Hugo W.B., Russel A.D.(ed). Pharmaceutical Microbiology
Подождите немного. Документ загружается.


Table 10.2 Antibacterial activity of commonly used disinfectants and antiseptics
Class of compound
Alcohols
Ethanol/isopropyl
Aldehydes
Glutaraldehyde
Formaldehyde
Biguanides
Chlorhexidine
Halogens
Hypochlorite/
chloramines
lodine/iodophor
Peroxygens
Peracetic acid
Hydrogen peroxide
Phenolics
Clear soluble fluids
Chloroxylenol
Bisphenols
Quaternary ammonium
compounds
Benzalkonium
Cetrimide
Activity against:
Bacterial
Mycobacteria spores
+ -
+ +
+ +
-
+ +
+ +
+ +
+
+ -
-
— —
-
-
General level*
of antibacterial activity
Intermediate
High
High
Intermediate
High
Intermediate, problems
with Ps. aeruginosa
High
Intermediate
High
Low
Low, poor against
Ps. aeruginosa
Intermediate
Intermediate
* Activity will depend on concentration, time of contact, temperature, etc. (see Chapter 11) but these
are activities expected if in-use concentrations were being employed.
resistant but this resistance is of much lesser magnitude than for bacterial spores.
The ability to rapidly destroy pathogenic fungi such as the opportunistic yeast,
Candida albicans, and filamentous fungi such as Trichophyton mentagrophytes, and
spores of common spoilage moulds such as Aspergillus niger, is put to advantage in
many applications of use. Many disinfectants have good activity against these fungi
(Table 10.3).
2.2.5
Viruses
Susceptibility of viruses to antimicrobial agents can depend on whether the viruses
possess a lipid envelope. Non-lipid viruses are frequently more resistant to disinfectants
and it is also likely that such viruses cannot be readily categorized with respect to their
sensitivities to antimicrobial agents. These viruses are responsible for many nosocomial
infections, e.g. rotaviruses, picornaviruses and adenoviruses (see Chapter 3), and it
may be necessary to select an antiseptic or disinfectant to suit specific circumstances.
Certain viruses, such as Ebola and Marburg which cause haemorrhagic fevers, are
highly infectious and their safe destruction by disinfectants is of paramount importance.
Chemical disinfectants, antiseptics and preservatives 205
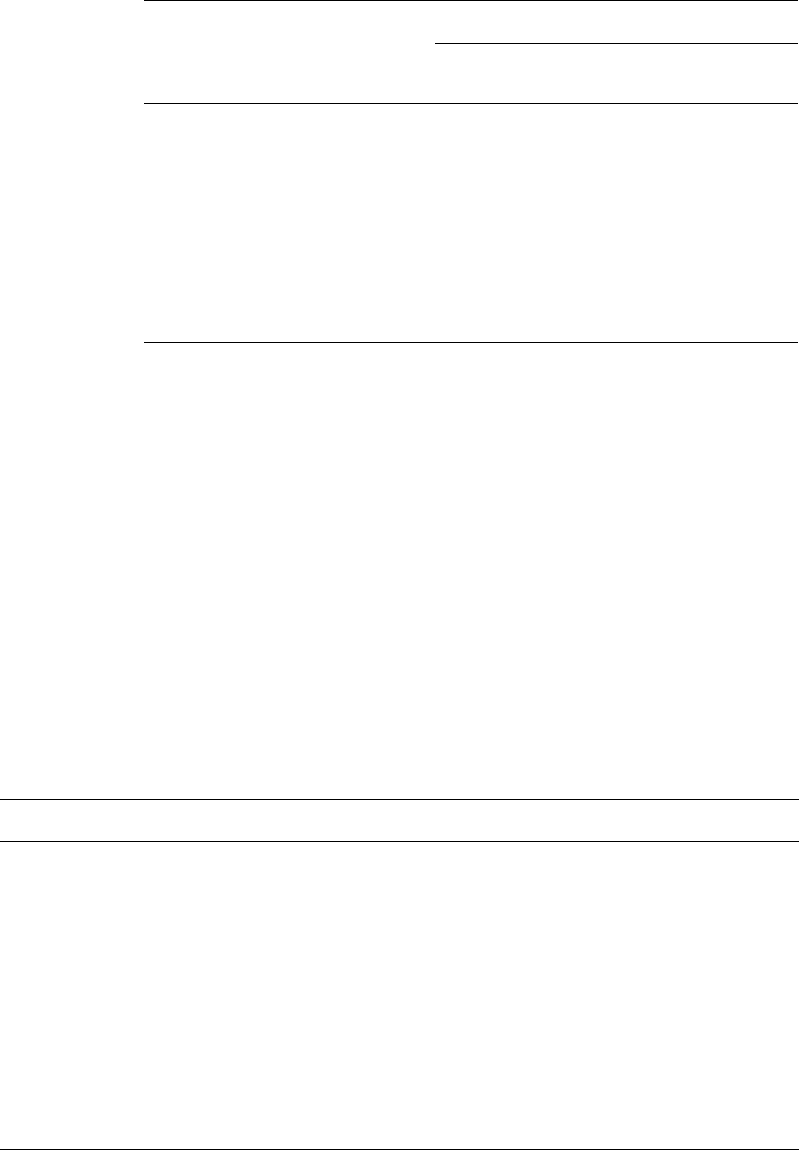
Table 10.3 Antifungal activity of disinfectants and antiseptics (adapted from Scott et al. 1986)
Antimicrobial agent
Phenolic (0.36%)
Chlorhexidine gluconate (0.02%,
alcoholic)
Iodine (1%, alcoholic)
Povidone-iodine (10%, alcoholic and
aqueous)
Hypochlorite (0.2%)
Cetrimide (1%)
Chlorhexidine gluconate (0.05%) +
cetrimide (0.5%)
Chlorhexidine gluconate (0.5%, aqueous)
Time (
imin) to
Aspergillus
niger
<2
<2
<2
10
10
<2
20
20
give >99.99% kill*
Trichophyton
of
mentagrophytes
<2
<2
<2
<2
<2
20
>20
>20
Candida
