Hugo W.B., Russel A.D.(ed). Pharmaceutical Microbiology
Подождите немного. Документ загружается.

to mix the air and steam. Air ballasting can also be employed to prevent bottle breakage.
2 Heating-up and exposure. When the sterilizer reaches its operating temperature
and pressure the sterilization stage begins. The duration of exposure may include a
heating-up time in addition to the holding time and this will normally be established
using thermocouples in dummy articles.
3 Drying or cooling. Dressings packs and other porous loads may become dampened
during the sterilization process and must be dried before removal from the chamber.
This is achieved by steam exhaust and application of a vacuum, often assisted by heat
from the steam-filled jacket if fitted. After drying, atmospheric pressure within the
chamber is restored by admission of sterile filtered air.
For bottled fluids the final stage of the sterilization process is cooling, and this needs to
be achieved as rapidly as possible to minimize thermal degradation of the product and
to reduce processing time. In modern sterilizers, this is achieved by circulating water
in the jacket which surrounds the chamber or by spray-cooling with retained condensate
delivered to the surface of the load by nozzles fitted into the roof of the sterilizer
chamber. This is often accompanied by the introduction of filtered, compressed air to
minimize container breakage due to high internal pressures (air ballasting). Containers
must not be removed from the sterilizer until the internal pressure has dropped to a safe
level, usually indicated by a temperature of less than 80°C. Occasionally, spray-cooling
water may be a source of bacterial contamination and its microbiological quality must
be carefully monitored.
4.3 Dry heat sterilization
The lethal effects of dry heat on microorganisms are due largely to oxidative processes
which are less effective than the hydrolytic damage which results from exposure to
steam. Thus, dry heat sterilization usually employs higher temperatures in the range
160-180°C and requires exposure times of up to 2 hours depending upon the temperature
employed (section 10).
Again, bacterial spores are much more resistant than vegetative cells, and their
recorded resistance varies markedly depending upon their degree of dryness. In many
early studies on dry heat resistance of spores their water content was not adequately
controlled, so conflicting data arose regarding the exposure conditions necessary to
achieve effective sterilization. This was partly responsible for variations in recommended
exposure temperatures and times in different pharmacopoeias.
Its application is generally restricted to glassware and metal surgical instruments
(where its good penetrability and non-corrosive nature are of benefit), non-aqueous
thermostable liquids and thermostable powders (see Chapter 21). In practice, the range
of materials which are actually subjected to dry heat sterilization is quite limited, and
consists largely of items used in hospitals. The major industrial application is in the
sterilization of glass bottles which are to be filled aseptically, and here the attraction of
the process is that it not only achieves an adequate sterility assurance level, but that it
also destroys bacterial endotoxins (products of Gram-negative bacteria, also known as
pyrogens, that cause fever when injected into the body). These are difficult to eliminate
by other means. For the purposes of depyrogenation of glass, temperatures of
approximately 250°C are used.
Principles and practice of sterilization 397
The F-value concept which was developed for steam sterilization processes has an
equivalent in dry heat sterilization although its application has been limited. The F
H
designation describes the lethality of a dry heat process in terms of the equivalent
number of minutes exposure at 170°C, and in this case a z value of 20°C has been
found empirically to be appropriate for calculation purposes; this contrast with the
value of 10°C which is typically employed to describe moist heat resistance.
4.3.1 Sterilizer design
Dry heat sterilization is usually carried out in a hot air oven which comprises an insulated
polished stainless steel chamber, with a usual capacity of up to 250 litres, surrounded
by an outer case containing electric heaters located in positions to prevent cool spots
developing inside the chamber. A fan is fitted to the rear of the oven to provide circulating
air, thus ensuring more rapid equilibration of temperature. Shelves within the chamber
are perforated to allow good air flow. Thermocouples can be used to monitor the
temperature of both the oven air and articles contained within. A fixed temperature
sensor connected to a chart recorder provides a permanent record of the sterilization
cycle. Appropriate door-locking controls should be incorporated to prevent interruption
of a sterilization cycle once begun.
