Hugo W.B., Russel A.D.(ed). Pharmaceutical Microbiology
Подождите немного. Документ загружается.

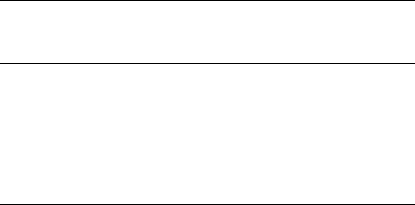
Table 20.5 Effect of membrane
disc filter diameter on filtration
volumes
fixed number of resterilizations; sintered filters may be resterilized many times. Filtration
sterilization is an aseptic process and careful monitoring of filter integrity is necessary
as well as final product sterility testing (Chapter 23).
Membrane filters, in the form of discs, can be assembled into pressure-operated
filter holders for syringe mounting and in-line use or vacuum filtration tower devices.
Filtration under pressure is generally considered most suitable since filling at high
flow rates directly into the final containers is possible without problems of foaming,
solvent evaporation or air leaks. The filtration capacity of a range of membrane filter
discs is given in Table 20.5. To increase the filtration area, and hence process volumes,
several discs can be used in parallel in multiple-plate filtration systems or, alternatively,
membrane filters can be fabricated into plain or pleated cylinders and installed in
cartridges. Membrane filters are often used in combination with a coarse-grade fibreglass
depth prefilter to improve their dirt-handling capacity.
7.2 Filtration sterilization of gases
The principal application for filtration sterilization of gases is in the provision of sterile
air to aseptic manufacturing suites, hospital isolation units and some operating theatres.
Filters employed generally consist of pleated sheets of glass microfibres separated and
supported by corrugated sheets of Kraft paper or aluminium; these are employed in
ducts, wall or ceiling panels, overhead canopies, or laminar airflow cabinets (Chapter
22). These high-efficiency particulate air (HEPA) filters can remove up to 99.997% of
particles greater than 0.3 fim in diameter and thus are acting as depth filters. In practice
their microorganism removal efficiency is rather better since the majority of bacteria
are found associated with dust particles and only the larger fungal spores are found in
the free state. Air is forced through HEPA filters by blower fans, and prefilters are used
to remove larger particles to extend the lifetime of the HEPA filter. The operational
efficiency and integrity of a HEPA filter can be monitored by pressure differential and
airflow rate measurements, and dioctylphthalate smoke particle penetration tests.
Other applications of filters include sterilization of venting or displacement air in
tissue and microbiological culture (carbon filters and hydrophobic membrane filters);
decontamination of air in mechanical ventilators (glass fibre filters); treatment of
exhausted air from microbiological safety cabinets (HEPA filters); and the clarification
and sterilization of medical gases (glass wool depth filters and hydrophobic membrane
filters).
Principles and practice of sterilization 407
Filter
(mm)
13
25
47
90
142
293
diameter
Effective filtration
area (cm
2
)
0.8
3.9
11.3
45
97
530
Typical batch
volume (litres)
<0.01
0.05-0.1
0.1-0.3
0.3-5
5-20
>20

Conclusions
A sterilization process should always be considered a compromise between achieving
good antimicrobial activity and maintaining product stability. It must, therefore, be
validated against a suitable test organism and its efficacy continually monitored during
use. Even so, a limit will exist as to the type and size of microbial challenge which can
be handled by the process without significant loss of sterility assurance. Thus,
sterilization must not be seen as a 'catch-all' or as an alternative to good manufacturing
practices but must be considered as only the final stage in a programme of
microbiological control.
Acknowledgements
The assistance of the following is gratefully acknowledged: F.J. Ley, Isotron
pic, Swindon (for discussions and permission to reproduce Fig. 20.9); M.S. Copson,
Albert Browne Ltd, Leicester (for discussions and permission to reproduce Fig.
20.6).