albicans
<2
<2
<2
<2
5
<2
>2
>2
* Initial viable counts were ca. 1 x 10
6
.
There is much concern for the safety of personnel handling articles contaminated
with pathogenic viruses such as hepatitis B virus (HBV) and human immunodeficiency
virus (HIV) which causes acquired immune deficiency syndrome (AIDS). Some
agents have been recommended for disinfection of HBV and HIV depending on the
circumstances and level of contamination; these are listed in Table 10.4. Disinfectants
must be able to treat rapidly and reliably accidental spills of blood, body fluids or
secretions from HIV infected patients. Such spills may contain levels of HIV as high as
10
4
infectious units/ml. Recent evidence from the Medical Devices Agency evaluation
of disinfectants against HIV indicated that few chemicals could destroy the virus in a
Table 10.4 Chemical disinfection of human immunodeficiency virus (HIV) and hepatitis B virus (HBV). Adapted from
ACDP (1990) and Anon (1991)
206 Chapter 10
Disinfecting agent
Chlorine-releasing preparations,
e.g. hypochlorite lOOOOppm av. Cl
2
,
at least 30min at room temperature
Hypochlorite 1000ppm av. Cl
2
Aldehydes, e.g. glutaraldehyde
2% (w/v), 30min at room
temperature
Alcohol 70% ethanol, at least 2min
for HIV but evaporation a
problem
Application
Spillage of HIV contaminated
blood and body fluid
Minor contamination of
inanimate surfaces
Reserved for non-corrosive
treatment of delicate items
Limited application
Comment
Use fresh solution
Deteriorates on storage and
may be adversely affected
by organic matter
Corrosive to metals
Bleaches fabrics
Must be freshly activated
Not recommended for surface
decontamination due to
vapour toxicity (see Table 10.5)
Use alternative if possible as
activity in presence of protein
questionable
short time in the presence of high serum levels; only two of 13 products (glutaraldehyde
and dichloroisocyanurate) were effective under the most stringent test conditions.
The virucidal activity of chemicals is difficult to determine in the laboratory. Tissue
culture techniques are the most common methods for growing and estimating viruses;
however, antimicrobial agents may also adversely affect the tissue culture: see also
Chapter 11.
2.2.6 Protozoa
Acanthamoeba spp. can cause acanthamoeba keratitis with associated corneal scarring
and loss of vision in soft contact lens wearers. The cysts of this protozoan, in particular,
present a problem in respect of lens disinfection. The chlorine-generating systems in
use are generally inadequate. Although polyhexamethylene biguanide shows promise
as an acanthamoebacide, only hydrogen peroxide-based disinfection is considered
completely reliable and consistent in producing an acanthomoebacidal effect.
