Hillert M. Phase Equilibria, Phase Diagrams and Phase Transformations: Their Thermodynamic Basis
Подождите немного. Документ загружается.


5.3 Alternative methods of transformation 85
through a system where a homogeneous molecular reaction takes place, suggesting that
the two processes could not be coupled. It is an interesting question whether one would
always find that there is no coupling if one could really identify the actual processes
on the microscale. If that is the case, then the reciprocal relation should always be a
mathematical consequence of a different choice of processes.
Exercise 5.3
Show how Eq. (5.21) can be derived.
Hint
Start with Eq. (5.16a) and insert in turn Eqs (5.15a) and (5.18).
Solution
J
∗
s
=
i
α
si
J
i
=
i
α
si
k
L
ik
X
k
=
i
α
si
k
L
ik
r
α
rk
X
∗
r
;
L
∗
sr
=
i
α
si
k
L
ik
α
rk
=
i
k
α
si
α
rk
L
ik
.
5.3 Alternative methods of transformation
So far, we have introduced a new set of processes by expressing the new fluxes as linear
combinations of the initial ones. One could just as well express the new forces as linear
combinations of the initial forces
X
∗
1
= β
11
X
1
+ β
12
X
2
(5.26a)
X
∗
2
= β
21
X
1
+ β
22
X
2
. (5.26b)
The initial processes are still defined by Eqs (5.15)but we shall invert them to make
them consistent with the new way of defining the new processes
X
1
= R
11
J
1
+ R
12
J
2
(5.27a)
X
2
= R
21
J
1
+ R
22
J
2
. (5.27b)
The R coefficients can be obtained from the L coefficients in Eqs (5.15)bystandard
methods. With a similar procedure as before, one now obtains
X
∗
s
=
r
R
∗
sr
J
∗
r
(5.28)
R
∗
sr
=
i
k
β
si
β
rk
R
ik
. (5.29)
The L
∗
coefficients can be evaluated by inverting Eq. (5.28).
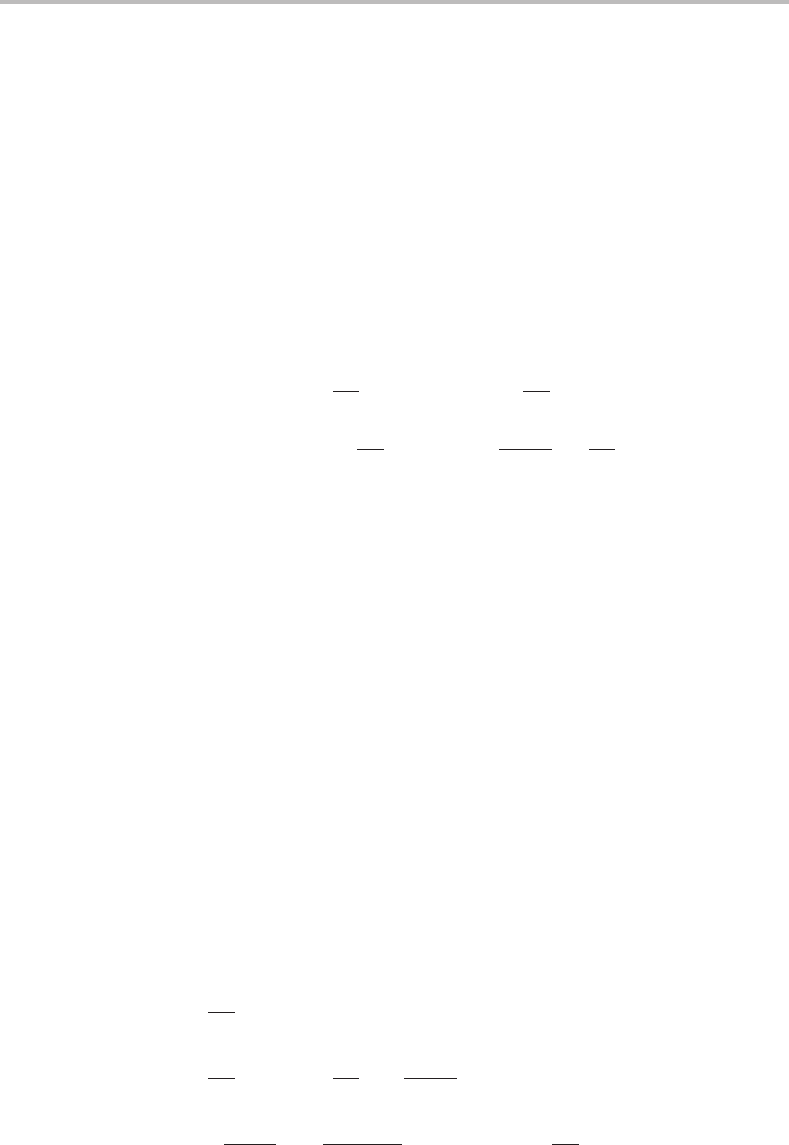
86 Thermodynamics of processes
One may also like to prescribe both flux and force for one process and not make any
prescription for the other process
J
∗
1
= α
11
J
1
+ α
12
J
2
(5.30)
X
∗
1
= β
11
X
1
+ β
12
X
2
. (5.31)
One should then express J
∗
2
and X
∗
2
using the four coefficients defined by these equations.
