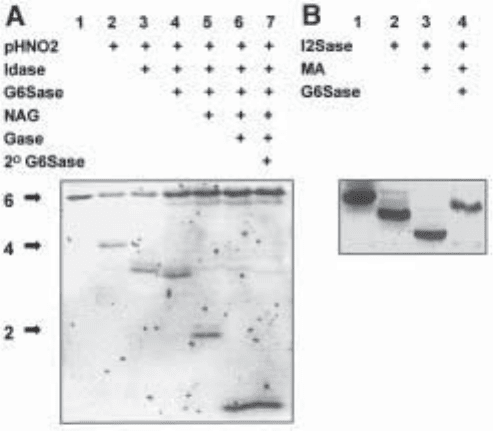
example of a decasaccharide from HS which has been purified by SAX-HPLC and
sequenced using IGS.
Usually the band shifts are downwards, due to the lower molecular mass and thus
higher mobility of the product. However, it should be noted that occasionally upward
shifts occur, probably due to subtle differences in charge/mass ratio (for examples, see
Figs. 1B, 2B and 3C). Note also that minor “ghost” bands sometimes appear after the
nitrous acid treatment. They are probably due to loss of an N-sulfate group, and nor-
mally these do not affect interpretation of the shifts in the major bands (11).
Fig. 3. Purification and IGS of a HS decasaccharide. (A) SAX-HPLC of a pool of HS
decasaccharides derived by heparitinase treatment of porcine mucosal HS. For details of this
technique, see Chapter 14. The arrowed peak was selected for sequencing. (B) IGS of the puri-
fied HS decasaccharide on a 16 cm 33% gel using the combinations of pHNO
2
and exoenzyme
treatments indicated (track 1, untreated, 20 pmol; other tracks correspond to ~400 pmol/per track
of starting sample for pHNO
2
digest). (C) Determining the sequence of the nonreducing disac-
charide unit of the HS decasaccharide using the mercuric acetate (MA) and G6Sase treatments
shown (approximately 40 pmol per track; track 1, untreated). Copyright© 1999 National Acad-
emy of Sciences, USA. From ref. (11).
Glycan Sequencing of HS and Heparin Saccharides 137
If the saccharide being sequenced was derived by bacterial lyase treatment, it will
have a ∆-4,5-unsaturated uronate residue at its nonreducing terminus. If this residue has
a 2-O-sulfate attached, this can be detected by susceptibility to I2Sase (see Fig. 2B), but
the sugar residue itself is resistant to both Idase and Gase. Its removal is required in order
to confirm whether there is a 6-O-sulfate on the adjacent non-reducing end glucosamine
(see Figs. 2B and 3C for examples). However, bacterial enzymes which specifically
remove the ∆-4,5-unsaturated uronate residues (and the 2-O-sulfate groups that may be
present on them) are now available commercially (see Table 1). Alternatively, they can
be removed chemically with mercuric acetate (20; see Figs. 2B and 3C).
In addition to the basic sequencing experiment, it is wise to confirm agreement of
the data with an independent analysis of the disaccharide composition of the saccha-
ride (see Chapter 14). It can sometimes be difficult to sequence the reducing terminal
monosaccharide, due to it being a poor substrate for the exoenzymes. In these cases it
has proved more effective to analyze the terminal 2AA-labeled disaccharide unit in
comparison to 2AA-labeled disaccharide standards (11).
4. Notes
1. Using large excesses of reagent as described, saccharides derived from HS and heparin by
bacterial lyase scission generally couple with 2-AA with efficiencies in the range 60–70%. In
contrast, saccharides derived from HS and heparin by low-pH nitrous acid scissioning (i.e.,
having an anhydromannose residue at their reducing ends) label more efficiently (~70–80%
coupling efficiency).
2. Unwanted reactants and solvent can also be removed from labeled saccharides by methods
such as dialysis, but the rapid gel filtration chromatography step described above using the
HiTrap desalting columns is convenient and usually allows good recoveries of loaded
sample, particularly for 2-AA-labeled saccharides (~80%).
3. It is useful to perform some trial incubations to test for optimal time points needed to gener-
ate a balance of all fragments in the partial nitrous acid digestion. With longer saccharides
(octasaccharides and larger) it is observed that the largest products are generated quickly
and thus a bias toward shorter incubations is required as saccharide length increases.
4. The enzyme conditions should provide for complete digestion of all susceptible residues.
This is important to the sequencing process, since incomplete digestion would create a more
complex banding pattern and would give a false indication of sequence heterogeneity. It is
useful to run parallel controls with standard saccharides to enable monitoring of reaction
conditions. When combinations of exoenzymes are required, these can be incubated simul-
taneously with the sample. If necessary, the activity of one enzyme can be destroyed before
a secondary digestion with a different enzyme by heating the sample at 100°C for 2–5 min.
5. Adequate separations, particularly over limited size ranges of saccharides, can be obtained
using single concentration gels, typically in the range 25–35% acrylamide. Improvements in
resolution can be made by using longer gel sizes. Different voltage conditions (usually in
the range 200–600 V) and running times are required for different gel formats, and should
be established by trial and error with the particular samples being analyzed. Gels up to 24 cm
in length can usually be run in 5–8 h using high voltages, whereas with longer gels it is more
convenient to use lower voltage conditions and run overnight. We have also found that
minigels can also be used effectively for separation of HS/heparin saccharides (see Fig. 1).
Note that it is also possible to run Tris-acetate gels with a Tris-MES electrophoresis buffer
(see Fig. 1; 11).