Recent sterilizer developments have led to the use of dry-heat sterilizing
tunnels where heat transfer is achieved by infra-red irradiation or by forced convection
in filtered laminar airflow tunnels. Items to be sterilized are placed on a conveyer belt
and pass through a high-temperature zone (250 - 300 + °C) over a period of several
minutes.
4.3.2 Sterilizer operation
Articles to be sterilized must be wrapped or enclosed in containers of sufficient strength
and integrity to provide good post-sterilization protection against contamination. Suitable
materials are paper, cardboard tubes or aluminium containers. Container shape and
design must be such that heat penetration is encouraged in order to shorten the heating-
up stage; this can be achieved by using narrow containers with dull non-reflecting
surfaces. In a hot-air oven, heat is delivered to articles principally by radiation and
convection; thus, they must be carefully arranged within the chamber to avoid obscuring
centrally placed articles from wall radiation or impending air flow. The temperature
variation within the chamber should not exceed ±5°C of the recorded temperature.
Heating-up times, which may be as long as 4 hours for articles with poor heat-conducting
properties, can be reduced by preheating the oven before loading. Following sterilization,
the chamber temperature is usually allowed to fall to around 40°C before removal of
sterilized articles; this can be accelerated by the use of forced cooling with filtered
air.
5 Gaseous sterilization
The chemically reactive gases ethylene oxide (CH
2
)
2
0, and formaldehyde (methanal,
H.CHO) possess broad-spectrum biocidal activity, and have found application in the
398 Chapter 20
sterilization of re-usable surgical instruments, certain medical, diagnostic and electrical
equipment, and the surface sterilization of powders. Sterilization processes using
ethylene oxide sterilization are far more commonly used on an international basis than
those employing formaldehyde.
Ethylene oxide treatment can also be considered as an alternative to radiation
sterilization in the commercial production of disposable medical devices (Chapter 21).
These techniques do not, however, offer the same degree of sterility assurance as heat
methods and are generally reserved for temperature-sensitive items.
The mechanism of antimicrobial action of the two gases is assumed to be through
alkylation of sulphydryl, amino, hydroxyl and carboxyl groups on proteins and imino
groups of nucleic acids. At the concentrations employed in sterilization protocols, type
A survivor curves (section 2.1, Fig. 20.1) are produced, the lethality of these gases
increasing in a non-uniform manner with increasing concentration, exposure temperature
and humidity. For this reason, sterilization protocols have generally been established
by an empirical approach using a standard product load containing suitable biological
indicator test strips (Chapter 23). Concentration ranges (given as weight of gas per unit
chamber volume) are usually in the order of 800-1200mgl
_1
for ethylene oxide and
15-100 mg l
-1
for formaldehyde, with operating temperatures in the region of 45-63°C
and 70-75°C, respectively. Even at the higher concentrations and temperatures, the
sterilization processes are lengthy and therefore unsuitable for the resterilization of
high-turnover articles. Further delays occur because of the need to remove toxic residues
of the gases before release of the items for use. In addition, because recovery of survivors
in sterility tests is more protracted with gaseous sterilization methods than with other
processes, an extended quarantine period may also be required.
As alkylating agents, both gases are potentially mutagenic and carcinogenic (as is
the ethylene chlorohydrin which results from ethylene oxide reaction with chlorine),
they also produce symptoms of acute toxicity including irritation of the skin, conjunctiva
and nasal mucosa; consequently, strict control of their atmospheric concentrations is
necessary and safe working protocols are required to protect personnel. Formaldehyde
can normally be detected by smell at concentrations lower than those permitted in the
atmosphere, whereas this is not true for ethylene oxide. Table 20.3 summarizes the
comparative advantages afforded by ethylene oxide and low-temperature steam
formaldehyde (LTSF) processes.
5.1 Ethylene oxide
Ethylene oxide gas is highly explosive in mixtures of >3.6% v/v in air; in order to
reduce this explosion hazard it is usually supplied for sterilization purposes as a 10%
mix with carbon dioxide, or as an 8.6% mixture with HFC 124 (2 chloro-1,1,1,2
tetrafluoroethane) which has replaced fluorinated hydrocarbons (freons). Alternatively,
pure ethylene oxide gas can be used at below atmospheric pressure in sterilizer chambers
from which all air has been removed.