In general, a complete set of β coefficients can be expressed in terms of the α coefficients
by inverting Eqs (5.18)
α
11
/β
22
=−α
12
/β
21
=−α
21
/β
12
= α
22
/β
11
= α
11
α
22
− α
12
α
21
. (5.32)
For the second process one could thus write
J
∗
2
= α
21
J
1
+ α
22
J
2
= α
22
α
21
α
22
J
1
+ J
2
= α
22
−
β
12
β
11
J
1
+ J
2
(5.33)
X
∗
2
= β
21
X
1
+ β
22
X
2
= β
22
−
α
12
α
11
X
1
+ X
2
=
α
11
β
11
α
22
−
β
12
β
11
X
1
+ X
2
. (5.34)
In these expressions only α
22
was not given by Eqs (5.30) and (5.31). However, α
22
is
eliminated in the product J
∗
2
X
∗
2
and, as in the discussion following Eq. (5.23), it may
thus be regarded as a trivial factor for the second new process. It may thus be concluded
that in order for a new process, defined by its flux and force, to be part of a new set of
conjugate processes it must be combined with a unique partner defined by Eqs (5.33)
and (5.34).
Finally, it may happen that for some particular reason one would like to prescribe the
flux for a new process but the force for the other process.
X
∗
1
= β
11
X
1
+ β
12
X
2
(5.35)
J
∗
2
= α
21
J
1
+ α
22
J
2
. (5.36)
Again, the α and β coefficients are related by Eq. (5.32)but this time only three of the
coefficients given by the initial equations are independent. Let α
21
be the dependent one.
Eq. (5.32) yields
α
21
=−α
22
β
12
/β
11
. (5.37)
This must be satisfied when the initial equations, Eqs (5.35) and (5.36) are formulated.
As a compensation, there is a degree of freedom when evaluating the remaining four
coefficients. Lets us choose α
11
as the arbitrary parameter. Eq. (5.32)would then yield
β
22
=
α
11
α
22
β
11
(5.38)
α
12
=
1
α
21
α
11
α
22
−
α
22
β
11
=
α
22
α
21
β
11
(
α
11
β
11
− 1
)
(5.39)
β
21
=−
α
12
β
11
α
22
=−
α
22
β
11
α
21
β
11
α
22
(
α
11
β
11
− 1
)
=−
1
α
21
(
α
11
β
11
− 1
)
. (5.40)
An application of this kind of transformation will be given in Section 17.5.

5.3 Alternative methods of transformation 87
So far, we have always transformed the initial set of processes into the same number
of new processes. However, there are cases where the new set will contain one process
more or one less. When transforming the phenomenological equations for diffusion in
order to change the frame of reference, one often introduces a set with one process less.
That case will be discussed in Section 5.8.Acase with an increase of the number of
processes will now be described.
It may happen that one describes the development of a system with a set of processes
that one is convinced are those that actually take place on a microscale. Nevertheless,
experimental measurements have yielded cross coefficients. There is thus a coupling
between the processes but it is possible that it may be caused by an additional process
that was not considered primarily because it was formally possible to represent the exper-
imental information without using it. By introducing the phenomenological equation for
that process into the representation of experimental information, it is possible that the
cross coefficients decrease in value or even become negligible. If several additional pro-
cesses seem possible, one may decide to use the one giving the lowest cross coefficients.
