Higman Chris Gasification (Газификация угля)
Подождите немного. Документ загружается.

310
Gasification
Molecular Sieves
The most common application of molecular sieves in connection with gasification
plants is the removal of water and CO
2
upstream of cryogenic units. Processes work-
ing at cryogenic temperatures, such as air separation or cryogenic gas separation,
require a feed gas completely free of these components, which would otherwise
freeze and deposit on the inlet heat exchangers and finally block them.
The classic cycle described above is usually employed. In air separation duty,
water and CO
2
are not the only considerations. The prepurification unit also prevents
the ingress of hydrocarbons into the cold box as a safety measure. Recently the
ingress of NO
x
into the cold box has also become an issue of concern. For air sepa-
ration. a combination of molecular sieve and silica gel is often used.
Pressure Swing Adsorption
Pressure swing adsorption (PSA) operates on an isothermal cycle, adsorbing at
high pressure and desorbing at low pressure. The principle application of PSA is for
hydrogen purification, although there are a number of others including air separation
(see Section 8-1).
The optimum pressure for hydrogen purification lies in the range 15–30 bar. At
higher pressures the hydrogen yield falls off, a point to be considered when integrating
a hydrogen off-take from a gasification plant optimized for a different application.
The hydrogen yield of a modern PSA unit usually lies between 80% and 92%.
Apart from the matter of pressure already mentioned, other influences are the quality
COMPONENT PARTIAL
PRESSURE, [bar]
LOADING,
[mol COMPONENT/mol SORBENT]
P
0
P
1
T
1
< T
0
5
4
3
2
1
T
2
>T
0
T
0
L
1
L
3
L
5
L
4
L
2
Figure 8-10. Adsorption Loading at Different Temperatures and Pressures
Auxiliary Technologies
311
of the feed gas (the higher the quantity of impurities to be removed, the more hydrogen
is lost with them) and the tail gas pressure. Where the tail gas is burned in dedicated
burners, as for instance in a steam reformer hydrogen plant, the typical tail gas pres-
sure is 0.3–0.4 bar gauge. Where the tail gas pressure is higher (e.g., 3–5 bar gauge),
the drop in hydrogen yield can become very significant.
Additionally, the hydrogen purity can affect the yield, though only to a small
degree. Typical purities range from 99 to 99.999 mol%. An additional common
hydrogen specification is a limit on the amounts of carbon oxides (CO and CO
2
).
Levels of 0.1 to 10 ppmv are easily achieved. In the design of an overall gasification-
to-hydrogen system, it is useful to have an idea about the performance of likely
impurities in the PSA unit. A comparison of a number of components is shown in
Table 8-3. In this connection it is important to note that although water is strongly
adsorbed and so will not contaminate the product. It is disadvantageous to have large
quantities in the feed gas since this requires excessively large beds. Usually, cooling to
below 40°C with subsequent condensate separation is sufficient to provide an economic
design.
A further design consideration is the number of adsorber vessels. Early plants used
four beds, as is still the practice on smaller plants. Larger modern plants use as many
as twelve adsorbers. Sophisticated cycles have been developed to minimize the loss of
hydrogen on depressurization from the adsorption step to the desorption step, by using
this hydrogen to repressurize a bed that has just completed its desorption step. Thus
there can be a trade-off between a higher investment for an increased number of vessels
(and valves) and operating savings from an increased hydrogen yield.
Zinc Oxide/Copper Oxide
Adsorption of H
2
S onto zinc oxide is an effective method for removing trace quanti-
ties of sulfur from gas to achieve a purity of less than 0.1 ppmv, as is required by
copper or nickel catalysts. It is therefore the standard method of desulfurization
Table 8-3
Relative Strength of Adsorption of Typical Impurities
Non-adsorbed Light Intermediate Heavy
H
2
O
2
CO C
3
H
6
He N
2
CH
4
C
4
H
10
Ar C
2
H
6
C
5
+
CO
2
H
2
S
C
3
H
8
NH
3
H
2
O
Source: Miller and Stoecker 1989
312
Gasification
upstream of natural gas steam reformers. The adsorption takes place via the reaction
of hydrogen sulfide with zinc oxide to form zinc sulfide. In situ regeneration is not
possible, and this places a limitation on the amount of sulfur that the process can
accept in the inlet gas.
