Hugo W.B., Russel A.D.(ed). Pharmaceutical Microbiology
Подождите немного. Документ загружается.

the bacteria undergo a number of changes in shape and ultimately die following
disruption (lysis) of the cells. Mammalian cells do not possess a cell wall and contain
no other macromolecular structures resembling peptidoglycan. Consequently, antibiotics
which interfere with peptidoglycan synthesis generally have excellent selective toxicity
since the target is vital to the bacteria but absent from mammalian cells.
2.1.1 D-Cycloserine
There are three stages of peptidoglycan biosynthesis (Fig. 8.1). The first occurs in the
cytoplasm where the precursors are synthesized. The formation and assembly of a
D-alanyl-D-alanine dipeptide is the site of action of D-cycloserine. Two molecules of
L-alanine are converted to the D-forms by an isomerase in the bacterial cytoplasm. A
ligase then joins the two D-alanines together. Both of these enzymes are inhibited by
binding cycloserine, which bears some structural similarities to D-alanine. Cycloserine
binds covalently to the pyridoxal phosphate cofactor of the enzymes, effectively
preventing them from forming D-alanyl-D-alanine. The D-alanyl-D-alanine dipeptide is
then coupled to three other amino acids (in Escherichia coli these are L-alanine, D-
glutamic acid and meso-diaminopimelic acid) which have been added sequentially to
the sugar nucleotide, uridine diphosphate (UDP)-TV-acetylmuramic acid. The sugar
pentapeptide produced (A^-acetylmuramylpentapeptide) is then transferred from the
nucleotide to a hydrophobic lipid carrier molecule (undecaprenyl phosphate) which is
located exclusively in the cytoplasmic membrane. The nucleotide uridine monophosphate
(UMP) remains in the cytoplasm. Another sugar nucleotide precursor, UDP-Af-
acetylglucosamine is also produced in the cytoplasm and donates a molecule of N-
acetylglucosamine to be coupled to the lipid carrier in the membrane forming a lipid
pyrophosphate-linked disaccharide pentapeptide. This is the second stage of the
biosynthetic pathway in which the disaccharide pentapeptide is transported across the
membrane on the lipid carrier to be inserted into the cell wall at a growing point. The
lipid carrier does not leave the cell membrane and is eventually recycled. It loses a
single phosphate group whilst returning to the cytoplasmic face of the membrane to
collect another disaccharide pentapeptide from the cytoplasm.
2.1.2 Glycopeptides—vancomycin and teicoplanin
It is in the third and final stage of the pathway that the glycopeptide antibiotics act.
Here the disaccharide pentapeptide is first incorporated into the expanding cell wall
linked to its lipid carrier. The growing glycan-peptide chain is transferred in turn to
each molecule of lipid carrier as it brings its disaccharide pentapeptide precursor
across the membrane. Each lipid carrier molecule thus acts in turn to hold the growing
linear glycan strand before returning through the membrane to the cytoplasmic face.
Incorporation of each disaccharide pentapeptide is catalysed by a transglycosylase and
this step is effectively blocked by the glycopeptides. These antibiotics bind very tightly
by hydrogen bonding to the terminal D-alanyl-D-alanine on each pentapeptide inhibiting
extension of the linear glycan peptide in the cell wall. Vancomycin is thought to bind
to the pentapeptides outside the cytoplasmic membrane. Possibly two vancomycin
molecules form a back-to-back dimer which bridges between pentapeptides preventing
Mechanisms of action of antibiotics 165
further peptidoglycan assembly. Teicoplanin is a lipoglycopeptide which may act slightly
differently by locating itself in the outer face of the cytoplasmic membrane and binding
the pentapeptide as the precursors are transferred through the membrane.
