Hermanson G. Bioconjugate Techniques, Second Edition
Подождите немного. Документ загружается.

710 18. Discrete PEG Reagents
provide increased water solubility and in vivo activity (Wilbur et al., 2000). Fifteen biotin
derivatives were conjugated to cyanocobalamin, which is a binder of cobalt that may be used
in radiotherapy for cancer. Structures with enhanced activity were formed with PEG spacers of
varying length between the cyanocobalamin and the biotin handle, which provided maximal
hydrophilicity to the entire complex.
Biotinylation reagents historically have used aliphatic chains to provide a spacer between a
modifi ed molecule and the bicyclic ring of biotin. This allows for enough molecular distance
for (strept)avidin to easily bind during detection or targeting applications. Even long spacers
on biotin reagents typically have been constructed of hydrophobic alkyl chains for the biot-
inylation of proteins. This can be detrimental to protein solubility, because biotin itself is spar-
ingly soluble in aqueous solution and adding an alkyl spacer to this group only decreases its
water solubility further. In particular, antibodies modifi ed with long hydrophobic biotin com-
pounds often aggregate and lose activity, especially at high modifi cation levels. Replacing the
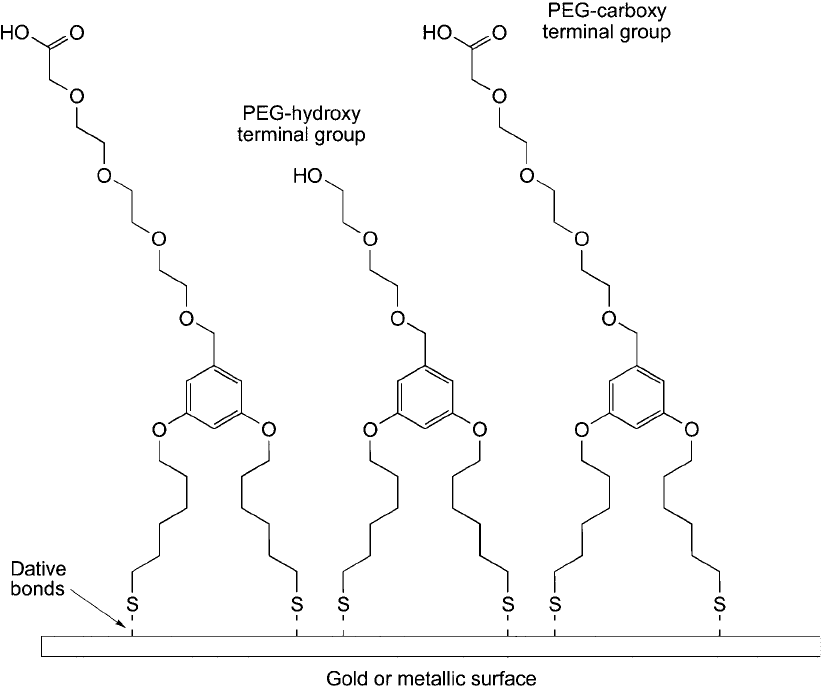
Figure 18.2 Dithiol linkers containing discrete PEG arms can be used to modify stably metallic surfaces for
coupling biomolecules.
alkyl chain with a discrete PEG spacer, however, can dramatically increase water solubility and
prevent antibody aggregation as well as signifi cantly boost long-term stability.
Discrete PEG reagents can provide benefi t for nearly any crosslinking compound or modi-
fi cation reagent designed for use in aqueous environments. Frequently, PEG chain lengths
between 4 and 24 repeating ethylene oxide units create increasingly hydrophilic character for
modifi ed biomolecules or surfaces. The following sections describe a number of these PEG
compounds, including crosslinkers, biotinylation compounds, and multi-armed PEG modifi ca-
tion agents designed to block molecules and surfaces. In addition, PEG-based compounds are
available that have functional groups on both ends for use as building blocks to create other
PEG compounds or as spacers to build unique surface functionality. Most of these reagents are
available commercially through Quanta BioDesign or Thermo Fisher Scientifi c.
Modifi cation or crosslinking agents containing a discrete PEG spacer are often not powders
or crystalline substances, but frequently they are thick, sticky, viscous liquids. Such materials
are diffi cult to dispense by weighing out a small portion from a vial. For this reason, it may
be best to dissolve an entire vial in a dry organic solvent prior to use or try to weigh out a
much larger quantity than may be initially required. Stock solutions may be stored for weeks at
0°C, but long-term stability of active groups is dependent on the quality of the solvent used.
