Hugo W.B., Russel A.D.(ed). Pharmaceutical Microbiology
Подождите немного. Документ загружается.

though it is worth noting that more than one mechanism may be present at any one
time in a resistant microorganism. This is particularly relevant in terms of the clinical
efficacy of antibiotics. The acquisition of a single resistance mechanism may render a
bacterium microbiologically resistant but therapeutically achievable levels of a drug
may be sufficient to overcome such resistance. The acquisition of a second resistance
mechanism may be necessary to achieve clinical resistance, i.e. the amount of antibiotic
necessary to overcome the resistance mechanism is greater than can be achieved
therapeutically.
Before considering specific mechanisms of resistance for particular classes of
antibiotic it is worth considering potential mechanisms of resistance in bacterial cells.
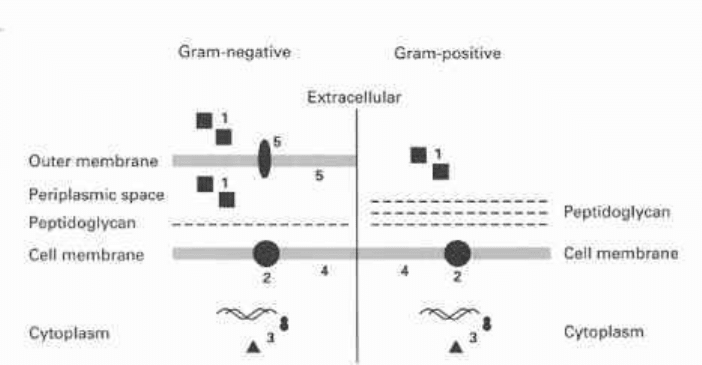
These are summarized in Fig. 9.1 and specific examples are listed in Table 9.3.
Gram-negative bacteria possess an outer membrane which can act as a barrier
to the penetration of antibiotics. The main route of entry of hydrophilic molecules is
via the porins, which form pores in the outer membrane. Qualitative or quantitative
alterations in these porins can result in the decreased accumulation of antibiotic.
The cytoplasmic (cell) membrane of Gram-positive and Gram-negative bacteria
can also act as an exclusion barrier. Alterations in membrane structure can reduce
penetration or the presence of proteins functioning as efflux pumps can actively
remove antibiotic molecules from the cytoplasm. Bacteria may produce enzymes
which inactivate antibiotics, rendering them ineffective. These may destroy or alter
the antibiotic molecule. Extracellular enzymes will be most effective in inactivating
antibiotics since they will be kept away from their target sites. Gram-negative bacteria
can also produce periplasmic enzymes which will act outside the cytoplasmic membrane.
These mechanisms of resistance rely on reducing or preventing access of antibiotic
to their target sites, but other mechanisms of resistance involving the target sites
themselves can be considered. Alterations in the target site which reduce the binding of
intracellular
Fig. 9.1 Schematic representation of possible mechanisms of resistance in Gram-negative and
Gram-positive bacteria. 1, antibiotic-inactivating enzymes; 2, antibiotic efflux proteins; 3, alteration
or duplication of intracellular targets; 4, alteration of the cell membrane reducing antibiotic uptake;
5, alterations in porins or lipopolysaccharide reducing antibiotic uptake or binding.
Bacterial resistance to antibiotics 185

Table 9.3 Mechanisms of antibiotic resistance
* Depends on chemical nature of drug and on type of organism.
t Penicillin-binding proteins.
antibiotics, but allow the target to retain its normal function, are well known. An
alternative is to bypass the antibiotic-sensitive step by duplicating the target site with
an antibiotic-resistant version. A third related mechanism is to overproduce the target
so that higher antibiotic concentrations are required to exert significant antibacterial
action. In certain species, an enzyme or metabolic pathway may be absent, rendering
the microorganism resistant to antibiotics effective against other bacterial species.