2.1.3 fi-Lactam antibiotics—penicillins, cephalosporins, carbapenems and monobactams
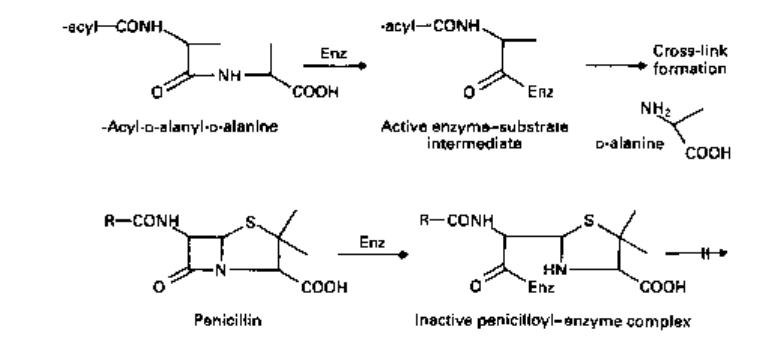
The /^-lactam antibiotics block the final crosslinking stage of the pathway which occurs
in the cell wall. Here the linear glycan strands are crosslinked via their peptide chains
to the mature peptidoglycan in the cell wall. The crosslinking is catalysed by a group of
enzymes called transpeptidases. These enzymes are located on the outer face of the
cytoplasmic membrane. They first remove the terminal D-alanine residue from each
pentapeptide on the linear glycan. This reaction involves breakage of the peptide between
the two D-alanine residues on the linear glycan. The energy released is thought to be
used in the formation of a new peptide bond between the remaining D-alanine on the
glycan chain and an acceptor amino group on existing crosslinked peptidoglycan. In
Escherichia coli this acceptor is the free amino group on raeso-diaminopimelic acid
(the third amino acid on each ./V-acetylmuramic acid). In other organisms, for example
Staphylococcus aureus, it is the free amino group on lysine (replacing diaminopimelic
acid) which acts as the acceptor. It should be noted that although there is considerable
variation in the composition of the peptide crosslink among different species of bacteria,
the essential transpeptidase mechanism is the same. Therefore virtually all bacteria can
be inhibited by interference with this group of enzymes. The /Mactam antibiotics
effectively inhibit the transpeptidases by acting as alternative substrates. They mimic
the D-alanyl-D-alanine residues and react with the transpeptidases (Fig. 8.2).
The /3-lactam bond is broken (instead of the equivalent peptide bond joining the
alanine residues) but the remaining ring system in the /3-lactam (a thiazolidine in
penicillins) is not released (Fig. 8.3). Instead, the transpeptidase remains linked to
the hydrolysed antibiotic with a half life of 10-15 minutes. Whilst bound to the
/^-lactam, the transpeptidase cannot participate in further rounds of peptidoglycan
Fig. 8.2 Interaction of transpeptidase (Enz) with its natural substrate, acyl-D-alanyl-D-alanine in the
first stage of the transpeptidation reaction to form an acyl-enzyme intermediate. A similar reaction
with a penicillin results in the formation of an inactive penicilloyl-enyme complex.
166 Chapter 8
crosslinking by reaction with its true substrate. All /J-lactam antibiotics (penicillins,
cephalosporins, carbapenems and monobactams) are thought to act in a similar way
through interaction of their /Mactam ring with transpeptidases. However, there is
considerable variation in the morphological effects of different /3-lactams upon bacterial
cells which is due to the existence of several types of transpeptidases. The transpeptidase
enzymes are usually referred to as penicillin-binding proteins (PBPs) because they can
be separated and studied after reaction with
14
C-labelled penicillin. This step is necessary
because there are very few copies of each enzyme present in a cell. They are usually
separated according to their size by electrophoresis and are numbered PBP1, PBP2,
etc. starting from the highest molecular weight species. In Gram-negative bacteria
the high molecular weight transpeptidases appear also to possess transglycosylase
activity, i.e. they have a dual function in the final stages of peptidoglycan synthesis.
Furthermore, the different transpeptidases have specialized functions in the cell; all
crosslink peptidoglycan but some are involved with maintenance of cell integrity, some
regulate cell shape and others produce new cross wall between elongating cells securing
chromosome segregation prior to cell division.
Recognition of the existence of multiple transpeptidase targets and their relative
sensitivity towards different /3-lactams helps to explain the different morphological
effects observed on treated bacteria. For example, benzylpenicillin (penicillin G),
ampicillin and cephaloridine are particularly effective in causing rapid lysis of Gram-
negative bacteria such as E. coli. These antibiotics act primarily upon PBP1B, the
major transpeptidase of the organism. Other /^-lactams have little activity against this
PBP, for example mecillinam binds preferentially to PBP2 and it produces a pronounced
change in the cells from a rod shape to an oval form. Many of the cephalosporins, for
example cephalexin, cefotaxime and ceftazidime bind to PBP3 resulting in the formation
of elongated, filamentous cells. The lower molecular weight PBPs, 4, 5 and 6, do not
possess transpeptidase activity. These are carboxypeptidases which remove the terminal
D-alanine from the pentapeptides on the linear glycans in the cell wall but do not catalyse
the crosslinkage. Their role in the cells is to regulate the degree of crosslinking by
denying the D-alanyl-D-alanine substrate to the transpeptidases but they are not essential
for cell growth. Up to 90% of the amount of antibiotic reacting with the cells may be
consumed in inhibiting the carboxypeptidases, with no lethal consequences to the cells.