The efficacy of ethylene oxide treatment depends upon achieving a suitable
concentration in each article and this is assisted greatly by the good penetrating powers
of the gas, which diffuses readily into many packaging materials including rubber,
plastics, fabric and paper. This is not without its drawbacks, however, since the level of
Principles and practice of sterilization 399

ethylene oxide in a sterilizer will decrease due to absorption during the process and the
treated articles must undergo a desorption stage to remove toxic residues. Desorption
can be allowed to occur naturally on open shelves, in which case complete desorption
may take many days, e.g. for materials like PVC, or it may be encouraged by special
forced aeration cabinets where flowing, heated air assists gas removal, reducing
desorption times to between 2 and 24 hours.
Organisms are more resistant to ethylene oxide treatment in a dried state, as are
those protected from the gas by inclusion in crystalline or dried organic deposits. Thus,
a further condition to be satisfied in ethylene oxide sterilization is attainment of a
minimum level of moisture in the immediate product environment. This requires a
sterilizer humidity of 30-70% and frequently a preconditioning of the load at relative
humidities of greater than 50%.
5.1.1 Sterilizer design and operation
An ethylene oxide sterilizer consists of a leak-proof and explosion-proof steel chamber,
normally of 100-300 litre capacity, which can be surrounded by a hot-water jacket
to provide a uniform chamber temperature. Successful operation of the sterilizer
requires removal of air from the chamber by evacuation, humidification and con-
ditioning of the load by passage of subatmospheric pressure steam followed by a
further evacuation period and the admission of preheated vaporized ethylene oxide
from external pressurized canisters or single-charge cartridges. Forced gas circulation
is often employed to minimize variations in conditions throughout the sterilizer chamber.
Packaging materials must be air-, steam- and gas-permeable to permit suitable conditions
for sterilization to be achieved within individual articles in the load. Absorption of
ethylene oxide by the load is compensated for by the introduction of excess gas at the
beginning or by the addition of more gas as the pressure drops during the sterilization
400 Chapter 20
Table 20.3 Relative merits of ethylene
processes
Advantages of ethylene
oxide over LTSF
Wider international
regulatory acceptance
Better gas penetration into
plastics and rubber
Relatively slow to form solid
polymers (with the potential to
block pipes etc.)
With long exposure times it
is possible to sterilize at
ambient temperatures
Very low incidence of
product deterioration
oxide and low-temperature steam formaldehyde (LTSF)
Advantage of LTSF
over ethylene oxide
Less hazardous because formaldehyde
is not flammable and is more readily
detected by smell
Cycle times may be shorter
The gas is obtained readily from
aqueous solution (formalin) which is a
more convenient source than gas in
cylinders
process. The ^ame may also be true for moisture absorption, which is compensated for
by supplementary addition of water to maintain appropriate relative humidity.
After treatment, the gases are evacuated either directly to the outside atmosphere
or through a special exhaust system. Filtered, sterile air is then admitted either for a
repeat of the vacuum/air cycle or for air purging until the chamber is opened. In this
way, safe removal of the ethylene oxide is achieved reducing the toxic hazard to the
operator. Sterilized articles are removed directly from the chamber and arranged for
desorption.