Appendix
Examples of typical conditions employed in the sterilization of pharmaceutical and
medical products
Sterilization method
Moist heat (autoclaving)
Dry heat
Ethylene oxide
Low-temperature steam and formaldehyde
Irradiation
Gamma-rays or accelerated
electrons
Filtration
Conditions
121°Cfor 15min
134°Cfor3min
160°Cfor 120min
170°Cfor60min
180°Cfor30min
Gas concentration:
800-1200 mgl"
1
45-63°C
30-70% relative humidity
1-4 hours sterilizing time
Gas concentration:
15-100 mgl"
1
Steam admission to 73°C
40-180min sterilizing time
depending on type of process
25kGy (2.5 Mrad) dose
=s0.22,um pore size, sterile
membrane filter
10 Further reading
Baird R.M. & Bloomfield S.F. (eds) (1996) Microbial Quality Assurance in Cosmetics, Toiletries and
Non-sterile Pharmaceuticals. London: Taylor & Francis.
British Pharmacopoeia (1993) London: HMSO.
British Standards Institution (1991) Specification for Steam Sterilizers for Aqueous Fluids in Rigid
Sealed Containers: BS 3970. London: BSI.
British Standards Institution (1990) Sterilizing and Disinfecting Equipment for Medical Products. BS
3970, Parts, 1, 3,4, 5. London: BSI.
Denyer S.P. & Baird R.M. (eds) (1990) Guide to Microbiological Control in Pharmaceuticals. Chichester:
Ellis Horwood. (Chapters 7, 8 and 9 provide additional information.)
European Pharmacopoeia, 3rd edn. (1997) Maisonneure: SA.
Gardner J.F. & Peel M.M. (1991) Introduction to Sterilisation, Disinfection and Infection Control.
Melbourne: Churchill Livingstone.
Gilbert P. & Allison D. (1996) Redefining the 'sterility' of sterile products. Eur J Parenteral Sci, 1,
19-23.
Health Technical Memorandum (1994) Sterilisers. HTM 2010. London: Department of Health.
Russell A.D. (1982) The Destruction of Bacterial Spores. London: Academic Press.
Russell A.D., Hugo W.B. & Ayliffe G.AJ. (eds) (1998) Principles and Practice of Disinfection,
Preservation and Sterilization, 3rd edn. Oxford: Blackwell Scientific Publications.
Stumbo CR. (1973) Thermobacteriology in Food Processing, 2nd edn. London: Academic Press.
United States Pharmacopeia (1995) 23rd revision. Rockville MD: US Pharmacopeial Convention.
Principles and practice of sterilization 409
Sterile pharmaceutical products
1
2
2.1
2.2
2.2.1
2.2.2
2.3
2.3.1
2.4
3
3.1
3.2
3.3
3.4
Introduction
Injections
Design philosophy
Intravenous infusions
Intravenous additives
Total parenteral nutrition (TPN)
Small-volume aqueous injections
Problems of drug stability
Small-volume oily injections
Non-injectable sterile fluids
Non-injectable water
Urological (bladder) irrigation solutions
Peritoneal dialysis and haemodialysis
solutions
Inhaler solutions
4 Ophthalmic preparations
4.1 Design philosophy
4.2 Eye-drops
4.3 Eye lotions
4.4 Eye ointments
4.5 Contact-lens solutions
4.5.1 Wetting solutions
4.5.2 Cleaning solutions
4.5.3 Soaking solutions
5 Dressings
6 Implants
7 Absorbable haemostats
7.1 Oxidized cellulose
7.2 Absorbable gelatin foam
7.3 Human fibrin foam
7.4 Calcium alginate
8 Surgical ligatures and sutures
8.1 Sterilized surgical catgut
8.2 Non-absorbable types
9 Instruments and equipment
10 Further reading
Introduction
Certain forms of drug administration and other pharmaceutical products, such as
dressings and sutures, must be sterile in order to avoid the possibility of nosocomial
(hospital-induced) infection arising from their usage. This applies particularly to
medicines which are administered parenterally but also to any material or instrument
likely to contact broken skin or internal organs. While inoculation of human pathogenic
bacteria, fungi or viruses poses the most obvious danger to the patient, it should also be
realized that microorganisms usually regarded as non-pathogenic which inadvertently
gain access to body cavities in sufficiently large numbers can also result in a severe,
often fatal, infection. Consequently, injections, ophthalmic preparations, irrigation fluids,
dialysis solutions, sutures and ligatures, implants, certain surgical dressings, as well as
instruments necessary for their use or administration, must be presented for use in a
sterile condition and in such a way that they remain sterile throughout the period of
use.