Mechanisms of action of antibiotics 167
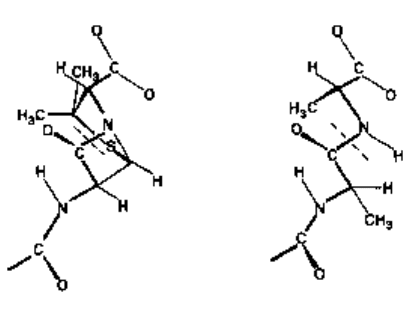
Fig. 8.3 A, comparison of the
structure of the nucleus of the
penicillin molecule with B, the
D-alanyl-D-alanine end group of
the precursor of bacterial
peptidoglycan. The broken lines
show the correspondence in
position between the labile bond
of penicillin and the bond broken
during the transpeptidation
reaction associated with the
crosslinking in peptidoglycan.
Gram-positive bacteria also have multiple transpeptidases, but fewer than Gram-
negatives. Shape changes are less evident than with Gram-negative rod-shaped
organisms. Cell death follows lysis of the cells mediated by the action of endogenous
autolytic enzymes (autolysins) present in the cell wall which are activated following
/3-lactam action. Autolytic enzymes able to hydrolyse peptidoglycan are present in
most bacterial walls, they are needed to reshape the wall during growth and to aid cell
separation during division. Their activity is regulated by binding to wall components
such as the wall and membrane teichoic acids. When peptidoglycan assembly is disrupted
through /3-lactam action, some of the teichoic acids are released from the cells which
are then susceptible to attack by their own autolysins.
2.2 Mycolic acid and arabinogalactan synthesis in mycobacteria
The cell walls of mycobacteria contain three structures: peptidoglycan, an arabino-
galactan polysaccharide and long chain hydroxy fatty acids (mycolic acids) which are
all covalently linked. Additional non-covalently attached lipid components found in
the wall include glycolipids, various phospholipids and waxes. The lipid-rich nature of
the mycobacterial wall is responsible for the characteristic acid-fastness on staining
and serves as a penetration barrier to many antibiotics. Isoniazid and ethambutol have
long been known as specific antimycobacterial agents but their mechanisms of action
have only recently become more clearly understood.
2.2.7 Isoniazid
Mycolic acids are produced by a diversion of the normal fatty acid biosynthetic pathway
in which short chain (16 carbon) and long chain (24 carbon) fatty acids are produced
by addition of 7 or 11 malonate extension units from malonyl coenzyme A to acetyl
coenzyme A. The long chain fatty acids are further extended and condensed to produce
the 60-70 carbon /3-hydroxymycolic acids. Isoniazid is thought to inhibit a desaturase
(dehydrogenase) enzyme which inserts a double bond into the fatty acid chain at the
24 carbon stage of mycolic chain extension. Isoniazid itself is a prodrug which is
activated inside mycobacteria by a catalase-peroxidase enzyme system called KatG.
Unidentified reactive radicals then attack sensitive targets such as the C
24
-desaturase
involved in mycolic acid synthesis. Mycobacterium tuberculosis becomes resistant
to isoniazid through loss of the activating KatG enzyme. Other targets involving
metabolism of the nucleotide nicotinamide adenine dinucleotide (NAD) and DNA
damage may also be involved in the killing mechanism.
2.2.2 Ethambutol
The antimicrobial action of ethambutol, like that of isoniazid, is specific for myco-
bacteria, suggesting a target in the unique components of the mycobacterial cell wall.
Cells treated with ethambutol accumulate an isoprenoid intermediate, decaprenyl-
arabinose which is the source of arabinose in the arabinogalactan polymer. This suggests
that ethambutol blocks assembly of the arabinogalactan through inhibition of an
arabinosyl transferase enzyme.