The following sections describe the biochemical mechanisms of resistance to
different classes of antibiotics, with the antibiotics grouped according to their mechanism
of action.
3.1
Inhibitors of nucleic acid synthesis
Antibiotics considered here can be divided into two mechanisms of action: those which
186 Chapter 9
Expression of resistance
Enzymatic inactivation
Enzymatic trapping
Enzymatic modification
Bacterial impermeability*
Antibiotic efflux
Decreased affinity of target
enzymes
Alteration in binding site
Example(s)
Some ^-lactam antibiotics
Chloramphenicol
Some /3-lactam antibiotics
Some aminoglycoside
antibiotics
Some /3-lactam antibiotics
Aminoglycoside antibiotics
Tetracyclines,
chloramphenicol, fusidic acid
Hydrophobic antibiotics:
novobiocin, actinomycin D,
erythromycin, rifampicin
Tetracyclines
/^-Lactam antibiotics
Trimethoprim
Sulphonamides
Streptomycin
Erythromycin
Glycopeptides
Comments
Hydrolysis of the /3-lactam
ring
Conversion to an inactive
compound
Penicillin-binding proteins
Alteration of the molecule by
phosphorylation,
adenylylation or acetylation
Mutational loss of porins
Reduced ability of cells to
take up drugs
Plasmid-mediated decreased
drug accumulation
Difficulty in entering
Gram-negative cells
Energy-dependent efflux of
accumulated drugs
Altered PBPst
Altered dihydroflolate
reductase
Altered dihydropteroate
synthetase
Protein S12 component of
30S ribosomal subunit
determines sensitivity or
resistance
Ribosomes from resistant cells
have lower affinity, resulting
from enzymatic methylation
of adenine in 23S rRNA
Acquired ligase produces
altered peptidoglycan
precursors with lower affinity
inhibit nucleotide metabolism and those which inhibit enzymes involved in nucleic
acid synthesis.
3.1.1 Sulphonamides
Chromosomal and plasmid-mediated resistance to the sulphonamides has been described
(Huovinen et al. 1995).
Two mechanisms of chromosomal resistance have been identified. A mutation of
dihydropteroate synthetase (DHPS) in Strep, pneumoniae produces an altered enzyme
with reduced affinity for sulphonamides. Hyperproduction of p-aminobenzoic acid
(PABA) overcomes the block imposed by inhibition of DHPS. The specific cause of
PABA hyperproduction is unknown, though chromosomal mutation is the probable cause.
Duplication of DHPS, with the second version of the enzyme being resistant to the
sulphonamides, is the cause of plasmid-acquired resistance. Two different enzymes
have been identified, both with lowered affinity for the antibiotic.
3.1.2 Trimethoprim
Trimethoprim is a 2,4-diaminopyrimidine and all three genetic bases of resistance have
been described (Huovinen et al. 1995).
Chromosomal mutations in E. coli result in overproduction of dihydrofolate reductase
(DHFR). Higher concentrations of trimethoprim, which may not be therapeutically
achievable, are therefore required to inhibit nucleotide metabolism. Other mutations
lower the affinity of DHFR for trimethoprim. These two mechanisms of resistance
may coexist in a single strain, effectively increasing the level of resistance to the
antibiotic.
Plasmid- and transposon-mediated resistance is akin to that described for the
sulphonamides, where the sensitive step is bypassed by duplication of the target with a
resistant version. Many different resistant enzymes have been identified thus far.
3.1.3 Quinolones
The quinolones exert their action by binding to DNA gyrase (bacterial topoisomerase
II) and inhibiting its functions. Acquired resistance to the quinolones arises due to
chromosomal mutations in the genes coding for DNA gyrase (Hooper & Wolf son 1993).