2.2.7 Prions
Prions (small proteinaceous infectious particles, also known as unconventional slow
viruses) are a unique class of infectious agent associated with causing spongiform
encephalopathies such as bovine spongiform encephalopathy (BSE) in cattle and
Creutzfeldt Jakob disease (CJD) in humans. There is considerable concern about the
transmission of these agents from infected animals or patients. Risk of infectivity is
highest in brain and spinal cord tissues. There are still many unknown factors regarding
destruction of prions. It appears that they are resistant to most disinfectant procedures
and that autoclaving or exposure to 1N sodium hydroxide is required for decontamination.
However, current advice is to destroy surgical instruments where procedures involve
brain, spinal cord or eye in patients with confirmed or suspected CJD.
2.3 Intended application
The intended application of an antimicrobial agent, whether for preservation, antisepsis
or disinfection, will influence its selection and also affect its performance. For example,
in medicinal preparations the ingredients in the formulation may antagonize preservative
activity. The risk to the patient will depend on whether the antimicrobial is in close
contact with a break in the skin or mucous membranes or is introduced into a sterile
area of the body.
In disinfection of instruments, the chemicals used must not adversely affect the
instruments, e.g. cause corrosion of metals, affect clarity or integrity of lenses, or change
texture of synthetic polymers. Many materials such as fabrics, rubber, plastics are capable
of adsorbing certain disinfectants, e.g. quaternary ammonium compounds (QACs), are
adsorbed by fabrics, while phenolics are adsorbed by rubber, the consequence of this
being a reduction in concentration of active compound. A disinfectant can only exert
its effect if it is in contact with the item being treated. Therefore access to all parts of an
instrument or piece of equipment is essential. For small items, total immersion in the
disinfectant must also be ensured.
Chemical disinfectants, antiseptics and preservatives 207
2.4 Environmental factors
Organic matter can have a drastic effect on antimicrobial activity either by adsorption
or chemical inactivation, thus reducing the concentration of active agent in solution or
by acting as a barrier to the penetration of the disinfectant. Blood, body fluids, pus,
milk, food residues or colloidal proteins, even present in small amounts, all reduce the
effectiveness of antimicrobial agents to varying degrees and some are seriously affected.
In their normal habitats, microorganisms have a tendency to adhere to surfaces and
are thus less accessible to the chemical agent. Some organisms are specific to certain
environments and their destruction will be of paramount importance in the selection
of a suitable agent, e.g. Legionella in cooling towers and non-potable water supply
systems, Listeria in the dairy and food industry and hepatitis in blood-contaminated
articles.
Dried organic deposits may inhibit penetration of the chemical agent. Where
possible, objects to be disinfected should be thoroughly cleaned. The presence of ions
in water can also affect activity of antimicrobial agents, thus water for testing biocidal
activity can be made artificially 'hard' by addition of ions.
These factors can have very significant effects on activity and are summarized in
Table 10.5.
2.5 Toxicity of the agent
In choosing an antimicrobial agent for a particular application some consideration must
be given to its toxicity. Increasing concern for health and safety is reflected in the
Control of Substances Hazardous to Health (COSHH) Regulations which specify the
precautions required in handling toxic or potentially toxic agents. In respect of
disinfectant use these regulations affect, particularly, the use of phenolics, formaldehyde
and glutaraldehyde. Toxic volatile substances, in general, should be kept in covered
containers to reduce the level of exposure to irritant vapour and they should be used
with an extractor facility. Limits governing the exposure of individuals to such substances
are now listed, e.g. 0.7mg/m
3
(0.2 ppm) glutaraldehyde for both short- and long-term
exposure. The aldehydes, glutaraldehyde less so than formaldehyde, may affect the
eyes and skin (causing contact dermatitis), and may induce respiratory distress. Face
protection and impermeable nitrile rubber gloves should be worn when using these
agents. Table 10.5 lists the toxicity of many of the disinfectants in use and other concerns
of toxicity are described for individual agents below.
Where the atmosphere of a workplace is likely to be contaminated, sampling and
analysis of the atmosphere may need to be carried out on a periodic basis with a frequency
determined by conditions.