For this reason, it is best to prepare solutions fresh.
Organic solvents that can be used with discrete PEG compounds include DMSO, DMF,
DMAC ( N,N -dimethylacetamide), and methylene chloride. The compounds also are very solu-
ble in many other commonly used organic solvents, which provide fl exibility for doing reactions.
DMAC is particularly convenient, because it is easily dried of contaminating water (using molec-
ular sieves), it doesn ’t decompose like DMF (producing amines), and it doesn ’t have the odors of
some of the other solvents. Methylene chloride can be used for water-insoluble molecules that are
to be reacted with the PEG compounds, but don ’t require subsequent water miscibility.
1. Homobifunctional PEG Crosslinkers
Compounds having the same functionality on both ends are homobifunctional in nature and
can be conjugated with the same target functionality on biomolecules, surfaces, or other mole-
cules. Chapter 4 describes traditional homobifunctional compounds in detail, but the discrete
PEG-based reagents are described here, because of their unique hydrophilic properties.
1.1. Bis-NHS Ester PEG Compounds
Bifunctional NHS esters can be used to conjugate two amine-containing molecules together.
The NHS ester groups react with amines to form amide linkages with loss of NHS. Two dif-
ferent PEG spacer lengths are available in this type of reagent, a PEG
5
compound and a PEG
9
derivative ( Figure 18.3 ). Unlike the popular BS
3
reagent (Chapter 4, Section 1.2), which is ini-
tially hydrophilic and water-soluble due to the presence of negatively charged sulfo-NHS esters
on both ends but after reacting leaves behind a 6-carbon hydrophobic spacer, these PEG com-
pounds attach two molecules together using a long and very hydrophilic bridge.
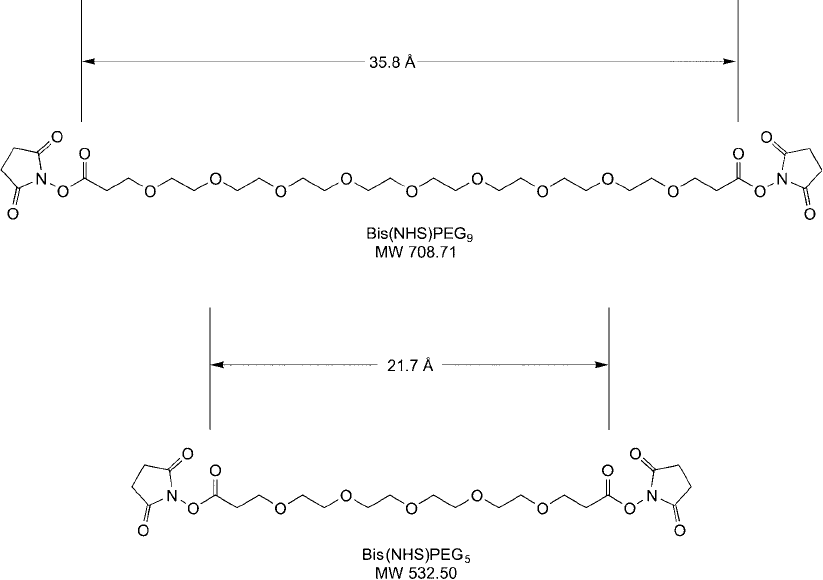
The bis-NHS–PEG
5
reagent provides a 21.7 Å spacer after attaching both ends to amines
on two molecules, which is nearly twice the spacer distance provided by BS
3
. The longer chain
1. Homobifunctional PEG Crosslinkers 711
712 18. Discrete PEG Reagents
Figure 18.3
Homobifunctional NHS ester compounds containing PEG spacers for water solubility.
bis-NHS–PEG
9
reagent has a very long 35.8 Å spacer. All of these distances are measured between
the carbonyl groups of the NHS esters, which would form the amide bonds and remain behind
after crosslinking two molecules together. The distances represent the energy minimized, maximal
molecular distance of a linear structure and may not refl ect how such structures exist in solution.
The reaction of the NHS esters occurs at physiological pH or under slightly basic conditions
to couple rapidly with amines and form amide bonds ( Figure 18.4 ). The NHS ester groups also
are subject to hydrolysis in aqueous solution, and the rate of hydrolysis increases with increas-
ing pH. The more hydrophilic the molecule the greater is the potential for hydrolysis. Nectar
reported that for long-chain polymeric PEGs containing NHS esters that the half-life of hydroly-
sis effectively triples upon lowing the pH one unit. In addition, the molecular constituents imme-
diately adjacent to the NHS ester affect the half-life of hydrolysis of activated PEG compounds.