The most common mutations arise in the gyrA gene where a single base-pair change
can be sufficient to cause resistance. Levels of resistance can be increased by the presence
of multiple mutations with a region of the gyrA gene known as the quinolone resistance-
determining region. The exact mechanism of resistance is unknown but is thought to
involve a subtle conformational change in DNA gyrase which reduces binding of
quinolones. Mutations in the gyrB gene have also been identified but these lead to
lower levels of resistance. With certain gyrB mutations, bacteria become resistant to
older quinolone analogues such as nalidixic acid, but become hypersusceptible to newer
quinolones such as ciprofloxacin. Mutations in other bacterial topoisomerases have
been identified in Staph, aureus. These are thought to be as important as DNA gyrase
mutations in quinolone resistance in this microorganism.
Bacterial resistance to antibiotics 187
Other chromosomal mutations resulting in quinolone resistance have been found
to decrease permeability of the antimicrobial agent. norB mutants show a decrease in
ompF porin. This is one of the major porins in Gram-negative bacteria. norC mutants
have altered ompF and lipopolysaccharide, though the mutations are not in the ompF
gene itself but appear to occur in a gene or genes whose products regulate OmpF. norC
mutants are less susceptible to some quinolones such as ciprofloxacin but more
susceptible to others.
Resistance to quinolones by efflux has been described in Staph, aureus and Proteus
mirabilis. This gene has been designated nor A in Staph, aureus and is homologous to
membrane transport proteins coupled to the electromotive force. These proteins have
the ability to remove small amounts of quinolone from cells normally and nor A may
have arisen as a result of mutations under selective pressure from quinolone use, resulting
in a transport protein with increased affinity for these agents.
3.1.4 Rifampicin
Rifampicin is the semisynthetic derivative used widely in the UK. Resistance to
rifampicin is primarily due to chromosomal mutations resulting in an altered RNA
polymerase which is less well inhibited by the drug. The mutations tend to be clustered
within short conserved regions of the j3 subunit gene of RNA polymerase. Similar
mutations have been found in all bacterial species studied thus far.
3.2 Inhibitors of protein synthesis
Inhibition of protein synthesis is the antibacterial mechanism shared by most groups of
antibiotics, though the exact action differs.
3.2.1 Aminoglycoside-aminocyclitol group
Three mechanisms of resistance to the aminoglycoside-aminocyclitol (AGAC) group
of antibiotics are recognized (Shaw et al. 1993).
Alteration of the antibiotic molecule is plasmid- or transposon-encoded. Three
classes of enzyme can alter the AGAC molecule. Aminoglycoside adenylyltransferases
(AADs) use adenosine triphosphate (ATP) as a cofactor in modifying certain hydroxyl
groups in the antibiotic molecule by adenylylating them (Fig. 9.2). Aminoglycoside
phosphotransferases (APH) also use ATP to modify certain hydroxyl groups by
phosphorylating them (Fig. 9.2). Aminoglycoside acetyltransferases (AACs) use acetyl
CoA as a cofactor and acetylate susceptible amino groups on the molecule (Fig. 9.2).
These three classes of enzyme have been further subdivided according to which site
on the AGAC molecule is modified. For example, APH(6) phosphorylates the 6-hydroxyl
group on the aminohexose group of streptomycin. Most AGAC antibiotics are susceptible
to more than one modification reaction. Relatively small amounts of the antibiotic are
modified, implying that resistance is determined by the relative rates of drug uptake
and modification. A less efficient modifying enzyme will permit unmodified antibiotic
to reach its ribosomal quantity. A more efficient enzyme, or greater quantities of the
enzyme, will result in resistance.