There are two generally accepted designs for zinc oxide desulfurization units. In a
guard bed function or where the sulfur load is low, a single bed is provided, sized to
adsorb the total quantity of sulfur to be expected between planned turnarounds, say
one or two years. Where the sulfur load is higher and a single bed would become
unmanageably large, a two-vessel series arrangement is provided and provision is
made for exchanging the adsorbent online. With this arrangement, the individual
bed can be sized smaller, such as for a six-month interval between bed replacement.
Zinc oxide can adsorb sulfur present as H
2
S almost completely. Performance with
other sulfur compounds (COS, mercaptans) is not as good. In cases where sulfur is
present other than as H
2
S, it is necessary to hydrogenate these components to H
2
S
upstream of the zinc-oxide bed. This is normally done over a cobalt-molybdenum
(CoMox) or nickel-molybdenum (NiMox) catalyst.
Zinc oxide adsorption is essentially a process for polishing or guard bed duty.
This becomes clear when considering a zinc-oxide bed for the carbon monoxide
plant described in Section 7.1.4. Operating in its optimum temperature range of 350
to 400°C, zinc oxide has a pick-up capacity of around 20% by weight. Assuming a
sulfur content of 100 ppmv in the natural gas, the total sulfur intake is about 10 tons/
year, requiring replacement of about 50 tons/year zinc oxide. Compare this with the
nearly 30 t/d sulfur intake of the 1000 t/d methanol plant of Section 7.1.2, and the
limitations become very apparent.
Given these numbers, zinc oxide in the gasification environment is limited either
to guard bed duty, for example, upstream of a low temperature shift or methanator
catalyst or to natural gas feeds. As discussed in Section 7.1.4, there are arguments
for desulfurizing either upstream or downstream of the partial oxidation reactor.
Where extreme sulfur cleanliness is required, copper oxide can be used for final
desulfurization down to 10 ppbv. Commercial adsorbents are available for this pur-
pose, either in a mixed ZnO/CuO formulation or as a separate polishing bed.
8.2.4 Membrane Systems
Permeable gas separation membranes in syngas service utilize differences in solu-
bility and diffusion of different gases in polymer membranes. The rate of transport
of a component through the membrane is approximately proportional to the differ-
ence in partial pressure of the component on the two sides of the membrane. Polymer
membranes have found increasing use in a number of applications, including natural
gas processing (CO
2
removal) and in the synthesis gas environment for hydrogen
separation out of the main syngas stream.
The design of a polymer membrane system exploits the different permeability
rates of the components in the feed gas. An idea of the relative rates through a typical
Auxiliary Technologies
313
hydrogen separation polymer can be gained from Table 8-4. Thus a good separation
can be achieved between, for example, hydrogen and CO or N
2
. Separation from
CO
2
will be only moderately satisfactory, however.
Membrane units are usually supplied packed, typically as a bundle of hollow
tube fibers. The feed is supplied to the shell side of the bundle and the permeate
(hydrogen rich stream), which passes through the fiber-tube walls, is collected on
the tube side. Design variables are the pressure difference selected and the total
surface area of the polymer.
For the system designer, the integration of a membrane unit has two important
characteristics. First, permeable membranes provide the only system leaving the
carbon monoxide at essentially the same pressure level as at the gas inlet (less
hydraulic losses only) and the hydrogen on the low-pressure side. This is exactly the
reverse of the pressure swing adsorber.
Second, as mentioned above, it must be recognized that since all permeable
membranes work on the basis of different rates of diffusion, they can only have a
limited selectivity. This can be disadvantageous, since in a hydrogen extraction
application, the product hydrogen is not very pure, and the diffusion of CO through
the membrane can be considered as a loss of high pressure gas.