168 Chapter 8
3
Protein synthesis
3.1 Protein synthesis and selective inhibition
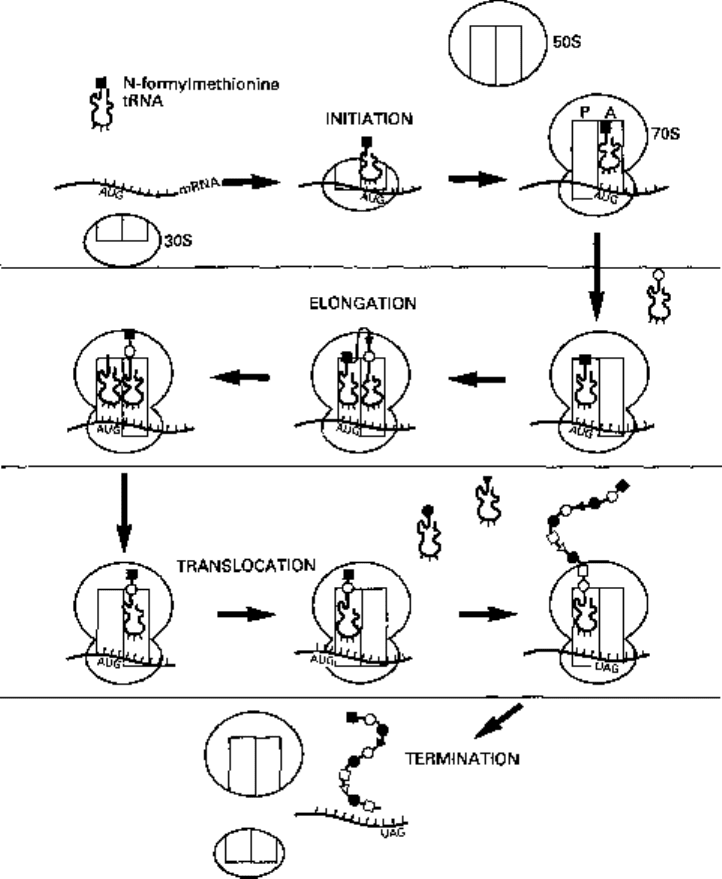
Figure 8.4 outlines the process of protein synthesis involving the ribosome, mRNA, a
series of aminoacyl transfer RNA (tRNA) molecules (at least one for each amino acid)
Fig. 8.4 Outline of the main events in protein synthesis; initiation, elongation, translocation and
termination. AUG is an initiation codon on the mRNA; it codes for Af-formylmethionine and initiates
the formation of the 70S ribosome. UAG is a termination codon; it does not code for any amino acid
and brings about termination of protein synthesis.
Mechanisms of action of antibiotics 169
and accessory protein factors involved in initiation, elongation and termination. As the
process is essentially the same in prokaryotic (bacterial) and eukaryotic cells (i.e. higher
organisms and mammalian cells) it is surprising that there are so many selective agents
which act in this area (see Table 8.1).
Bacterial ribosomes are smaller than their mammalian counterparts. They consist
of one 30S and one 50S subunit (the S suffix denotes the size which is derived from the
rate of sedimentation in an ultracentrifuge). The 30S subunit comprises a single strand
of 16S rRNA and over 20 different proteins which are bound to it. The larger 50S
subunit contains two single strands of rRNA (23S and 5S) together with over 30 different
proteins. The subunits pack together to form an intact 70S ribosome. The equivalent
subunits for mammalian ribosomes are 40S and 60S making an 80S ribosome. Some
agents exploit subtle differences in structure between the bacterial and mammalian
ribosomes. The macrolides, azalides and chloramphenicol act upon the 50S subunits in
bacteria but not the 60S subunits of mammalian cells. By contrast, the tetracyclines
derive their selective action through active uptake by microbial cells and exclusion
from mammalian cells. They are equally active against both kinds of ribosomes by
binding to the respective 30S and 40S subunits.
3.2 Aminoglycoside-aminocyclitol antibiotics
Most of the information on the mechanisms of action of aminoglycoside-aminocyclitol
(AGAC) antibiotics comes from studies with streptomycin. One effect of the AGACs
is to interfere with the initiation and assembly of the bacterial ribosome (Fig. 8.4).