3 Types of compound
The following section presents in alphabetical order by chemical grouping the agents
most often employed for disinfection, antisepsis and preservation. This information is
summarized in Table 10.6.
208 Chapter 10

Table 10.5 Properties of commonly used disinfectants and antiseptics
Class of
compound
Alcohols
Ethanol
Aldehydes
Glutaraldehyde
Biguanides
Chlorhexidine
Chlorine
compounds
Hypochlorite
Iodine
preparations
lodophors
Phenolics
Clear soluble
fluids
Black/white
fluids
Chloroxylenol
QACs
Cetrimide and
benzalkonium
Chloride
Effect of
organic
matter
Slight
Slight
Severe
Severe
Severe
Slight
Moderate/
severe
Severe
Severe
pH optimum
pH8
pH7-8
Acid/neutral
pH
Acid pH
Acid pH
Alkaline pH
Toxicity and
OES*
Avoid broken
skin, eyes
OES: 1000ppm/1900
mgrrr
3
, 8h only
Respiratory complaints and
contact dermatitis reported
Eyes, sensitivity
OES: 0.2 ppm/0.7 mg rrr
3
,
10min only
Avoid contact with eyes and
mucous membranes
Sensitivity may develop
Irritation of skin, eyes and
lungs
OES: 1ppm/3mgirr
3
,
10min; 0.5ppm/1.5mgm"
3
,
8h
Eye irritation
OES:0.1ppm/1mg
m~
3
, 10min only
Protect skin and eyes
Very irritant
Sensitivity. May
irritate skin
OES: 10ppm/38 mgrrr
3
,
10min; 5ppm/19mgnrr
3
, 8h
Avoid contact with eyes
Other factors
Poor penetration,
good cleansing
properties
Non-corrosive,
useful for heat
sensitive
instruments
Incompatible with
soap and anionic
detergents
Inactivated by hard
water, some
materials and plastic
Corrosive to metals
May corrode
metals
Adsorbed by
rubber/plastic
Greatly reduced by
dilution
Adsorbed by
rubber/plastic
Incompatible with
soap and anionic
detergents
Adsorbed by fabrics
* From the Control of Substances Hazardous to Health (COSHH) Regulations (1988).
OES, occupational exposure standard; QAC, quaternary ammonium compound.
Chemical disinfectants, antiseptics and preservatives 209

Table 10.6 Examples of the
Antimicrobial agent
Acids and esters.
e.g. benzoic acid,
parabens
Alcohols,
e.g. ethyl or isopropyl
Aldehydes,
e.g. glutaraldehyde
Biguanides,
e.g. chlorhexidinef
(gluconate, acetate etc)
Chlorine,
e.g. hypoclorite
Hydrogen peroxide
main antimicrobial
groups as antiseptics, disinfectants and preservatives
Antiseptic activity
Concentration
50-90%
in water
10%
0.02%
0.2%
0.5% (in 70%
alcohol)
1.0%
4.0%
<0.5%
avCI
2
1.5%
3-6%
Typical formulation/
application
Skin prep.
Gel for warts
Bladder irrigation
Mouthwash
Skin prep.
Dusting powder,
cream dental gel
Pre-op. scrub in
surfactant
Solution for skin and
wounds
Stabilized cream
Solution for wounds
and ulcers, mouthwash
Disinfectant Activity
Concentration
50-90%
in water
2.0%
0.05%
0.5% (in 70%
alcohol)
1-10%
3.0%
Typical formulation/
application
Clean surface prep.,
thermometers
Solution for instruments
Storage of instruments,
clean instrument
disinfection (30min)
Emergency instrument
disinfection (2min)
Solution for surfaces and
instruments
Disinfection of soft contact
lenses
Preservative activity
Concentration
0.05-0.1%
0.25%
0.0025%
0.01%
Typical formulation/
application
For oral and topical
formulations
Solution for hard
contact lenses
Eye-drops

* Also used in combination with other agents e.g. chlorhexidine, iodine.
t Several forms available having x% chlorhexidine and 10x% cetrimide.
QAC, quaternary ammonium compound.