188 Chapter 9
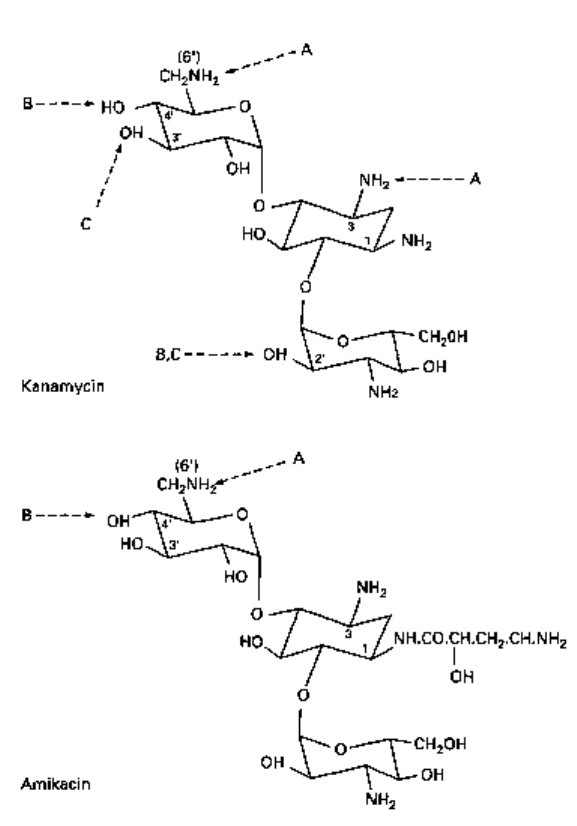
Fig. 9.2 Modification of AGACs (e.g. kanamycin and amikacin) by resistance enzymes.
A, acetylation (AAC); B, adenylylation (AAD); C, phosphorylation (APH).
AGAC-modifying enzymes are active outside the cytoplasmic membrane, in the
periplasmic space in Gram-negative bacteria and extracellularly in Gram-positives.
Table 9.4 summarizes some of the enzymes involved in AGAC resistance and their
spectrum of activity.
A second mechanism of resistance to the AGACs involves an alteration of the
ribosomal target site. Mutations in the gene coding for ribosomal protein S12 (rpsL in
E. coli) prevent the antibiotics from binding to their target. In mycobacteria, which
possess only one ribosomal RNA operon, mutations in rpoB, coding for 16S rRNA,
also inhibit binding of the drugs.
Acquired resistance due to decreased permeability by mutations affecting membrane
transport have also been reported in other bacteria.
Bacterial resistance to antibiotics 189
Table 9.4 Examples of aminoglycoside-aminocyclitol susceptibility to modifying enzymes
3.2.2 Tetracyclines
Three types of resistance mechanism have also been identified with this class of antibiotic
(Chopra et al. 1992).
Plasmid- or transposon-encoded tetracycline efflux proteins have been described
in a number of bacteria. These efflux proteins are thought to span the cytoplasmic
membrane and are dependent on the proton-motive force for their action. It is thought
that the efflux proteins bind tetracyclines and initiate proton transfer, although no
functional domains have been identified. Eight distinct tetracycline efflux proteins have
been identified thus far.
Plasmid- or transposon-encoded ribosomal protection factors are a second mechan-
ism of resistance to the tetracyclines. These proteins are believed to alter the tetracycline
binding site on the 30S ribosomal subunit, lowering the affinity for the drugs.
OmpF mutations in Gram-negative bacteria such as E. coli (see above) can result
in low level resistance to the tetracyclines by reducing their uptake.
3.2.3 Chloramphenicol
Plasmid- or transposon-encoded chloramphenicol acetyltransferases (CATs) are respon-
sible for resistance by inactivating the antibiotic. CATs convert chloramphenicol to an
acetoxy derivative which fails to bind to the ribosomal target. Several CATs have been
characterized and found to differ in properties such as electrophoretic mobility and
catalytic activity.
Three other mechanisms of chloramphenicol resistance have been described. First,
a transposon-encoded chloramphenicol efflux protein has been identified in E. coli.
Second, some bacterial strains have been found to possess drug-resistant ribosomes,
and third, low level resistance can arise by chromosomal mutations which reduce the
amount of porins and therefore impair uptake. This last mechanism is essentially that
described for the AG AC antibiotics.