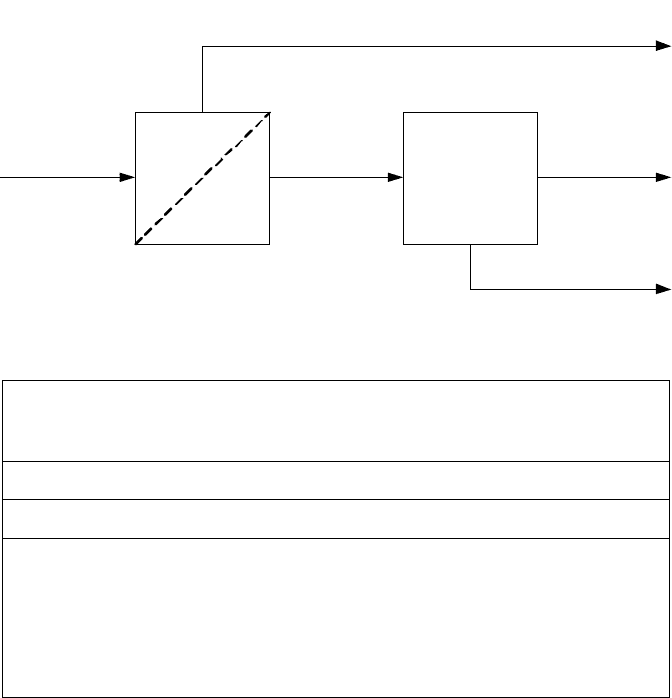
Nonetheless, skilled integration of membrane and PSA technologies can together
provide some extremely attractive solutions. Consider the following situation where
20,000 Nm
3
/h pure hydrogen is required from a main stream of syngas in an IGCC
(Figure 8-11 and Table 8-5). The membrane is used to produce a raw hydrogen at
reduced pressure (but still adequate for PSA feed) with only a small loss of other
syngas components for the gas turbine. The raw hydrogen has a purity of about 70–
90 mol%, depending on syngas composition and pressure, which allows the PSA to
have a significantly higher efficiency than would be the case with syngas feed. Fur-
thermore, the much smaller quantity of tail gas to be adsorbed allows the PSA unit
to be smaller too.
Care should be exercised with liquid carry over from an upstream AGR system.
In some cases these can damage the membrane. Proper separation at the AGR outlet
should however be sufficient to prevent problems (Collodi 2001).
Table 8-4
Relative Permeability Rates of Typical Syngas Components
Quick Intermediate Slow
H
2
CO
2
CO
He CH
4
H
2
S N
2
Source: Kubek, Polla, and Wilcher 1997
314
Gasification
Hot Gas Cleanup
For power applications the energy loss involved in cooling synthesis gas down to
ambient or lower temperatures as required by current acid gas removal systems is
reason enough for the interest in so-called “hot gas cleanup.” Actually, hot gas
cleanup is a misnomer, and these technological developments should rightly be
called “warm gas cleanup,” since the target operating temperature range is between
250 and 500°C.
Impurities that need to be considered in a warm gas cleanup system include
particulates (fly ash and char) as well as gaseous compounds such as H
2
S, COS,
NH
3
, HCN, HCl, and alkali species. At temperatures above about 500°C, alkaline
species will pass through a particulate filter, and this together with materials issues
is the principle reason why no attempts at hotter cleanup have been made.
MEMBRANE PSA
SYNGAS
DEPLETED
SYNGAS
RAW H
2
PURE
HYDROGEN
TAIL GAS
Figure 8-11. Membrane and PSA Combination
Table 8-5
Mass Balance for Membrane/PSA Combination
Syngas In Syngas Out Raw H
2
Pure H
2
Tail Gas
mol% mol% mol% mol% mol%
CO
2
8.6 8.4 9.4 31.4
CO 43.8 52.4 7.9 26.3
H
2
45.3 36.5 82.4 100.0 41.2
CH
4
2.3 2.7 0.3 1.1
Total (kmol/h) 6635 5360 1275 893 382
Pressure (bar) 50 49 25 24 1.3
Auxiliary Technologies
315
Technologies for warm gas cleanup using zinc-based sorbents have been built at
demonstration scale in Polk County and Piñon Pine without great success (Simbeck,
2002; U.S. Department of Energy 2002). In fact, neither of these units was ever oper-
ated. Both of these were designed essentially as desulfurization units with removal
efficiencies of up to 98%, which at the time of design conformed to existing power
station emission regulations. They did not address some of the other species, such as
nitrogen compounds and halides, nor for that matter mercury. Furthermore, the sulfur
removal efficiencies made them unsuitable for most chemical applications.
Nonetheless, the potential in terms of efficiency improvement remains and continues
to provide an incentive for research and development to find appropriate systems.