Principles of the methods employed to sterilize pharmaceutical products are
described in Chapter 20. The British Pharmacopoeia (1993) recommends autoclaving
and filtration as suitable methods applicable to aqueous liquids, and dry heat for non-
aqueous and dry solid preparations. The choice is determined largely by the ability of
the formulation and container to withstand the physical stresses applied by moist heat
21
treatment. The use of ionizing radiation or ethylene oxide is also appropriate in specific
instances. The primary considerations relate to the ability of active ingredients to
withstand the applied stress and of the container to maintain the product in a sterile
condition until use. It should be realized that all products intended to be sterilized must
be rendered and kept thoroughly clean and therefore of low microbial content prior to
sterilization. Thus, the process itself is not overtaxed and is generally well within safety
limits to guarantee sterility with minimal stress applied to the product. Because of the
clinical consequences (such as granuloma in the lung) of injecting solid particles into
the bloodstream, the numbers of particles present in injections and in other solutions
used in body cavities must be restricted. The British Pharmacopoeia (1993) set
limits for injections based on operation of a particle-detecting apparatus. The European
Pharmacopoeia (1997) describes a microscope method for particulate contamination
of injections and intravenous infusions, i.e. extraneous, mobile, undissolved particles,
other than bubbles, unintentionally present in the solutions. The test method provides
a qualitative method for identifying and detecting the characteristics of such particles
together with an indication of their possible origin. It might then be possible to
develop means of avoiding such contamination. Limits are given in the United States
Pharmacopoeia (1995) for large-volume injections, using this method.
Injections
Design philosophy
Any injectable product must be designed and produced to the highest possible
pharmaceutical standards. Not only must the product have the minimum possible levels
of particles and pyrogenic substances, but also the formulation and packaging must
maintain product integrity throughout the production processes, the shelf-life and during
administration. The formulation must be such as to ensure that the product remains
physically and chemically stable over the designated shelf-life. To achieve this,
excipients such as buffers and antioxidants may be required to ensure chemical stability,
and solubilizers, such as propylene glycol or polysorbates, may be necessary for drugs
with poor aqueous solubility to maintain the drug in solution. The packaging must
prevent water, excipient or drug loss during sterilization and storage and, in addition,
retain microbiological integrity. Axiomatically, ingress of microorganisms must be
prevented. The packaging must not contribute any significant amounts of extractable
chemicals to the contents, for example vulcanizing agents from rubber or plasticizers
from polyvinyl chloride (PVC) infusion containers.
Most injections are formulated as aqueous solutions, with Water for Injections BP
as the vehicle. The formulation of injections depends upon several factors, namely the
aqueous solubility of the active ingredient, the dose to be employed, thermal stability
of the solution, the route of injection and whether the product is to be prepared as a
multidose one (i.e. with a dose or doses removed on different occasions) or in a single-
dose form (as the term suggests, only one dose is contained in the injection). Nowadays,
most injections are prepared as single-dose forms and this is mandatory for certain
routes, e.g. spinal injections such as the intrathecal route and large-volume intravenous
infusions (section 2.2). Multidose injections may require the inclusion of a suitable
Sterile pharmaceutical products 411

preservative to prevent contamination following the removal of a dose on different
occasions. Single-dose injections are usually packed in glass ampoules containing 1, 2
or 5 ml of product; to ensure removal of the correct volume by syringe, it is necessary
to add an appropriate overage to an ampoule. Thus, a 1-ml ampoule will actually contain
1.1 ml of product, with 2.15 ml in a 2-ml ampoule. Full details are to be found in the
British Pharmacopoeia (1993).
Some types of injections must be made iso-osmotic with blood serum. This applies
particularly to large-volume intravenous infusions if at all possible; hypotonic solutions
cause lysis of red blood corpuscles and thus must not be used for this purpose.
Conversely, hypertonic solutions can be employed: these induce shrinkage, but not
lysis, of red cells which recover their shape later. Intraspinal injections must also be
isotonic, and to reduce pain at the site of injection so should intramuscular and
subcutaneous injections. Adjustment to isotonicity can be determined by the following
methods.
1 Depression of freezing-point, which depends on the number of dissolved particles
present in solution. A useful equation is given by:
W=
0
-
52
~
a
b
in which W is the percentage (w/v) of adjusting substance, a the freezing-point of
unadjusted solution and b the depression of the freezing-point of water by 1 % w/v of
adjusting substance.