For instance, the addition of a 4-carbon aliphatic chain between the PEG polymer and the NHS
ester results in a half-life of hydrolysis of about 44 minutes at pH 8. Reducing this to only a
2-carbon unit decreases the half-life of the NHS ester to about 3 minutes. Therefore, to aid in NHS
ester stability maintaining a reaction pH in the range of 7.0–7.5 is optimal for most applications.
The following protocol describes a general method for using bis-NHS ester PEG compounds.
Optimization of concentrations should be done for each application to assure the best possible
results. See also the protocol in Chapter 28, Section 1, which describes the use of homobifunctional
NHS-ester compounds to study protein interactions. These bis-NHS–PEG compounds may
provide a superior crosslinker for studying such interactions due to their water solubility and
the fact that the PEG bridge won ’t get buried in hydrophobic pockets on proteins or within
hydrophobic membrane structures.
Protocol
1. Dissolve the bis-NHS–PEG compound in a dry, water-miscible organic solvent to make a
concentrated stock solution. To prepare a 10 mM solution of the bis-NHS–PEG
9
reagent,
dissolve 7 mg/ml of DMAC; for the bis-NHS–PEG
5
reagent dissolve 5.3 mg/ml of DMAC.
2. Dissolve the molecules to be conjugated in 0.1 M sodium phosphate, pH 7.2 (for aqueous
reactions) or in DMSO, DMAC, or methylene chloride (for organic reactions). If proteins
are to be conjugated, a concentration of 1–10 mg/ml in buffer will work well in this proto-
col. For more dilute protein solutions, greater quantities of the bis-NHS–PEG compound
may have to be added than recommended here to obtain similar levels of crosslinking.
3. Add a quantity of the crosslinker solution to the protein solution to provide a 1- to 10-
fold molar excess of reagent over the concentration of protein. The use of lower molar
ratios will limit the potential for oligomerization of proteins in solution. A series of reac-
tions using different concentrations of crosslinker may have to be done to determine the
optimal level to use for a particular application.
4. React for 30–60 minutes at room temperature.
5. Remove excess crosslinker by dialysis or gel fi ltration.
1. Homobifunctional PEG Crosslinkers 713
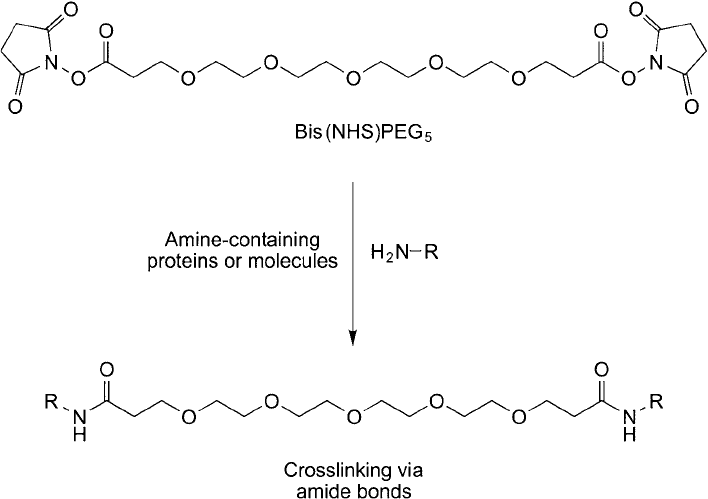
Figure 18.4 The reaction of bis-NHS–PEG
5
with amines on proteins yields amide bond linkages with amine-
containing molecules.