8.2.5 Further Developments
One current program is addressing some of these issues. It includes the use of
membrane technology for bulk desulfurization, zinc oxide or similar chemisorption
process for fine sulfur removal, a sodium carbonate-based sorbent for HCl removal,
and a high-temperature molecular sieve for ammonia removal (Gupta 2001). How-
ever, the use of polymer membrane technology is likely to limit its ability to operate
at high temperature and thus any efficiency gains on this basis. Mercury capture is
not specifically mentioned as being part of this program, although on the basis of
currently published flowsheets it could possibly be incorporated as a separate stage.
8.2.6 Biomass Syngas Treating
Treating the syngas generated from biomass has special problems—particularly those
associated with the presence of tars in the gas. Attempts have been made to reduce the tar
content by cracking (Morris and Waldheim 2002; Bajohr etal. 2002). Other attempts have
been made at using an oil wash (Boerrigter, den Uil, and Calis 2002; Hofbauer 2002). To
date, success has been limited to achieving a quality suitable for power applications.
Considerable work is still required to achieve a chemicals application syngas quality.
8.3 CO SHIFT
Besides having an important influence on the composition of the raw syngas from
the gasifier itself, the CO shift reaction
CO + H
2
OCO
2
+H
2
−41 MJ/kmol (2-7)
can be and is operated as an additional and separate process from the gasifier at
much lower temperatures in order to modify the H
2
/CO ratio of the syngas or maxim-
ize the total hydrogen production from the unit. As can be seen from the reaction
2-7, one mole of hydrogen can be produced from every mole of CO. The reaction
←
→
316
Gasification
itself is equimolar and is therefore largely independent of pressure. The equilibrium
for hydrogen production is favored by low temperature.
The CO shift reaction will operate with a variety of catalysts between 200°C to
500°C. The types of catalyst are distinguished by their temperature range of operation
and the quality (sulfur content) of the syngas to be treated.
8.3.1 Clean Gas Shift
High Temperature (HT) Shift
Conventional (high temperature) shift uses an iron oxide–based catalyst promoted
typically with chromium and more recently with copper. The operating range of
these catalysts is between 300 and 500°C. Much above 500°C sintering of the
catalyst sets in and it is deactivated. HT shift catalyst is tolerant of sulfur up to a
practical limit of about 100 ppmv, but it is likely to loose mechanical strength, par-
ticularly if subjected to changing amounts of sulfur.
An important aspect in the design of CO shift in the gasification environment, where
inlet CO contents of 45% (petroleum residue fed) to 65% (coal) are common, is the
handling of the heat of the reaction, particularly under end-of-run conditions where an
inlet temperature of 350°C or more may be necessary. On the one hand, the reaction
must be performed in several stages to avoid excessive catalyst temperatures and to have
an advantageous equilibrium. On the other hand, optimum use must be made of the heat.
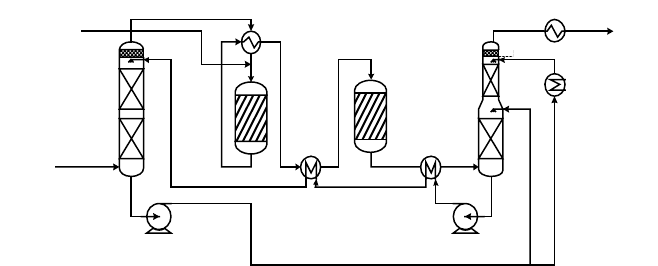
One such arrangement is shown in Figure 8-12. Desulfurized syngas containing
about 45 mol% CO, which leaves the AGR at about 54 bar and ambient temperature,
is heated and water saturated at a temperature of about 215°C by water that has been
preheated with hot reactor effluent gas. The saturated gas is further preheated to the
catalyst inlet temperature of between 300°C and 360°C. The steam loading from
the saturator is such that only the stoichiometric steam demand for the reaction is
required to be added from external sources. In the first stage, the CO is reduced to a
DESULFURIZED
GAS
SATURATOR REACTOR I
PROCESS
STEAM
REACTOR II
SHIFT GAS
DESATURATOR
HEAT
RECOVERY
Figure 8-12. CO Shift with Saturator-Desaturator Circuit (Source: Higman 1994)
Auxiliary Technologies
317
level of about 7–8 mol% at an outlet temperature of about 500°C. The outlet gas is
cooled to a temperature of about 380°C in the gas and water preheaters before entering
the second catalyst bed. Here the residual CO is reduced to about 3.2 mol%. The gas
is then cooled in a direct-contact desaturator tower. There are a number of different
designs, particularly for the first reactor, that incorporate the gas-gas heat exchanger
as an internal. In such reactors, the exchanger is arranged centrally inside an annular
catalyst bed with an axial (Lurgi) or axial-radial (Casale) gas-flow pattern. Alternative
methods of controlling the catalyst outlet temperature include interbed condensate
injection (e.g., Toyo). The use of an isothermal steam raising reactor has been pro-
posed, and although such a solution has been employed in a steam reformer plant,
none is recorded at the high CO inlet concentrations involved in a gasification plant.