That one would then be regarded as the coupling process.
Suppose one has studied two processes finding that they are coupled as described by
L
12
= L
21
= 0intheir phenomenological equations,
J
1
= L
11
X
1
+ L
12
X
2
(5.41a)
J
2
= L
21
X
1
+ L
22
X
2
. (5.41b)
With these equations one has thus been able to give an adequate description of how the
system develops. However, one is convinced that the two processes actually occur in
the system and feels that they should be independent of each other if it were not for
the presence of a third process that is responsible for the coupling. Assuming that there
should be no cross terms if the system is represented by all three processes one would
write
J
∗
1
= L
∗
1
X
∗
1
(5.42a)
J
∗
2
= L
∗
2
X
∗
2
(5.42b)
J
∗
3
= L
∗
3
X
∗
3
. (5.42c)
It should be noticed that it is necessary to redefine the fluxes of the two initial processes
when the third one is introduced even though those processes are the same as before and
should still have the same forces.
X
∗
1
= X
1
(5.43a)
X
∗
2
= X
2
. (5.43b)
Of course, there should be some relation between the third process and the initial ones
because an adequate description of the system can be given already by the initial ones.
Suppose the relation is
X
∗
3
= kX
1
+lX
2
= kX
∗
1
+lX
∗
2
. (5.44)

88 Thermodynamics of processes
The entropy production must be the same in both descriptions. By eliminating X
∗
3
one
obtains
σ = J
∗
1
X
∗
1
+ J
∗
2
X
∗
2
+ J
∗
3
X
∗
3
= (J
∗
1
+ kJ
∗
3
)X
∗
1
+ (J
∗
2
+lJ
∗
3
)X
∗
2
= J
1
X
1
+ J
2
X
2
. (5.45)
By inserting Eqs (5.43) one can identify the relations between the two sets of processes
by comparing terms.
J
1
= J
∗
1
+ kJ
∗
3
(5.46a)
J
2
= J
∗
2
+lJ
∗
3
. (5.46b)
Introducing the phenomenological equations for the three new processes from Eqs (5.42)
one obtains,
J
1
= J
∗
1
+ kJ
∗
3
= L
∗
1
X
∗
1
+ kL
∗
3
(kX
∗
1
+lX
∗
2
) = (L
∗
1
+ k
2
L
∗
3
)X
1
+ klL
∗
3
X
2
. (5.47a)
J
2
= J
∗
2
+lJ
∗
3
= L
∗
2
X
∗
2
+lL
∗
3
(kX
∗
1
+lX
∗
2
) = klL
∗
3
X
1
+ (L
∗
2
+l
2
L
∗
3
)X
2
. (5.47b)
It is satisfactory to see that the reciprocal relation is obeyed. Comparison with Eqs (5.41)
yields
L
11
= L
∗
1
+ k
2
L
∗
3
; L
12
= klL
∗
3
= L
21
; L
22
= L
∗
2
+l
2
L
∗
3
(5.48)
or inverted,
L
∗
3
= L
12
/kl = L
21
/kl (5.49a)
L
∗
2
= L
22
−l
2
L
12
/kl = L
22
− L
12
l/k (5.49b)
L
∗
1
= L
11
− k
2
L
12
/kl = L
11
− L
12
k/l. (5.49c)
The introduction of a third, coupling process is thus another way of describing the
development of the system without any cross terms. The advantage of this method is that
the initial processes will be part of the final description. That may be desirable if they
have a strong physical basis.
The fact that it is always possible to introduce a set of processes without cross terms
may be of theoretical interest even without trying to identify their physical background,
as demonstrated by the following example. It may seem self-evident that there could be
no spontaneous processes in a system without any entropy being produced. σ = 0 for
the whole system should thus be a condition of equilibrium for the whole system and for
all parts of it. However, it has been argued that one process having σ
j
> 0 and another
one having σ
k
< 0 could together yield σ = 0. On the other hand, the fact that one can
always describe what happens in the system with a set of processes without coupling
proves that there is no basis for that argument. Each one of those processes will only
be driven by its own thermodynamic force because all cross coefficients are zero. All
those processes will have to give positive contributions to the entropy production if they
progress. One could thus apply the second law to the individual processes if they are

5.4 Basic thermodynamic considerations for processes 89
not coupled. One may conclude that there can be no spontaneous changes in a system if
σ = 0 for the whole system.