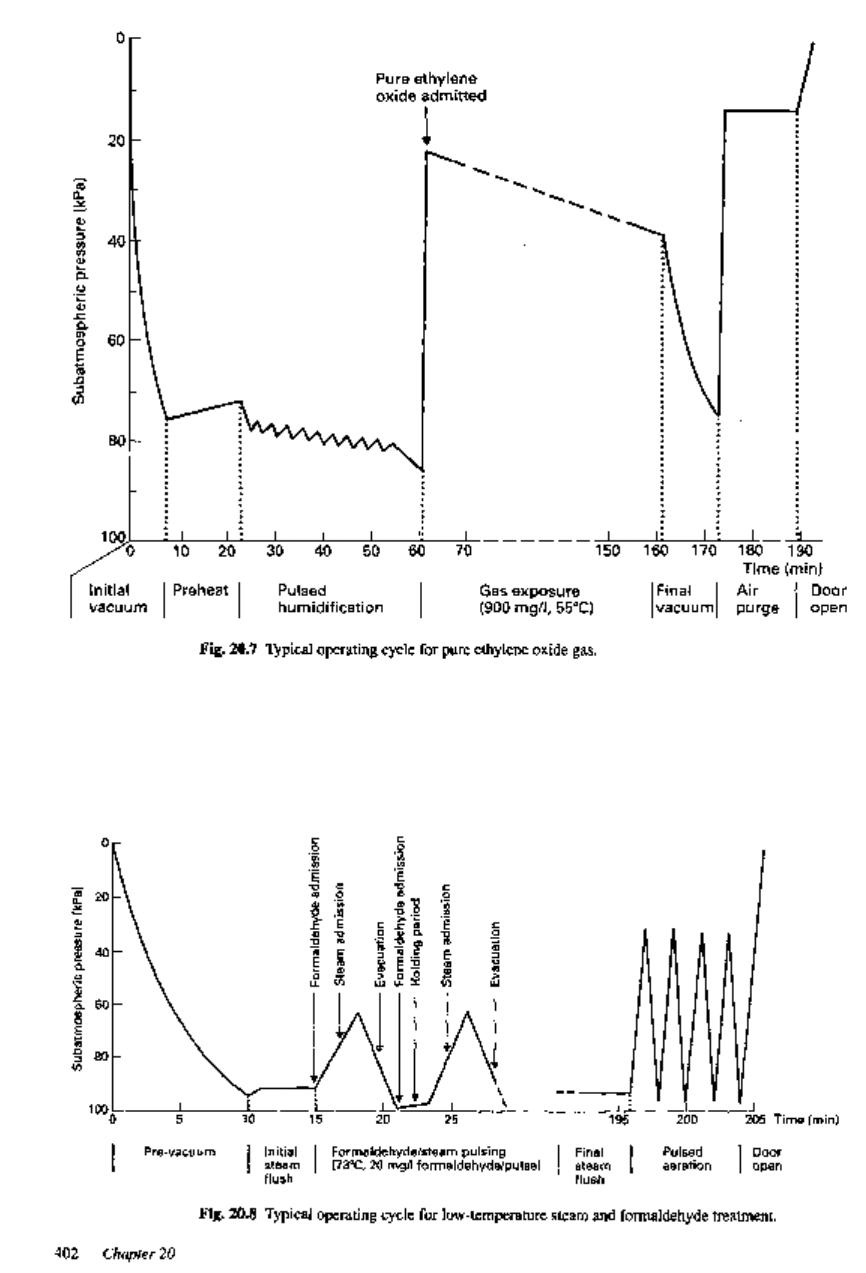
The operation of an ethylene oxide sterilizer should be monitored and controlled
automatically. A typical operating cycle for pure ethylene oxide gas is given in Fig.
20.7, and general conditions are summarized in section 10.
5.2 Formaldehyde
Formaldehyde gas for use in sterilization is produced by heating formalin (37% w/v
aqueous solution of formaldehyde) to a temperature of 70-75°C with steam, leading to
the process known as LTSF. Formaldehyde has a similar toxicity to ethylene oxide and
although absorption to materials appears to be lower similar desorption routines are
recommended. A major disadvantage of formaldehyde is low penetrating power and
this limits the packaging materials that can be employed to principally paper and cotton
fabric.