Typical catalyst lifetime for the first bed in a gasification situation is two to three
years, which is considerably shorter than for a steam-reforming situation. This is gen-
erally attributed to the high operating temperatures associated with high CO concen-
trations in the inlet gas. On a moles-converted basis over the lifetime of the catalyst,
the performance in the gasification context is comparable with that of steam reforming.
Low Temperature (LT) Shift
Low temperature shift operates in the temperature range 200°C to 270°C and uses
a copper-zinc-aluminum catalyst. It is used in most steam reforming-based ammonia
plants to reduce residual CO to about 0.3 mol%, a requirement for a downstream
methanator, but has generally not been applied in gasification-based units. On the one
hand, it is highly sulfur-sensitive, and even with 0.1 ppmv H
2
S in the inlet gas, will
over time become poisoned. A second reason for its lack of use particularly in oil-
gasification plants, is the effect of the higher pressure on the water dewpoint in the
gas. Operation near the dewpoint will cause capillary condensation and consequent
damage to the catalyst. With a dewpoint of about 215°C and a temperature rise of
25–30°C, there is not much margin for error below the upper temperature limit of 270°C
when recrystallization of the copper catalyst begins. The first application of low
temperature shift at high pressure was in Shell’s Pernis gasification facility, which
has now performed successfully for several years (de Graaf et al. 2000).
Medium Temperature (MT) Shift
An improved copper-zinc-aluminum catalyst able to operate at higher exit
temperatures (300°C) than conventional LT shift has been developed, particularly
for use in isothermal reactors. No application in gasification plants is known.
8.3.2 Raw Gas Shift
For applications where it is desired to perform CO shift on raw syngas, a cobalt-
molybdenum catalyst, variously described as a “sour shift” or “dirty shift” catalyst,
318
Gasification
can be used. In some parts of the literature this catalyst is described as sulfur tolerant.
This is actually a misnomer, since the catalyst requires sulfur in the feed gas to
maintain it in the active sulfided state. It is generally applied after a water quench of
the raw syngas, which typically will provide a gas at about 250°C saturated with
sufficient water to conduct the shift reaction without any further steam addition.
For an ammonia application the raw gas shift is typically configured as two or three
adiabatic beds with intermediate cooling resulting in a residual CO of about 1.6 or
0.8 mol%, respectively.
An important side-effect of the raw gas shift catalyst is its ability to handle a
number of other impurities characteristic of gasification. COS and other organic
sulfur compounds are largely converted to H
2
S, which eases the task of the down-
stream AGR. HCN and any unsaturated hydrocarbons are hydrogenated.
Carbonyls are decomposed and deposited as sulfides, which increases the pres-
sure drop over the bed. Selective removal of arsenic in the feed is also claimed
(BASF undated).
8.4 SULFUR RECOVERY
The sulfur compounds from the feedstock of a gasification-based process are
generally removed from the synthesis gas as a concentrated stream of hydrogen
sulfide and carbon dioxide known as acid gas. Depending on the design of the
upstream AGR unit, the acid gas may contain other sulfur species such as COS as
well as ammonia and hydrogen cyanide. It is unacceptable to emit H
2
S, a highly
toxic, foul-smelling gas, to the atmosphere, so it is necessary to fix it in one form or
other. There are essentially two alternative products in which one can fix the sulfur,
either as liquid or solid elemental sulfur, or as sulfuric acid. The choice of product
will depend on the local market. Where there is a strong local phosphate industry,
then there will be a good local market for sulfuric acid. If this is not the case, then
elemental sulfur will probably be the better choice, since bulk transport of this mater-
ial is much easier than of the concentrated acid.