2 Sodium chloride equivalent, which is produced by dividing the value for the
depression of freezing-point produced by a solution of the substance by the cor-
responding value of a solution of sodium chloride of the same strength.
For details of these and other methods, the Pharmaceutical Codex (1993) should
be consulted.
2.2 Intravenous infusions
These consist of large-volume injections or drips (500 ml or more) that are infused at
various rates (e.g. 50-500 ml h
_1
) into the venous system; they are sterilized in an
autoclave (see Chapter 20). The most commonly used infusions are isotonic sodium
chloride and glucose. These are used to maintain fluid and electrolyte balance, for
replacement of extracellular body fluids (e.g. after surgery or during prolonged periods
of fluid loss), as a supplementary energy source (1 litre of 5% w/v glucose = 714kJ)
and as a vehicle for drugs. Such solutions are prepared using freshly distilled water as
a vehicle under rigidly controlled conditions to minimize pyrogen (see Chapters 1 and
18) and particle content, and filtered to remove remaining particles immediately before
distribution to the final clean container.
Other important examples are blood and blood products, which are collected and
processed in sterile containers, and plasma substitutes, for example dextrans and
degraded gelatin. Dextrans, glucose polymers consisting essentially of (1 -^6) a-links,
are produced as a result of the biochemical activities of certain bacteria of the genus
Leuconostoc, e.g. L. mesenteroides (see Chapter 25).
A small range of intravenous infusions, e.g. those containing amino acids or
412 Chapter 21
chlormethiazole, are prepared in glass containers. These are sealed with a rubber closure
held on by an aluminium screw cap or crimp-on ring. The rubber should be non-
fragmenting, not release soluble extractives, and be sufficiently soft and pliable to
seal around the giving set needle inserted immediately prior to use. Although bottles
are sterilized by autoclaving, it is still possible for the infusion in glass bottles to become
contaminated with microorganisms before use. For instance, during the final part of
the autoclave process, bottles may be spray-cooled with water to hasten the cooling
process and therefore reduce the total autoclaving time. However, due to the poor fit
between bottle lip and rubber plug (a skirted insert type is used) it is possible for the
spray-cooling water to spread by capillary movement between bottle thread and screw
cap and even enter the bottle contents. This process is encouraged if the bottle contains
a vacuum as a consequence of rubber seal failure during heating-up. It should also be
remembered that autoclaving leads to considerable heat and pressure stresses on the
container. Failure may result from any imperfection in the bottle or plug. Microbes
may also gain access to the contents of bottles during storage if hair-line cracks (a
result of bad handling and rough treatment) are present, through which fluid may seep
outwards and microorganisms inwards to contaminate the fluid. Finally, contamination
may occur during use due to poor aseptic techniques when setting up the infusion, via
an ineffective air inlet (allowing replacement of infused fluid with air) or when changing
the giving set or bottle.
Most infusions are now packed in plastic containers. The plastic material should be
pliable, thermoresistant, transparent and non-toxic. Plasticized PVC and polyethylene
are commonly used. The former is transparent and very pliable, allowing the pack to
collapse as the contents are withdrawn (consequently no air inlet is required). These
packs are also amenable to the inclusion of ports into the bag, allowing greater safety
during use. Such ports can be protected by sterile overseals. Two problems arise: (i) the
possibility of toxic extractives, e.g. diethyl phthalate, from the plastic entering the
fluid if poor quality PVC is used; and (ii) moisture permeability leading to loss of
water if the packs are not protected by a water-impermeable outer wrap. Bags of high-
quality polyethylene are readily moulded (although separate ports cannot be included),
translucent and free from potential toxic extractives. As stated, these packs normally
collapse readily during infusion. An important advantage of all plastic packs is that the
containers are hermetically sealed prior to autoclaving and, therefore, spray-cooling
water cannot enter the pack unless there is seal failure, an easily detected occurrence.
However, the autoclaving of plastic bags is more complex than that of bottled fluids
because a steam-air mixture is necessary to prevent bursting of the bags when heated
(air-ballasting); adequate mixing of the steam and air is therefore required to prevent
layering of gases inside the chamber.