Exercise 5.4
In an attempt to eliminate the cross terms in two processes one introduces a third process
by requiring that (1) J
1
= J
∗
1
+ J
∗
3
and (2) J
2
= J
∗
2
− J
∗
3
.Evaluate the L* coefficients
if this is possible.
Hint
Start by finding the condition for preserving the rate of entropy production.
Solution
σ = J
∗
1
X
∗
1
+ J
∗
2
X
∗
2
+ J
∗
3
X
∗
3
= J
1
X
1
+ J
2
X
2
= (J
∗
1
+ J
∗
3
)X
1
+(J
∗
2
− J
∗
3
)X
2
= J
∗
1
X
1
+
J
∗
2
X
2
+ J
∗
3
(X
1
− X
2
), which yields X
∗
1
= X
1
; X
∗
2
= X
2
; X
∗
3
= X
1
− X
2
.
Insert Eqs (5.42)inEq. (1) or (2): J
1
= J
∗
1
+ J
∗
3
= L
∗
1
X
∗
1
+ L
∗
3
X
∗
3
= L
∗
1
X
1
+
L
∗
3
(X
1
− X
2
) = (L
∗
1
+ L
∗
3
)X
1
− L
∗
3
X
2
; J
2
= J
∗
2
− J
∗
3
= L
∗
2
X
∗
2
− L
∗
3
X
∗
3
= L
∗
2
X
2
−
L
∗
3
(X
1
− X
2
) =−L
∗
3
X
1
+ (L
∗
2
+ L
∗
3
)X
2
.
Comparison with Eqs (5.41) yields L
11
= L
∗
1
+ L
∗
3
; L
12
=−L
∗
3
= L
21
;
L
22
= L
∗
2
+ L
∗
3
or inverted: L
∗
3
=−L
12
=−L
21
; L
∗
2
= L
22
− L
∗
3
= L
22
+ L
12
;
L
∗
1
= L
11
− L
∗
3
= L
11
+ L
12
.
5.4 Basic thermodynamic considerations for processes
In order to apply thermodynamics to the kinetics of processes, one must be aware of some
fundamental principles and new assumptions must be made. For inhomogeneous systems
the basic extensive quantities, N
i
, U, V and S are obtained by integration of the local
value of the corresponding intensive property, usually expressed by the molar quantity.
It is thus necessary to assume that one can define the local value of those quantities. The
molar quantity is not just an average over a larger system. It is an intensive quantity and
depends on the local values of T, P and composition and also on the arrangement of the
atoms. However, it may also depend on the gradient of those quantities. There are no
gradient effects in a homogeneous system and in the present work they will be neglected
for inhomogeneous systems unless specifically stated. An exception is the treatment
of a phenomenon called spinodal decomposition for which important restrictions are
described by including the effect of composition gradients on the Gibbs energy. See
Section 15.4.
N
i
, U and V obey the law of additivity and their values in a system are conserved
quantities in the sense that they can only change by interaction with the surroundings.
Their values for the whole system are thus conserved if there is no exchange of heat, work
or volume with the surroundings. The entropy, S, can change by internal processes but

90 Thermodynamics of processes
S −
ip
S is a conserved quantity if
ip
S is defined as the internally produced entropy.
Other extensive quantities are derived from the primary ones by adding or subtracting
terms containing P, T and µ
i
, e.g. the terms PV and TS. The law of additivity applies to
such quantities only under special precautions as discussed in Section 3.4.
Finally, it should be noted that, when applying thermodynamics to systems with inter-
nal processes, one assumes that thermodynamic properties, evaluated under equilibrium
or frozen-in conditions, apply not only to stable and metastable systems but also to unsta-
ble systems undergoing changes. In the present work that assumption will be applied as an
approximation without further discussion. We shall for instance evaluate thermodynamic
properties by assuming that any momentary situation is frozen-in.