190 Chapter 9
Aminoglycoside
Streptomycin
Spectinomycin
Gentamicin
Kanamycin
Tobramycin
Neomycin
Amikacin
Inactivation by
APH(3"), APH(6), AAD(6),
AAD{3")(9)
AAD(3")(9), AAD(9)
APH{2"), AAD(2"), AAC(3),
AAC(2')
APH(3'), APH(2"), AAD<2"),
AAD(4')(4"), AAC(6')
APH(2"), AAD(4')(4"),
AAD(2"), AAC(3), AAC(2'),
AAC(6')
APH(3'), AAD(4')(4"),
AAC(3), AAC(2'), AAC(6')
AAD(4')(4"), AAC(6')
3.2.4
Macrolide, lincosamide and streptogramin (MLS) antibiotics
These three classes of antibiotics are often grouped together because of their similar
mode of action. They share a common mechanism of resistance, but there are some
mechanisms specific to each group (Leclerq & Courvalin 1991).
Plasmid- or transposon-mediated resistance common to the MLS group is due to
RNA methylase genes {ermA, ermB and ermC) which code for the methylation of an
adenine residue in 23 S rRNA. Methylation prevents the drugs from binding to the 50S
ribosomal subunit and confers resistance to all MLS antibiotics.
A gene designated msrA has been identified in Staph, aureus which confers resistance
to macrolides and streptogramins but not to lincosamides. Its function is unknown but
the DNA sequence is homologous to genes coding for known efflux proteins.
Chromosomal mutations in E. coli have been identified as causing macrolide
resistance. eryA alters protein L4 with a concomitant loss of binding to ribosomes.
eryB alters protein L22 with a loss of macrolide binding, though the mutation is not in
the structural gene for L22. eryC mutants are thought to alter the processing of rRNA
and a 30S ribosomal subunit protein, though the precise mechanism of resistance is
unclear. Macrolide resistance in mycobacteria is associated with point mutations in
23S rRNA. Plasmid-mediated inactivation of erythromycin (a 14-membered macrolide)
is common in Gram-negative bacteria and has also been described in some Gram-
positives. The lactone ring is hydrolysed by esterase in Gram-negatives, although
no similar enzymes have been identified in Gram-positives. Erythromycin can also
be phosphorylated, the altered molecule being rendered inactive. Plasmid-mediated
resistance to the lincosamides is common in staphylococci. An enzyme nucleotidylates
the antibiotics at a specific position rendering them inactive. Staphylococcal resistance
to streptogramins is due to inactivation of the antibiotics by plasmid-encoded enzymes.
Streptogramin A is inactivated by an acetyltransferase and streptogramin B by a
hydrolase.
3.2.5 Fusidic acid
Gram-negative bacteria are intrinsically resistant to low levels of fusidic acid (a
steroid) due to exclusion by the outer membrane. Nevertheless, acquired resistance
does occur which has the effect of increasing the level of resistance to the antibiotic.
Acquired resistance also occurs in Gram-positive bacteria normally susceptible to fusidic
acid.
Plasmid-mediated resistance in Gram-positive bacteria is thought to involve
decreased uptake of the drug, although the precise mechanism is unknown. Resistance
to fusidic acid in Gram-negative bacteria is also poorly understood. A CAT-type
enzyme has been identified in resistant strains but no modification or inactivation of
the antibiotic has been observed. It is believed that the CAT forms a tight stoichiometric
complex with the antibiotic, sequestering it and thus rendering it inactive (Davies
1994).
Chromosomal mutations have also been described which produce a modified
translocation factor protein with lowered affinity for fusidic acid.
Bacterial resistance to antibiotics 191
3.2.6 Mupirocin
3.3
3.3.1
Mupirocin is a topical antibiotic that inhibits isoleucyl tRNA synthetase with the
subsequent inhibition of protein synthesis. Mupirocin has become a mainstay in the
treatment of Staph, aureus infection and colonization during hospital outbreaks, and it
is in this organism that acquired resistance has arisen (Gilbart et al. 1993).