During assembly of the initiation complex, Af-formylmethionyl-tRNA (fmet-tRNA)
binds initially to the ribosome binding site on the untranslated 5' end of the mRNA
together with the 30S ribosomal subunit. Three protein initiation factors (designated
IFj_
3
) and a molecule of guanosine triphosphate (GTP) are involved in positioning the
fmet-tRNA on the AUG start codon of mRNA. IFj and IF
3
are then released from the
complex, GTP is hydrolysed to guanosine diphosphate (GDP) and released with IF
2
as
the 50S subunit joins the 30S subunit and mRNA to form a functional ribosome. The
fmet-tRNA occupies the peptidyl site (P site) leaving a vacant acceptor site (A site) to
receive the next aminoacyl-tRNA specified by the next codon on the mRNA.
Streptomycin binds tightly to one of the protein components of the 30S subunit. Binding
of the antibiotic to the protein, which is the receptor for IF
3
, prevents initiation and
assembly of the ribosome.
Streptomycin binding to the 30S subunit also distorts the shape of the A site on
the ribosome and interferes with the positioning and the aminoacyl-tRNA molecules
during peptide chain elongation. Streptomycin therefore exerts two effects: inhibition
of protein synthesis by freezing the initiation complex, and misreading of the codons
through distortion of the 30S subunit. Simple blockage of protein synthesis would
be bacteriostatic rather than bacteriocidal. Since streptomycin and the other AGACs
exert a potent lethal action it seems that the formation of toxic, non-functional proteins
through misreading of the codons on mRNA is a more likely mechanism of action.
This can be demonstrated with cell-free translation systems in which isolated bacterial
ribosomes are supplied with an artificial mRNA template such as polyU or polyC
and all the other factors, including aminoacyl-tRNAs needed for protein synthesis.
170 Chapter 8
In the absence of an AGAC the ribosomes will produce the artificial polypeptides,
polyphenylalanine (as specified by the codon UUU) or polyproline (as specified by the
codon CCC). However, when streptomycin is added, the ribosomes produce a mixture
of polythreonine (codon ACU) and polyserine (codon UCU). The misreading of the
codons does not appear to be random: U is read as A or C and C is read as A or U. If
such misreading occurs in whole cells the accumulation of non-functional or toxic
proteins would eventually prove fatal to the cells. There is some evidence that the
bacterial cell membrane is damaged when the cells attempt to excrete the faulty proteins.
The effectiveness of the AGACs is enhanced by their active uptake by bacteria
which proceeds in three phases. First, a rapid uptake occurs within a few seconds of
contact which represents binding of the positively charged AGAC molecules to the
negatively charged surface of the bacteria. This phase is referred to as the energy-
independent phase (EIP) of uptake. In the case of Gram-negative bacteria the AGACs
damage the outer membrane causing release of some lipopolysaccharide, phospholipid
and proteins but this is not directly lethal to the cells. Second, there follows an energy-
dependent phase of uptake (EDP I) lasting about 10 minutes, in which the AGAC is
actively transported across the cytoplasmic membrane. A second energy-dependent
phase (EDP II) which leads to further intracellular accumulation follows after some
AGAC has bound to the ribosomes in the cytoplasm. Although the precise details of
uptake by EDP I and EDP II are not clear, both require organisms to be growing
aerobically. Anaerobes do not take up AGACs by EDP I or EDP II and are consequently
resistant to their action.
Tetracyclines
This group of antibiotics is actively transported into bacterial cells, possibly as the
magnesium complex, achieving a 50-fold concentration inside the cells. Mammalian
cells do not take up the tetracyclines and it is this difference in uptake that determines
the selective toxicity. Resistance to the tetracyclines is usually associated with failure
of the active uptake system or with an active efflux pump which removes the drug
from the cells before it can interfere with ribosome function. Protein synthesis by both
bacterial and mammalian ribosomes is inhibited by the tetracyclines in cell-free systems.
The action is upon the smaller subunit. Binding of just one molecule of tetracycline
to the bacterial 30S subunit occurs at a site involving the 3' end of the 16S rRNA, a
number of associated ribosomal proteins and magnesium ions. The effect is to block
the binding of aminoacyl-tRNA to the A site of the ribosome and halt protein synthesis.
Tetracyclines are bacteriostatic rather than bacteriocidal, consequently they should not
be used in combination with j8-lactams, which require cells to be growing and dividing
to exert their lethal action.
Chloramphenicol
Of the four possible optical isomers of chloramphenicol, only the o-threo form is active.