Most of the applications in this chapter are based on the combined law in the form
dS = (1/T )dU + (P/T )dV − (µ
i
/T )dN
i
+ d
ip
S, (5.50)
where d
ip
S is the entropy production which must be positive for a system undergoing
spontaneous changes. We shall first consider cases where d
ip
S is caused by homogeneous
processes that will not disturb the uniformity of a system which is uniform from the
beginning. Transport processes require a quite different approach. They concern quanti-
ties that can be exchanged with the surroundings and in the limit they could sometimes
establish a stationary state of flow through the system. Even though such processes may
concern quantities already present in the combined law, it should be realized that in the
combined law they represent direct exchanges with the surroundings and not processes
of flow inside the system. The discussion of transport processes will thus be preceded
by considering a discontinuous system composed of two subsystems and with transport
between them.
Starting with processes in homogeneous systems we shall presume that all thermo-
dynamic properties are uniform. Internal processes may tend to change some properties
but the processes must progress uniformly in the whole system in order not to change the
homogeneous character. In a system that is not completely isolated there may be compli-
cations, e.g. due to the heat of reaction leaking out to the surroundings and causing heat
flow from the interior of the system to its surface. In a system open for heat transfer to
a reservoir of constant temperature it is common to assume that the temperature is kept
constant although there must be temperature gradients in order for the heat of reaction
to leave the system. Evidently, one assumes that those gradients and the corresponding
thermodynamic forces for the heat flow are so small that their production of entropy is
negligible. That will now be our assumption.
It may often be more convenient to use the combined law in the form based on Gibbs
energy if T and P are kept constant. By further assuming that there is no exchange of
matter with the surroundings we get for spontaneous changes of the state,
dG = V dP − SdT + µ
i
dN
i
− T d
ip
S =−T d
ip
S =− D
j
dξ
j
< 0. (5.51)
D
j
is the driving force for process j.Itisdefined as a generalization of the driving force,
D,inSection 1.8.
D
j
≡ T
∂
ip
S
∂ξ
j
=−
∂G
∂ξ
j
. (5.52)

5.4 Basic thermodynamic considerations for processes 91
Introducing the flux of process j, J
j
= dξ
j
/dt,weget for the time derivative
−
˙
G = T
d
ip
S
dt
= T σ = D
j
J
j
> 0. (5.53)
The rate of entropy production, σ ,was defined by Eq. (5.3) and the second law requires
that it is positive. That explains why dG in Eq. (5.51)was stated as negative. It should
be emphasized that the driving force is only defined for constant T and is then related to
the more generally applicable thermodynamic force X
j
by
D
j
= TX
j
. (5.54)
That is shown by comparison with Eq. (5.7). The term driving force for D
j
is here used
in an attempt to avoid confusion with the thermodynamic force, X
j
.
The internal production of entropy, σ ,isawell defined quantity and T σ is regarded
as the dissipation of Gibbs energy. It is thus connected to the internal processes. On the
other hand, −
˙
G in Eq. (5.53) describes the change of the properties of the system with
no regard to how the change occurred. The equality of the two may seem self-evident
but is extremely useful and may be illustrated in diagrams of the molar Gibbs energy
versus molar content. Then it is necessary to express both the dissipation and the change
of Gibbs energy in the same dimensions as the diagram, i.e. J/mol. Equation (5.53)is
expressed in J/s and it would thus be necessary to divide the whole equation by some
flux, J
o
, measured as mol/s. One would then obtain the change of Gibbs energy per mole
of the flux,
− G
m
=−
˙
G/J
o
= f
j
D
j
, (5.55)
where f
j
≡ J
j
/J
o
.For simple case with just one process, J
o
will normally be defined as
the flux of that process and −G
m
will be equal to D
j
. One can then evaluate the driving
force D
j
from −G
m
and it is even common to regard it as the driving force itself. For
more complicated cases one may even regard it as the total driving force. However, it
should be remembered that −G
m
is the change of the properties of the system and D
j
or f
j
D
j
represents dissipation.
There are many kinds of homogeneous processes, some of which can be described as
a change of order, e.g., short-range order of the atoms relative to each other and even the
transition to a state of long-range order if it occurs by a so-called second-order transition,
which is homogeneous. An extreme case of order among the atoms is the formation of
molecules by so-called chemical reactions. They will be treated separately in the next
section.
Exercise 5.5
Suppose T and V are kept constant. What form of the combined law should it then be
natural to use? How would the time derivative of that characteristic state function be
related to the rate of entropy production if there is no exchange of matter?