High level mupirocin resistance, where strains can be up to 1000 times more resistant
than susceptible strains, is due to the presence of an additional isoleucyl tRNA synthetase
which is resistant to the antibiotic. Resistance is plasmid-encoded, but the resistant
gene differs significantly from the normal susceptible version. The origins of high
level mupirocin resistance are unclear but the low homology with existing genes would
suggest that resistance is acquired from microorganisms other than Staph, aureus. High
level mupirocin resistance is an example of duplication of the target site, the new version
being resistant to the antibiotic. Low level mupirocin resistance results in strains
approximately 50 times more resistant to the antibiotic than susceptible strains. No
extra copies of the isoleucyl tRNA synthetase gene are found, indicating that resistance
has been acquired by mutations in the normal chromosomal gene. Presumably, mupirocin
has less affinity for the altered isoleucyl tRNA synthetase.
Inhibitors of peptidoglycan synthesis
^-Lactams
Acquired resistance to /Mactam antibiotics can occur by three different mechanisms:
inactivation of the antibiotic, alteration of the target site and reduced permeability
(Sanders 1992; Georgopapadakou 1993).
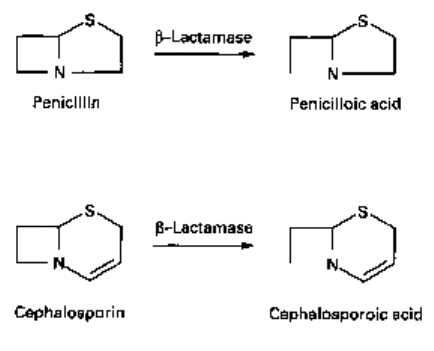
j8-Lactams are inactivated by enzymes called /^-lactamases which hydrolyse the
cyclic amide bond in the antibiotic molecule (Fig. 9.3). Penicillins are converted to
penicilloic acid which is unable to bind to penicillin-binding proteins (PBPs: see Chapter
8). A similar reaction occurs with cephalosporins, except that the cephalosporoic acid
derivative is unstable and tends to break up. A wide variety of/^-lactamases with different
structures and substrate profiles has been identified in recent years and classified
according to the scheme in Table 9.5. Many /3-lactamases are plasmid- or transposon-
Fig. 9.3 Hydrolysis of
/Mactams. Cephalosporoic acid
is unstable (see also Fig. 5.1
and sections 2.1 and 2.2 in
Chapter 5).
192 Chapter 9

Table 9.5 Examples of
b-lactamases
* Based on Bush et al. (1995).
t Plasmid-encoded /3-lactamases (TEM, PSE, OXA, SHV).
encoded but the Group 1 enzymes are mainly chromosomal. The origin of these is
unclear but they may have diverged from existing PBPs. Transfer of these chromosomal
enzymes by conjugation may be possible, but no evidence for this exists. Nevertheless,
some reports indicate that these Group 1 genes may be mobilized into plasmids and
then transferred to other bacteria. Some Group 1 enzymes are constitutive and expressed
at low levels, but in other species these enzymes are inducible by /^-lactams themselves.
Mutations in regulatory genes can lead to constitutive high levels of expression. Such
mutations are of clinical significance since they have led to the development of resistance
to newer /3-lactams previously thought to be resistant to ^-lactamases.
It is worth noting that in Gram-negative organisms, /3-lactamases are found in the
periplasmic space where they inactivate /^-lactams before the antibiotics can bind to
their PBP targets on the cytoplasmic membrane. In Gram-positive organisms, however,
^-lactamases are excreted extracellularly and therefore resistance is very much a
characteristic of the population rather than individual /3-lactamase-producing cells. If
enough enzyme is synthesized, levels of pMactam may be reduced sufficiently to permit
growth of non-pMactamase-producing strains.