5.2.1 Sterilizer design and operation
An LTSF sterilizer is designed to operate with subatmospheric pressure steam. Air is
removed by evacuation and steam admitted to the chamber to allow heating of the load
and to assist in air removal. The sterilization period starts with the release of
formaldehyde by vaporization from formalin (in a vaporizer with a steam jacket) and
continues through either a simple holding stage or through a series of pulsed evacuations
and steam and formaldehyde admission cycles. The chamber temperature is maintained
by a thermostatically controlled water jacket, and steam and condensate are removed
via a drain channel and an evacuated condenser. At the end of the treatment period
formaldehyde vapour is expelled by steam flushing and the load dried by alternating
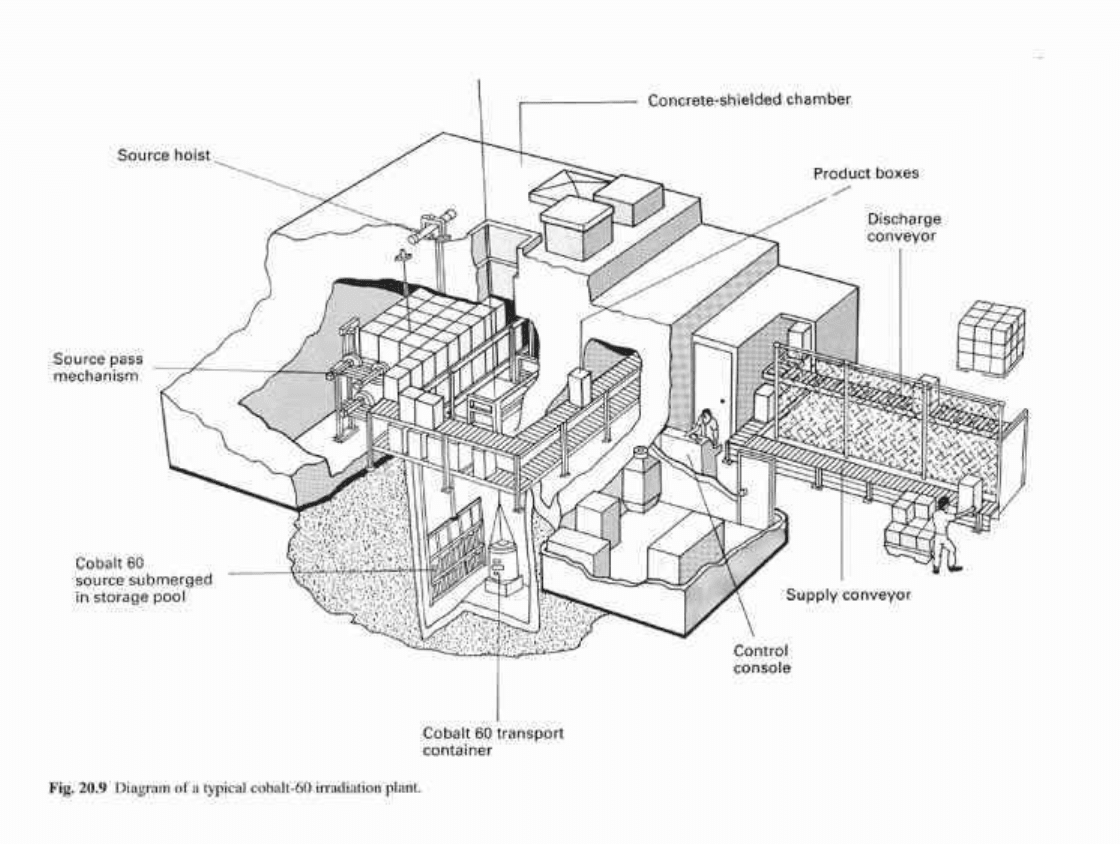
stages of evacuation and admission of sterile, filtered air. A typical pulsed cycle of
operation is shown in Fig. 20.8 and general conditions are summarized in section 10.
6 Radiation sterilization
Several types of radiation find a sterilizing application in the manufacture of
pharmaceutical and medical products, principal among which are accelerated electrons
(particulate radiation), gamma-rays and ultraviolet (UV) light (both electromagnetic
radiations). The major target for these radiations is believed to be microbial DNA, with
damage occurring as a consequence of ionization and free radical production (gamma-
rays and electrons) or excitation (UV light). This latter process is less damaging and
less lethal than ionization, and so UV irradiation is not as efficient a sterilization method
as electron or gamma-irradiation. As mentioned earlier (section 2), vegetative bacteria
Principles and practice of sterilization 401
generally prove to be the most sensitive to irradiation (with notable exceptions, e.g.