92 Thermodynamics of processes
Solution
Using Helmholtz energy, the combined law is written as dF =−V dP − SdT +
µ
i
dN
i
− T d
ip
S =−T d
ip
S =− D
j
dξ
j
< 0. The time derivative of Helmholtz
energy is the same as of Gibbs energy but the conditions are different −
˙
F = T d
ip
S/dt =
T σ = D
j
J
j
> 0.
5.5 Homogeneous chemical reactions
The combined law for dG contains the terms µ
j
dN
j
where the summation includes
a set of c components. Each one is regarded as independent if it cannot be formed by a
reaction between the other ones. Basically, the set should only contain the c independent
components. For homogeneous chemical reactions one sometimes includes more com-
ponents. One may then choose which ones should be regarded as independent. The other
ones will be regarded as dependent and each one of them can be formed by a reaction
between the independent ones. Those reactions are regarded as independent reactions
but many more reactions could occur between the components. If one considers s com-
ponents altogether, there will be (s − c) dependent components and the same number of
independent reactions. That number will be represented by r = s − c.
A component could be a pure element but could also be a molecule or some hypo-
thetical aggregate of atoms. Let us express the chemical composition of component j by
the letter J and write
J =
c
i=1
a
j
i
I , (5.56)
where a
j
i
represents the stoichiometric coefficients and I represents a pure element
or the composition of any other basic unit used for representing the composition of
the components. Let these units be the independent components and J be a dependent
component. It is evident that Eq. (5.56) should then be the reaction formula for the
formation of J if it is turned around.
c
i=1
a
j
i
I = J. (5.57)
We could thus regard the a
j
i
coefficients as the reaction coefficients for the reaction
between J and the c independent components. They have here been normalized by
making the coefficient for J equal to 1. There will be such a reaction for each dependent
component, i.e. r reactions. They may be chosen as the r independent reactions among
the many more possible reactions between the s components.
One reason to include dependent components in µ
j
dN
j
is that it may facilitate
the modelling of the thermodynamic properties, e.g. for a gas containing several kinds
of molecules. A related reason is that one may like to consider frozen-in states where
the rates of internal reactions are negligible. In that case one may add some amount of
any component without having to consider its possible reactions with other components

5.5 Homogeneous chemical reactions 93
inside the system. The dependent components can then be treated as independent and
we may define the chemical potential of any component
µ
i
=
∂G
∂ N
i
T,P,N
l
. (5.58)
Even though the system is frozen-in, we may evaluate the driving force for the r reactions
by which each one of the r additional components could react with the independent ones.
It may be derived as follows.
Considering the changes of all the s components, both independent and dependent
ones, we may write the combined law as
dG = V dP − SdT +
s
i=1
µ
i
dN
i
(5.59)
We would now like to introduce the extent of the internal reactions, ξ
j
,inthis equation.
One may define the reactions in such a way that each reaction represents the formation
of a single dependent component from the set of independent ones, but without the other
dependent components being involved. In a closed system, the change of an independent
component may be given by the loss caused by several reactions, and may thus be related
to the increase of several dependent components.
dN
i
=−
r
j=1
a
j
i
dN
j
. (5.60)
For the dependent components dN
j
is only caused by their own independent reactions.