2.2.1 Intravenous additives
A common practice in hospitals is to add drugs to infusions immediately prior to, or
during, administration. The most common additives are potassium chloride, lignocaine,
heparin, certain vitamins and antibiotics.
Potentially, this can be a hazardous practice. For instance, the drug may precipitate
in the infusion fluid because of the pH (e.g. amphotericin) or the presence of calcium
Sterile pharmaceutical products 413
salts (e.g. thiopentone). The drug may degrade rapidly (e.g. ampicillin in 5% w/v
glucose). Multiple additions may lead to precipitation of one or both of the drugs or to
accelerated degradation. Finally, drug loss may occur because of absorption by the
container. For instance, insulin is absorbed by glass or PVC, glyceryl trinitrate and
diazepam are absorbed by PVC. Apart from these problems, if the addition is not
carried out under strict aseptic conditions the fluid can become contaminated with
microorganisms during the procedure. Thus, any addition should be made in a laminar-
flow work station or isolator, preferably in the pharmacy, and the fluid administered
within 24 hours, unless prepared under strict aseptic conditions.
Another approach to the problem of providing an intravenous drug additive service
is to add the drug to a small-volume (50-100 ml) infusion in a collapsible plastic
container and store the preparation at -20°C in a freezer. The infusion can be removed
when required and thawed rapidly by microwave. Many antibiotics are stable for several
months when stored in minibags at -20°C and are unaffected by the thawing process in
a suitable microwave oven. Other antibiotics, e.g. ampicillin, are degraded when frozen.
2.2.2 Total parenteral nutrition (TPN)
Total parenteral nutrition is the use of concentrated mixtures of amino acids, vitamins,
inorganic salts and an energy source (carbohydrate or fat emulsion, e.g. soyabean oil
with lecithin as emulsifying agent) for the long-term feeding of patients who are
unconscious or unable to take food. Many hospital pharmacies operate a TPN service.
All or most of the ingredients to feed a patient for 1 day are combined in one large (3-
litre capacity) collapsible plastic bag. The contents are infused over a 12-24 hour period.
Transfer of amino acid, glucose and electrolyte infusions, and the addition of vitamins
and trace elements, must be carried out with great care under aseptic conditions to
avoid microbial contamination. These solutions often provide good growth conditions
for bacteria and moulds. Fats are administered as oil-in-water emulsions, comprising
small droplets of a suitable vegetable oil (e.g. soyabean) emulsified with egg lecithin
and sterilized by autoclaving. In many cases, the fat emulsion may be added to the 3-
litre bag.
2.3 Small-volume aqueous injections
This category comprises single-dose injections, usually of 1-2 ml but as high as 50 ml,
dispensed in borosilicate glass ampoules, plastic (polyethylene or polypropylene)
ampoules or, rarely, multidose glass vials of 5-15 ml capacity stoppered with a rubber
closure through which a hypodermic needle can be inserted, e.g. insulins, vaccines.
The closure is designed to reseal after withdrawal of the needle. It is unwise to include
too many doses in a multidose container because of the risk of microbial contamination
during repeated use. Bactericides must be added to injections in multidose containers
to prevent contamination during withdrawal of successive doses, except as detailed
below. Bactericides may not be used in injections in which the total volume to be
injected at one time exceeds 15 ml. This may occur if the solubility of a drug is such
that a therapeutic dose is only soluble in this order of volume (e.g. Bemegride Injection).
There is also an absolute prohibition on the inclusion of bactericides in injections of
414 Chapter 21
the following categories: intra-arterial, intracardiac, intrathecal or subarachnoid,
intracisternal and peridural.
Small-volume injections may be sterilized by the following methods.
1 Heating in an autoclave for injections packed in glass ampoules.
2 Filtration followed by aseptic sealing (plastic containers). Since the product is not
sterilized in its final container, a bactericide may be included to reduce the risks of
contamination.
2.3.1 Problems of drug stability
1 Thermostability. The choice of sterilization method depends on the thermostability
of the active ingredient, autoclaving being applied only to drugs that are heat stable in
aqueous solution.
2 Chemical stability. Some medicaments undergo chemical change in aqueous
solutions. If the change is due to oxidation, a reducing agent such as sodium
metabisulphite is included (e.g. Adrenaline Injection BP).