Iodine compounds,
e.g. free iodine,
povidone-iodine
Phenolics,
e.g. tar acids (clear
soluble phenolics),
non-coal tar
(chloroxylenol),
bisphenol (triclosan)
QACs,
e.g. cetyltrimethyl
ammonium
bromide (cetrimide)
1.0%
1.0%
2.5%
7.5%
10%
0.5%
1.3%
2.0%
0.1%
0.5%
1.0%
Aqueous or alcoholic
(70%) solution
Mouthwash
Dry powder spray
Scalp and skin
cleanser
Pre-op. scrub, fabric
dressing
Dusting powder
Solution
Skin cleanser
Solution for wounds
and burns
Cream
Skin solution
10.0%
1-2%
0.1%
1.0%
Aqueous or ale. solution
Solution
Storage or sterile
instruments
Instruments (1 h)
0.01%
Eye-drops
3.1
Acids and esters
Antimicrobial activity, within a pharmaceutical context, is generally found only in the
organic acids. These are weak acids and will therefore dissociate incompletely to give
the three entities HA, H
+
and A" in solution. As the undissociated form, HA, is the
active antimicrobial agent, the ionization constant, K
a
, is important and the pK
a
of the
acid must be considered especially in formulation of the agent.
3.1.1 Benzoic acid
This is an organic acid, C
6
H
5
COOH, which is included, alone or in combination with
other preservatives, in many pharmaceuticals. Although the compound is often used as
the sodium salt, the non-ionized acid is the active substance. A limitation on its use is
imposed by the pH of the final product as the pK
a
of benzoic acid is 4.2 at which pH
50% of the acid is ionized. It is advisable to limit use of the acid to preservation of
pharmaceuticals having a maximum final pH of 5.0 and if possible less than 4.0.
Concentrations of 0.05-0.1 % are suitable for oral preparations. A disadvantage of the
compound is the development of resistance by some organisms, involving in some
cases metabolism of the acid resulting in complete loss of activity. Benzoic acid also
has some use in combination with other agents, salicylic acid among others, in the
treatment of superficial fungal infections.
3.1.2 Sorbic acid
This compound is a widely used preservative as the acid or its potassium salt. The pK
a
is 4.8 and, as with benzoic acid, activity decreases with increasing pH and ionization.
It is most effective at pH 4 or below. Pharmaceutical products such as gums, mucilages
and syrups are usefully preserved with this agent.
3.1.3 Sulphur dioxide, sulphites and metabisulphites
Sulphur dioxide has extensive use as a preservative in the food and beverage industries.
In a pharmaceutical context, sodium sulphite and metabisulphite or bisulphite have a
dual role acting as preservatives and antioxidants.
3.1.4 Esters of p-hydroxybenzoic acid (parabens)
A series of alkyl esters (Fig. 10.1) of/?(4)-hydroxybenzoic acid was originally prepared
to overcome the marked pH-dependence on activity of the acids.
These parabens, the methyl, ethyl, propyl and butyl esters, are less readily ionized
having pK
a
values in the range 8-8.5 and exhibit good preservative activity even at pH
212 Chapter 10
Fig. 10.1 p-Hydroxybenzoates (R is methyl, ethyl, propyl, butyl
or benzyl).
levels of 7-8, although optimum activity is again displayed in acidic solutions. This
broader pH range allows extensive and successful use of the parabens as pharmaceutical
preservatives. The agents are active against a wide range of fungi but are less active
against bacteria, especially the pseudomonads which may utilize the parabens as a
carbon source. They are frequently used as preservatives of emulsions, creams and
lotions where two phases exist. Combinations of esters are most successful for this
type of product in that the more water-soluble methyl ester (0.25%) protects the aqueous
phase whereas the propyl or butyl esters (0.02%) give protection to the oil phase. Such
combinations are also considered to extend the range of activity. As inactivation of
parabens occurs with non-ionic surfactants, due care should be taken in formulation
of these.
3.2 Alcohols
3.2.1 Alcohols used for disinfection and antisepsis
The aliphatic alcohols, notably ethanol and isopropanol, which are used for disinfection
and antisepsis, are bactericidal against vegetative forms, including Mycobacterium spp.,
but are not sporicidal. Alcohols have poor penetration of organic matter and their use is
therefore restricted to clean conditions. They possess properties such as a cleansing
action and volatility, are able to achieve a rapid and large reduction in skin flora and
have been widely used for skin preparation prior to injection or other surgical procedures.