The combined law for a closed isobarothermal system will thus be
dG = V dP − SdT +
c
i=1
µ
i
−
r
j=1
a
j
i
dN
j
+
r
j=1
µ
j
dN
j
= V dP − SdT −
r
j=1
D
j
dξ
j
. (5.61)
The driving force for the formation of the dependent component j is here defined as
D
j
=
c
i=1
a
j
i
µ
i
− µ
j
. (5.62)
The combined law for an open system could thus be written
dG = V dP − SdT +
c
i=1
µ
i
dN
i
+
r
j=1
µ
j
dN
j
−
r
j=1
D
j
dξ
j
. (5.63)
It should be emphasized that dN
i
and dN
j
here represent only the amounts received from
the surroundings. The changes due to all internal reactions are included in dξ
j
. Under
freezing-in conditions one should omit D
j
dξ
j
. When the internal reactions are very
fast, there is almost internal equilibrium and the driving forces necessary for maintaining
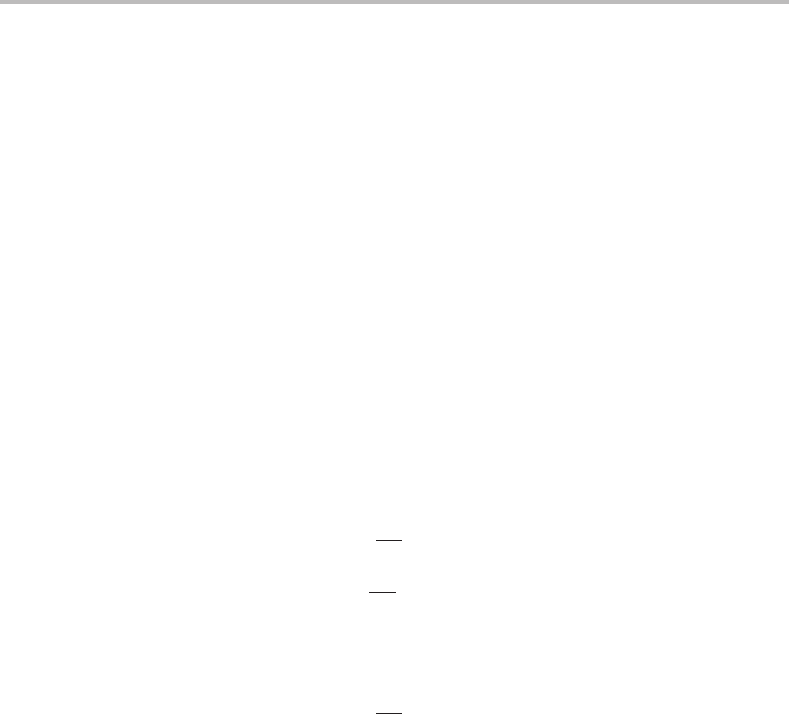
94 Thermodynamics of processes
equilibrium are so small that D
j
dξ
j
can again be omitted. Then
µ
j
=
c
i=1
a
j
i
µ
i
(5.64)
dG = V dP − SdT +
c
i=1
µ
i
dN
i
+
r
j=1
a
j
i
dN
j
. (5.65)
Again, dN
i
and dN
j
represent only the amounts received from the surroundings. When
treating intermediate cases one should take into account the rate of all reactions, starting
with the r reactions by which the r dependent components can form from the independent
ones and then adding those in which two or more of the dependent ones take part. We
shall now consider the very simplest case where there are one independent component
and two dependent ones. The dependent components will be denoted by 1 and 2 and
their reactions will also be identified by 1 and 2. For consistency with the derivations in
Section 5.4 the superscripts used for D and ξ in the above equations will now be given
as subscripts. Without any coupling between the first two reactions their rates will thus
be given as
J
∗
1
=
dξ
1
dt
= L
∗
1
X
∗
1
= K
∗
1
D
∗
1
(5.66)
J
∗
2
=
dξ
2
dt
= L
∗
2
X
∗
2
= K
∗
2
D
∗
2
, (5.67)
where D
∗
i
= TX
∗
i
according to Eq. (5.54) and K
∗
i
= L
∗
i
/T .For the additional reaction,
where both dependent components take part, we write
J
∗
3
=
dξ
3
dt
= L
∗
3
X
∗
3
= K
∗
3
D
∗
3
. (5.68)
Suppose this reaction produces component 1 and consumes component 2. Then we know
that its driving force must be
D
∗
3
= D
∗
1
− D
∗
2
. (5.69)
By direct measurements of the amounts of the two dependent components one would not
get any direct information on the third reaction but one can represent the experimental
information using the phenomenological equations for those reactions.
J
1
= K
11
D
1
+ K
12
D
2
(5.70a)
J
2
= K
21
D
1
+ K
22
D
2
. (5.70b)
The goal is to evaluate the kinetic coefficients in the three processes, assumed to have no
cross terms, from the experimentally determined coefficients in Eqs (5.70a and b). This
problem was discussed in more general terms in Section 5.2.Bycomparing the relations
between the driving forces given by Eqs (5.44) and (5.69)wefind that the present case
corresponds to k = 1 and l =−1. From Eq. (5.49)wethus find
K
∗
3
=−K
12
=−K
21
; K
∗
2
= K
22
+ K
12
; K
∗
1
= K
11
+ K
12
(5.71)