Bacterial resistance to antibiotics 193
Group of
enzyme*
1
2a
2b
2be
2br
2c
2d
2e
2f
3
4
Preferred
substrate
Cephalosporin
Penicillins
Penicillins,
cephalosporins
Penicillins,
cephalosporins
monobactams
Penicillins
Penicillins,
carbenicillin
Penicillins,
cloxacillin
Cephalosporin
Penicillins,
cephalosporins,
carbapenems
Most /3-lactams,
including
carbapenems
Penicillins
Inhibited by:
Clavulanic acid
-
+
+
+
+/-
+
+/-
+
+
-
EDTA
-
-
-
-
-
-
-
+
?
Representative
enzymes
AmpCfrom Gram-negatives
Penicillinases from Gram-positives
TEM-1t,TEM-2, SHV-1
from Gram negatives
TEM-3 to TEM-26
TEM-30 to TEM-36
PSE-1, PSE-3, PSE-4
OXA-1
to OX A-11
Inducible cephalosporinases
from Proteus vulgaris
NMC-Afrom Enterobacter
cloacae, Sme-I from Serratia
marcescens
L1 from Xanthomonas
maltophilia, CcrA from
Bacteroides fragilis
Penicillinase from
Pseudomonas cepacia

A second mechanism of resistance involves alterations in PBPs which affect
binding of /3-lactams. These changes have been found to occur by multiple substitutions
through recombination rather than point mutations. Acquired penicillin resistance in
Strep, pneumoniae is because of such gene mosaics which code for an altered yet
functional PBP with reduced affinity for penicillin. Sections of the susceptible PBP
gene have been replaced by other DNA sequences, presumably via transformation.
Clinically, one of the most important examples of /3-lactam resistance is that found
in methicillin-resistant Staph, aureus (MRSA) strains. These are causing increasing
concern in hospitals, especially because methicillin resistance is often accompanied by
multiple resistance to unrelated antibiotics. Methicillin is resistant to /^-lactamases and
is a mainstay in the treatment of Staph, aureus since over 90% of hospital strains produce
/3-lactamase. Methicillin resistance is due to a novel PBP with low affinity for /3-lactams.
It is capable of functioning when all other PBPs have been inhibited and is sufficient to
catalyse all the reactions necessary for cell growth. Resistance is mediated by the mec
gene, whose origin is unknown. This is an example of resistance by duplication of an
antibiotic target, the new version being resistant to the antibiotic.
A third resistance mechanism is akin to that described for the AGAC antibiotics
and chloramphenicol, whereby changes in the outer membrane porins of Gram-negative
bacteria reduce the penetration of /3-lactams resulting in low levels of resistance.
3.3.2 Glycopeptides
Glycopeptide antibiotics interfere with peptidoglycan synthesis by binding to the D-
alanyl-D-alanine terminus of peptidoglycan precursors. Resistance to glycopeptides
was thought unlikely because the changes in integral structures and functions of the
cell wall and the enzymes involved in its synthesis would render bacteria non-viable.
As is often the case, bacteria have a nasty habit of surprising us!
Acquired resistance to the glycopeptides is transposon-mediated and has so far
been largely confined to the enterococci. This has been a problem clinically because
many of these strains have been resistant to all other antibiotics and were thus effectively
untreatable. Fortunately, the enterococci are not particularly pathogenic and infections
have been confined largely to seriously ill, long-term hospital patients. Two types of
acquired glycopeptide resistance have been described (Woodford et al. 1995). The
VanA phenotype is resistant to vancomycin and teicoplanin, whereas VanB is resistant
ORF1 ORF2 vanR vanS vanH vanA vanX vanY vanZ
IR IR
Transposase Response Dehydrogenase Dipeptidase unknown
regulator ,
lrw
,. „ „ . TcR?
Resolvase HPK Ligase D, D-carboxy-
peptidase
I I I I
Transposition Regulation Required for Accessory
glycopeptide proteins
resistance
Fig. 9.4 Organization of glycopeptide-resistance genes in transposon Tnl546. IR, invested repeats;
HPK, histidine protein kinase; TcR, low level teicoplanin resistance.
194 Chapter 9