Deinococcus {Micrococcus) radiodurans), followed by moulds and yeasts, with bacterial
spores and viruses as the most resistant (except in the case of UV light where mould
spores prove to be most resistant). The extent of DNA damage required to produce cell
death can vary and this, together with the ability to carry out effective repair, probably
decides the resistance of the organism to radiation. With ionizing radiations (gamma-
ray and accelerated electrons), microbial resistance decreases with the presence of
moisture or dissolved oxygen (as a result of increased free radical production) and also
with elevated temperatures.
Radiation sterilization with high-energy gamma-rays or accelerated electrons has
proved to be a useful method for the industrial sterilization of heat-sensitive products.
However, undesirable changes can occur in irradiated preparations, especially those in
aqueous solution where radiolysis of water contributes to the damaging processes. In
addition, certain glass or plastic (e.g. polypropylene, PTFE) materials used for packaging
or for medical devices can also suffer damage. Thus, radiation sterilization is generally
applied to articles in the dried state; these include surgical instruments, sutures,
prostheses, unit-dose ointments, plastic syringes and dry pharmaceutical products
(Chapter 21). With these radiations, destruction of a microbial population follows the
classic survivor curves (see Fig. 20.1) and a D-value, given as a radiation dose, can be
established for standard bacterial spores (e.g. Bacillus pumilus) permitting a suitable
sterilizing dose to be calculated. In the UK it is usual to apply a dose of 25 kGy (2.5 Mrad)
for pharmaceutical and medical products, although lower doses are employed in the
USA and Canada.
UV light, with its much lower energy, causes less damage to microbial DNA. This,
coupled with its poor penetrability of normal packaging materials, renders UV light
unsuitable for sterilization of pharmaceutical dosage forms. It does find applications,
however, in the sterilization of air, for the surface sterilization of aseptic work areas,
and for the treatment of manufacturing-grade water.
6.1 Sterilizer design and operation
6.1.1 Gamma-ray sterilizers
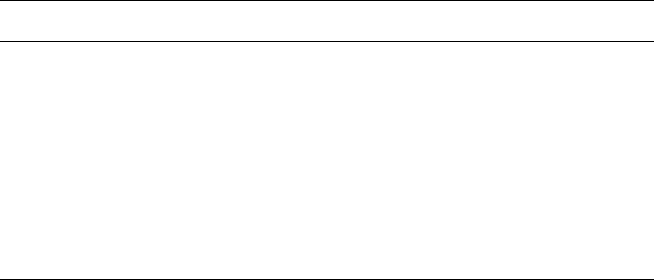
Gamma-rays for sterilization are usually derived from a cobalt-60 (
60
Co) source
(caesium-137 may also be used), with a half-life of 5.25 years, which on disintegration
emits radiation at two energy levels of 1.33 and 1.17 MeV. The isotope is held as pellets
packed in metal rods, each rod carefully arranged within the source and containing up
to 20kCi (740 x 10
12
Bq) of activity; these rods are replaced or rearranged as the activity
of the source either drops or becomes unevenly distributed. A typical
60
Co installation
may contain up to 1 MCi (3.7 x 10
16
Bq) of activity. For safety reasons, this source is
housed within a reinforced concrete building with walls some 2 m thick, and it is only
raised from a sunken water-filled tank when required for use. Control devices operate
to ensure that the source is raised only when the chamber is locked and that it is
immediately lowered if a malfunction occurs. Articles being sterilized are passed
through the irradiation chamber on a conveyor belt or monorail system and move
around the raised source, the rate of passage regulating the dose absorbed (Fig. 20.9).
Principles and practice of sterilization 403
Radiation monitors are continually employed to detect any radiation leakage during
operation or source storage, and to confirm a return to satisfactory background levels
within the sterilization chamber following operation. The dose delivered is dependent
upon source strength and exposure period, with dwell times typically up to 20 hours
duration.
The difference in radiation susceptibilities of microbial cells and humans may be
gauged from the fact that a lethal human dose would be delivered by an exposure of
seconds or minutes.