However, the contact time of an alcohol-soaked swab with the skin prior to venepuncture
is so brief that it is thought to be of doubtful value.
Ethanol (CH
3
CH
2
OH) is widely used as a disinfectant and antiseptic. The presence
of water is essential for activity, hence 100% ethanol is ineffective. Concentrations
between 60 and 95% are bactericidal but a 70% solution is usually employed for the
disinfection of skin, clean instruments or surfaces. At higher concentrations, e.g. 90%,
ethanol is also active against most viruses, including HIV. Ethanol is also a popular
choice in pharmaceutical preparations and cosmetic products as a solvent and
preservative.
Isopropyl alcohol (isopropanol, CH
3
CHOH.CH
3
) has slightly greater bactericidal
activity than that of ethanol but is also about twice as toxic. It is less active against
viruses, particularly non-enveloped viruses, and should be considered a limited-spectrum
virucide. Used at concentrations of 60-70%, it is an acceptable alternative to ethanol
for preoperative skin treatment and is also employed as a preservative for cosmetics.
3.2.2 Alcohols as preservatives
The aralkyl alcohols and more highly substituted aliphatic alcohols (Fig. 10.2) are
used mostly as preservatives. These include:
1 Benzyl alcohol (C
6
H
5
CH
2
OH). This has antibacterial and weak local anaesthetic
properties and is used as an antimicrobial preservative at a concentration of 2%, although
its use in cosmetics is restricted.
2 Chlorbutol (trichlorobutanol; trichloro-r-butanol; trichlorobutanol). Typical in-use
concentration: 0.5%. It has been used as a preservative in injections and eyedrops. It is
Chemical disinfectants, antiseptics and preservatives 213
214 Chapter 10
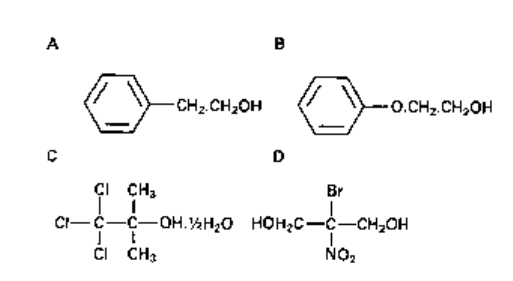
Fig. 10.2 Structural formulae of alcohols used in preserving and disinfection: A, 2-phenylethanol;
B, 2-phenoxyethanol; C, chlorbutol (trichlonw-butanol); D, Bronopol (2-bromo-2-nitropropan-l,3-
diol).
unstable, decomposition occurring at acid pH during autoclaving, while alkaline
solutions are unstable at room temperature.
3 Phenylethanol (phenylethy 1 alcohol; 2-phenylethanol). Typical in-use concentration:
0.25-0.5%. It is reported to have greater activity against Gram-negative organisms and
is usually employed in conjunction with another agent.
4 Phenoxyethanol (2-phenoxyethanol). Typical in-use concentration: 1%. It is more
active against Ps. aeruginosa than against other bacteria and is usually combined
with other preservatives such as the hydroxybenzoates to broaden the spectrum of
antimicrobial activity.
5 Bronopol (2-bromo-2-nitropropano-l,3-diol). Typical in-use concentration: 0.01-
0.1%. It has a broad spectrum of antibacterial activity, including activity against
Pseudomonas spp. The main limitation on the use of bronopol is that when exposed to
light at alkaline pH, especially if accompanied by an increase in temperature, solutions
decompose, turning yellow or brown. A number of decomposition products including
formaldehyde are produced. In addition, nitrite ions may be produced and react with
any secondary and tertiary amines present forming nitrosamines, which are potentially
carcinogenic.
Aldehydes
A number of aldehydes possess antimicrobial properties, including sporicidal activity;
however, only two, formaldehyde and glutaraldehyde, are used for disinfection. Both
these aldehydes are highly effective biocides and their use as 'chemosterilants' reflect
this.
Glutaraldehyde
Glutaraldehyde (CHO(CH
2
)
3
CHO) has a broad spectrum of antimicrobial activity and
rapid rate of kill, most vegetative bacteria being killed within a minute of exposure,
although bacterial spores may require 3 hours or more. The latter depends on the
intrinsic resistance of spores which may vary widely. It has the further advantage
of not being affected significantly by organic matter. The glutaraldehyde molecule