Aqueous solutions of some drugs are so unstable that chemical stabilization is
impossible. In this case the drug itself, not its aqueous solution, is sterilized by dry heat
(160°C for 2 hours or its equivalent at higher temperatures) in its final container and
dissolved immediately before use by the addition of sterile water (Water for Injections
BP). For drugs which are both thermolabile and unstable in aqueous solution, a sterile
solution of the drug is freeze-dried in the final container and is reconstituted as above
just before use (e.g. many antibiotics, Hyaluronidase BP).
Details of time-temperature regimens as dictated by injection volume and heat
transfer to the whole of the product (section 2.2) and of possible interactions between
active ingredients and containers must be considered (see also Chapter 20).
2.4 Small-volume oily injections
Certain small-volume injections are available where the drug is dissolved in a viscous
oil because it is insoluble in water; non-aqueous solvent must be used. In addition,
drags in non-aqueous solvents provide a depot effect, for example for hormonal
compounds. The intramuscular route of injection must be used. The vehicle may be a
metabolizable fixed oil such as arachis or sesame oil (but not a mineral oil) or an ester
such as ethyl oleate which is also metabolizable. The latter is less viscous and therefore
easier to administer but the depot effect is of shorter duration. The drug is normally
dissolved in the oil, filtered under pressure and distributed into ampoules. After sealing,
the ampoules are sterilized by dry heat, for example, at 160°C for 2 hours. A bactericide
is probably ineffective in such a medium and therefore offers very little protection
against contamination in a multidose oily injection.
3 Non-injectable sterile fluids
There are many other types of solution required in a sterile form for use particularly in
hospitals.
Sterile pharmaceutical products 415
3.1 Non-injectable water
This is sterile water, not necessarily of injectable water standards, which is used widely
during surgical procedures for wound irrigation, moistening of tissues, washing of
surgeons' gloves and instruments during use and, when wanned, as a haemostat. Isotonic
saline may also be used. Topical water (as it is often called) is prepared in 500-ml and
1-litre polyethylene or polypropylene containers with a wide neck, to allow for ease
for pouring, and tear-off cap. Hospitals in the UK probably use larger quantities of
topical fluids than of intravenous infusions.
3.2 Urological (bladder) irrigation solutions
These are used for the rinsing of the urinary tract to aid tissue integrity and cleanliness
during or after surgery. Either water or glycine solution is used, the latter eliminating
the risk of intravascular haemolysis when electrosurgical instruments are used. These
are sterile solutions produced in collapsible or semi-rigid plastic containers of up to 3-
litre capacity.
3.3 Peritoneal dialysis and haemodialysis solutions
Peritoneal dialysis solutions are admitted into the peritoneal cavity as a means of
removing accumulated waste or toxic products following renal failure or poisoning.
They contain electrolytes and glucose (1.4-7% w/v) to provide a solution equivalent to
potassium-free extracellular fluid. Lactate or acetate is added as a source of bicarbonate
ions. Slightly hypertonic solutions are usually employed to avoid increasing the water
content of the intravascular compartment. A more hypertonic solution, containing a
higher glucose concentration, is used to achieve a more rapid removal of water. In fact,
the peritoneal cavity behaves as if it were separated from the body organs by a semi-
permeable membrane. Warm peritoneal solution (up to 5 litres) is perfused into the
cavity for 30-90 minutes and then drained out completely. This procedure can then be
repeated as often as required. Since the procedure requires large volumes, these fluids
are commonly packed in 2.5-litre containers. It is not uncommon to add drugs (for
instance potassium chloride or heparin) to the fluid prior to use.
Haemodialysis is the process of circulating the patient's blood through a machine
via tubing composed of a semi-permeable material such that waste products permeate
into the dialysing fluid and the blood then returns to the patient. Haemodialysis solutions
need not be sterile but must be free from heavy bacterial contamination.
3.4 Inhaler solutions
In cases of severe acute asthmatic attacks, bronchodilators and steroids for direct delivery
to the lungs may be needed in large doses. This is achieved by direct inhalation via a
nebulizer device; this converts a liquid into a mist or fine spray. The drug is diluted in
small volumes of Water for Injections BP before loading into the reservoir of the machine.
This vehicle must be sterile and preservative-free and is therefore prepared as a terminally
sterilized unit dose in polyethylene nebules.
416 Chapter 21