6.1.2 Electron accelerators
Two types of electron accelerator machine exist, the electrostatic accelerator and the
microwave linear accelerator, producing electrons with maximum energies of 5 MeV
and 10 MeV, respectively. Although higher energies would achieve better penetration
into the product, there is a risk of induced radiation, and so they are not used. In the
first, a high-energy electron beam is generated by accelerating electrons from a hot
filament down an evacuated tube under high potential difference, while in the second,
additional energy is imparted to this beam in a pulsed manner by a synchronized
travelling microwave. Articles for treatment are generally limited to small packs and
are arranged on a horizontal conveyor belt, usually for irradiation from one side but
sometimes from both. The sterilizing dose is delivered more rapidly in an electron
accelerator than in a
60
Co plant, with exposure times for sterilization usually amounting
to only a few seconds or minutes. Varying extents of shielding, depending upon the
size of the accelerator, are necessary to protect operators from X-rays generated by the
bremsstrahlung effect.
6.1.3 Ultraviolet irradiation
The optimum wavelength for UV sterilization is around 260 nm. A suitable source for
UV light in this region is a mercury lamp giving peak emission levels at 254 nm. These
sources are generally wall- or ceiling-mounted for air disinfection, or fixed to vessels
for water treatment. Operators present in an irradiated room should wear appropriate
protective clothing and eye shields.
7 Filtration sterilization
The process of filtration is unique amongst sterilization techniques in that it removes,
rather than destroys, microorganisms. Further, it is capable of preventing the passage
of both viable and non-viable particles and can thus be used for both the clarification
and sterilization of liquids and gases. The principal applications of sterilizing-grade
filters are the treatment of heat-sensitive injections and ophthalmic solutions, biological
products and air and other gases for supply to aseptic areas (see Chapters 21 and 22).
They may also be required in industrial applications where they become part of
venting systems on fermenters, centrifuges, autoclaves and freeze-dryers. Certain types
of filter (membrane filters) also have an important role in sterility testing, where they
can be employed to trap and concentrate contaminating organisms from solutions under
Principles and practice of sterilization 405
test. These filters are then placed on the surface of a solid nutrient medium and incubated
to encourage colony development (Chapter 23).
The major mechanisms of filtration are sieving, adsorption and trapping within the
matrix of the filter material. Of these, only sieving can be regarded as absolute since it
ensures the exclusion of all particles above a defined size. It is generally accepted that
synthetic membrane filters, derived from cellulose esters or other polymeric materials,
approximate most closely to sieve filters, while fibrous pads, sintered glass and sintered
ceramic products can be regarded as depth filters relying principally on mechanisms of
adsorption and entrapment. Some of the characteristics of filter media are summarized
in Table 20.4. The potential hazard of microbial multiplication within a depth filter and
subsequent contamination of the filtrate (microbial grow-through) should be recognized.
7.1 Filtration sterilization of liquids
In order to compare favourably with other methods of sterilization, the microorganism
removal efficiency of filters employed in the processing of liquids must be high. For
this reason, membrane filters of 0.2-0.22 fim nominal pore diameter are chiefly used,
while sintered filters are used only in restricted circumstances, i.e. for the processing
of corrosive liquids, viscous fluids or organic solvents. It may be tempting to assume
that the pore size is the major determinant of filtration efficiency and two filters of
0.2jimi pore diameter from different manufacturers will behave similarly. This is not so
because, in addition to the sieving effect, trapping within the filter matrix, adsorption
and charge effects all contribute significantly towards the removal of particles.
Consequently, the depth of the membrane, its charge and the tortuosity of the channels
are all factors which can make the performance of one filter far superior to that of
another. The major criterion by which filters should be compared, therefore, is their
titre reduction values, i.e. the ratio of the number of organisms challenging a filter
under defined conditions to the number penetrating it. In all cases, the filter medium
employed must be sterilizable, ideally by steam treatment; in the case of membrane
filters this may be for once-only use, or, in the case of larger industrial filters, a small
406 Chapter 20
Table 20.4 Some characteristics of membrane and depth filters
Characteristic
Absolute retention of microorganisms
rated pore size
Rapid rate of filtration
High dirt-handling capacity
Grow-through of microorganisms
Shedding of filter components
Fluid retention
Solute adsorption
Good chemical stability
Good sterilization characteristics
+, applicable; -, not applicable.
greater than
Membrane
+
+
-
Unlikely
-
-
-
Variable (depends
on membrane)
+
Depth
_
-
+
+
+
+
+
+
+