This antibiotic selectively inhibits protein synthesis in bacterial ribosomes by binding
to the 50S subunit in the region of the A site involving the 23S rRNA. The normal
binding of the aminoacyl-tRNA in the A site is affected by chloramphenicol in such a
Mechanisms of action of antibiotics 171
way that the peptidyl transferase cannot form a new peptide bond with the growing
peptide chain on the tRNA in the P site. Studies with aminoacyl-tRNA fragments
containing truncated tRNA chains suggest that the shape of the region of tRNA closest
to the amino acid is distorted by chloramphenicol. The altered orientation of this region
of the aminoacyl-tRNA in the A site is sufficient to prevent peptide bond formation.
Chloramphenicol has a broad spectrum of activity which covers Gram-positive and
Gram-negative bacteria, mycoplasmas, rickettsia and chlamydia. It has the valuable
property of penetrating into mammalian cells and is therefore the drug of choice for
treatment of intracellular pathogens, including Salmonella typhi, the causative organism
of typhoid. Although it does not inhibit 80S ribosomes, the 70S ribosomes of mammalian
mitochondria are sensitive and therefore some inhibition occurs in rapidly growing
mammalian cells with high mitochondrial activity.
3.5 Macrolides and azalides
Erythromycin is a member of the macrolide group of antibiotics; it selectively inhibits
protein synthesis in a broad range of bacteria by binding to the 50S subunit. The site at
which it binds is close to that of chloramphenicol and involves the 23S rRNA. Resistance
to chloramphenicol and erythromycin can occur by methylation of different bases within
the same region of the 23 S rRNA. The sites are therefore not identical but binding of
one antibiotic prevents binding of the other. Unlike chloramphenicol, erythromycin
blocks translocation. This is the process by which the ribosome moves along the mRNA
by one codon after the peptidyl transferase reaction has joined the peptide chain to
the aminoacyl-tRNA in the A site. The peptidyl-tRNA is moved (translocated) to the P
site, vacating the A site for the next aminoacyl-tRNA. Energy is derived by hydrolysis
of GTP to GDP by an associated protein elongation factor, EF-G. By blocking the
translocation process, erythromycin causes release of incomplete polypeptides from
the ribosome. It is assumed that the azalides, such as azithromycin (Chapter 5), have a
similar action to the macrolides. The azalides have improved intracellular penetration
over the macrolides and are resistant to the metabolic conversion which reduces the
serum half life of erythromycin.
3.6 Lincomycin and clindamycin
These agents bind selectively to a region of the 50S ribosomal subunit close to that of
chloramphenicol and erythromycin. They block elongation of the peptide chain by
inhibition of peptidyl transferase.
3.7 Fusidic acid
This steroidal antibiotic does not act upon the ribosome itself, but upon one of the
associated elongation factors, EF-G. This factor supplies energy for translocation by
hydrolysis of GTP to GDP. Another elongation factor, EF-Tu promotes binding of
aminoacyl-tRNA molecules to the A site through binding and hydrolysis of GTP. Both
EF-G and EF-Tu have overlapping binding sites on the ribosome. Fusidic acid binds the
EF-G: GDP complex to the ribosome after one round of translocation has taken place.
172 Chapter 8
This prevents further incorporation of aminoacyl-tRNA by blocking the binding of
EF-Tu:GTP. Like the tetracyclines, fusidic acid owes its selective antimicrobial action
to active uptake by bacteria and exclusion from mammalian cells. The equivalent elonga-
tion factor in mammalian cells, EF-2 is susceptible to fusidic acid in cell-free systems.
Mupirocin
The target of mupirocin is one of a group of enzymes which couple amino acids to
their respective tRNAs for delivery to the ribosome and incorporation into protein. The
particular enzyme inhibited by mupirocin is involved in producing isoleucyl-tRNA.
The basis for the inhibition is a structural similarity between one end of the mupirocin
molecule and isoleucine. Protein synthesis is halted when the ribosome encounters the
isoleucine codon through depletion of the pool of isoleucyl-tRNA.
Chromosome function and replication
The basis for selective inhibition of chromosome replication and function
As with protein synthesis, the mechanisms of chromosome replication and function
are essentially the same in prokaryotes and eukaryotes. There are, however, important
differences in the detailed functioning and properties of the enzymes involved and
these differences are exploited by a number of agents as the basis of selective inhibition.