8.4.1 The Claus Process
The basic Claus process for substoichiometric combustion of H
2
S to elemental
sulfur was developed as a single-stage process on the basis of reaction 8-5 at the end
of the nineteenth century. During the 1930s it was modified into a two-stage process
in which initially one third of the H
2
S was combusted to SO
2
and water, and in a
second low-temperature catalytic stage, the SO
2
was reacted with the remaining
H
2
S to sulfur. Operating the second stage at a comparatively low temperature
(200–300°C) used the more favorable equilibrium to achieve much higher sulfur
yields than had been possible with the original process.
Auxiliary Technologies
319
H
2
S + 1½ O
2
←→ SO
2
+H
2
O (8-3)
2 H
2
S + SO
2
←→ 2 H
2
O + 3/8 S
8
(8-4)
3 H
2
S + 1½ O
2
←→ 3 H
2
O +3/8 S
8
(8-5)
Today there are innumerable Claus processes available, all of them ultimately vari-
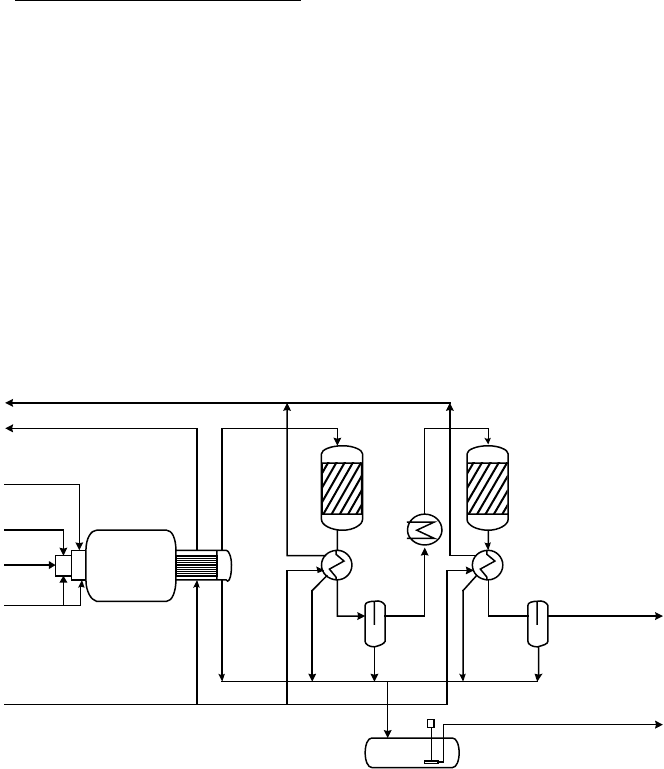
ants of the modified Claus process. A typical standard Claus process is shown in
Figure 8-13. In the first combustion stage all the H
2
S is combusted with an amount
of air corresponding to the stoichiometry of reaction 8-5 at a temperature in the
range 1000–1200°C. The thermodynamics of these three main reactions is such that
about half the total sulfur is present in the outlet gas as elemental sulfur vapor, the
rest as an equal mix of H
2
S and SO
2
. The hot gas is cooled by raising steam and the
sulfur already formed is condensed out. The removal of sulfur at this point assists in
driving reaction 8-4 further to the right in the subsequent catalytic stage. The gas is
reheated and passed over an alumina catalyst at a temperature of about 200–300°C,
and cooled again to condense the sulfur formed. This may be performed a number of
times to remove further amounts of sulfur. Typically, two (as shown in Figure 8-13)
or three catalytic stages are used.
Oxygen Claus Processes
A standard air-blown Claus plant is limited in the dilution of H
2
S possible in the acid
gas. At concentrations less than about 25–30 mol% H
2
S, the temperature in the
COOLER I
COOLER II
PRE-
HEATER
ACID GAS
CLAUS
REACTOR I
CLAUS
REACTOR II
WHB
CLAUS
FURNACE
AIR
O
2
SWS GAS
MP STEAM
LP STEAM
BFW
TAIL GAS
PRODUCT SULFUR
SULFUR TANK
Figure 8-13. Typical Two-Stage Claus Unit (Source: Weiss 1997)