The microbial chromosome is large in comparison with the cell that contains it
(approximately 1000 times the length of E. coli). During replication the circular double
helix must be unwound to allow the DNA polymerase enzymes to synthesize new
complementary strands. The shape of the chromosome is manipulated by the cell
by the formation of regions of supercoiling. Positive supercoiling (coiling in the
same sense as the turns of the double helix) makes the chromosome more compact.
Negative supercoiling (generated by twisting the chromosome in the opposite sense to
the helix) produces localized strand separation which is required both for replication
and transcription. In a bacterium such as E. coli, four different topoisomerase enzymes
are responsible for maintaining the shape of DNA during cell division. They act by
cutting one or both of the DNA strands, they remove or generate supercoiling, then
reseal the strands. Their activity is essential for the microbial cell to relieve the complex
tangling of the chromosome (both knotting and chain link formation) which results
from progression of the replication fork around the circular chromosome. Type I
topoisomerases cut one strand of DNA and pass the other strand through the gap before
resealing. Type II enzymes cut both strands and pass another double helical section of
the DNA through the gap before resealing. In E. coli topoisomerase I and III are both
type I enzymes whilst topoisomerases II and IV are type II enzymes. Topoisomerase II
is also known as DNA gyrase and is the site of action of the quinolones.
The basic sequence of events for microbial chromosome replication is as follows.
Synthesis of precursors
Purines, pyrimidines and their nucleosides and nucleoside triphosphates are synthesized
Mechanisms of action of antibiotics 173
in the cytoplasm. At this stage the antifolate drugs (sulphonamides and dihydrofolate
reductase inhibitors) act by blocking the production of thymine. The antifungal agent
5-fluorocytosine interferes with these early stages of DNA synthesis. Through con-
version to 5-fluorouracil then to 5-fluorodeoxyuridylic acid (5-F-dUMP) it blocks
thymidylic acid production through inhibition of the enzyme thymidylate synthetase.
The antiviral nucleosides acycloguanosine (acyclovir) and iododeoxyuridine (idoxuridine)
are converted to their respective nucleoside triphosphates in the cytoplasm of infected
cells. They proceed to inhibit viral DNA replication either by inhibition of the DNA
polymerase or by incorporation into DNA with subsequent termination of chain
extension. Finally the anti-human immunodeficiency virus (HIV) drug azidothymidine
(AZT) acts in an analogous manner, being converted to the corresponding triphosphate
and inhibiting viral RNA synthesis by the HIV reverse transcriptase.
4.1.2 Unwinding of the chromosome
As described in section 4.1, the DNA double helix must unwind to allow access of the
polymerase enzymes to produce two new strands of DNA. This is facilitated by DNA
gyrase, the target of the quinolones. Some agents interfere with the unwinding of the
chromosome by physical obstruction. These include the acridine dyes, of which the
topical antiseptic proflavine is the most familiar, and the antimalarial acridine, mepacrine.
They prevent strand separation by insertion (intercalation) between base pairs from
each strand, but exhibit very poor selective toxicity.
4.1.3 Replication of DNA strands
The unwound DNA strands are kept unfolded during replication by binding a protein
called Albert's protein. A series of enzymes produce new strands of DNA using each of
the separated strands as templates. An RNA polymerase forms short primer strands of
RNA on each template strand at specific initiator sites. DNA polymerase III then
synthesizes and joins short DNA strands on to the RNA primers. These DNA strands
are called Okasaki fragments. DNA polymerase I (which possesses nucleotidase activity)
removes the RNA primers and replaces them with DNA strands. Finally a DNA ligase
joins together the DNA strands producing two daughter chromosomes. The entire process
is carefully regulated with proofreading stages to check that each nucleotide is correctly
incorporated as specified by the template sequence. There are no therapeutic agents yet
known which interfere directly with the DNA polymerases.
4.1.4 Transcription
The process of transcription, the copying of a single strand of mRNA sequence using
one strand of the chromosome as a template, is carried out by RNA polymerase. This is
a complex of four proteins (two a, one /3 and one /3'subunits) which make up the core
enzyme. Another small protein, the cfactor, joins the core enzyme, which binds to the
promoter region of the DNA preceding the gene which is to be transcribed. The correct
positioning and orientation of the polymerase is obtained by recognition of specific
marker sites on the DNA at positions -10 and -35 nucleotide bases before the initiation
174 Chapter 8