714 18. Discrete PEG Reagents
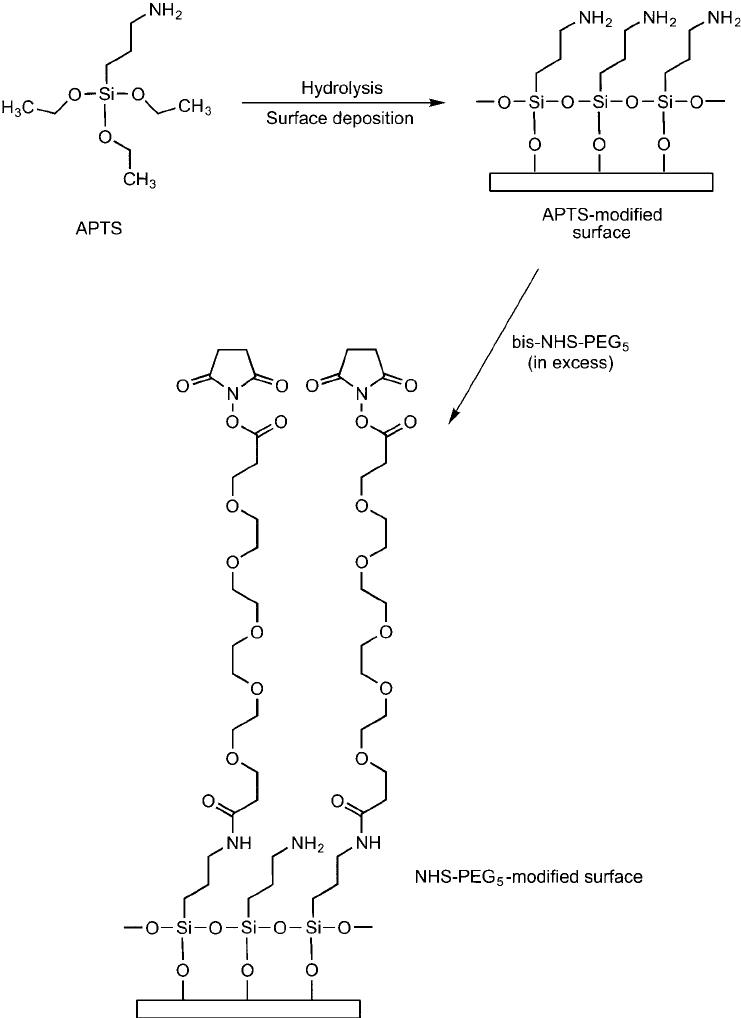
The bis-NHS–PEG compounds also may be used for modifying surfaces or particles that con-
tain amine groups. If the bis-NHS–PEG reagent is reacted in large excess to the concentration of
amines, then a single end of each crosslinker will couple to the amine groups on the surface and
result in a PEG spacer terminating in a reactive NHS ester for further conjugation. Surfaces mod-
ifi ed with an amino silane group, such as 3-aminopropyltriethoxysilane (APTS) are particularly
good for further modifi cation with PEG-based reagents. For instance, APTS modifi ed glass slides
or silica particles contain aminopropyl groups that can be modifi ed with the bis-NHS–PEG com-
pounds. This will create a hydrophilic monolayer having low nonspecifi c binding character and
that can be used to covalently link to biomolecules via the terminal NHS ester group ( Figure 18.5 ).
The following procedure can be used with planar or spherical surfaces containing amine
groups. The reaction is done in organic solvent to preserve the activity of the terminal NHS ester
after modifi cation of the surface. For particle modifi cation, care should be taken in choosing a
solvent that won ’t damage the particle core structure, such as the potential for certain organic sol-
vents to dissolve polymeric particles. Silica particles, however, are very robust to solvent conditions
and can be used without damage. See also Chapter 14 for other particle conjugation methods.
Protocol
1. In ad fume hood, wash the amine-surface with organic solvent to remove any contami-
nants or water (especially when working with particles). Suggested solvents to use for
this reaction are highly pure and dry DMAC, DMSO, or DMF. Particles can be washed
by repeated centrifugation and resuspension.
2. Dissolve bis-NHS–PEG
5
into the solvent of choice at a concentration of 1 mg/ml also
containing an equal molar concentration of triethylamine as base. Add the crosslinker
solution to the surface or to the particles to coat them fully. When working with parti-
cles, centrifuge them to remove solvent prior to resuspending in the crosslinker solution.
3. React for 30–60 minutes at room temperature with mixing.
4. Wash the surface or particles with solvent at least thrice to remove excess crosslinker.
Planar surfaces activated with the NHS–PEG groups may be sealed in a pouch and stored
dry in the presence of a desiccant. Activated particles may be stored as a suspension in dry
solvent under a head of nitrogen at 4°C until used for further conjugation. The addition of an
amine-containing protein or other amine-molecule will cause covalent coupling to the surface
NHS ester groups to form amide bonds.
1.2. Bis-Maleimide–PEG Compounds
Homobifunctional crosslinkers containing thiol-reactive maleimides on each end of a PEG
spacer are available in several sizes. These compounds are hydrophilic and react with sulfhydr-
yls to produce thioether linkages, which are stable under most conditions. The following com-
pounds can be obtained from Thermo Fisher or Quanta BioDesign.
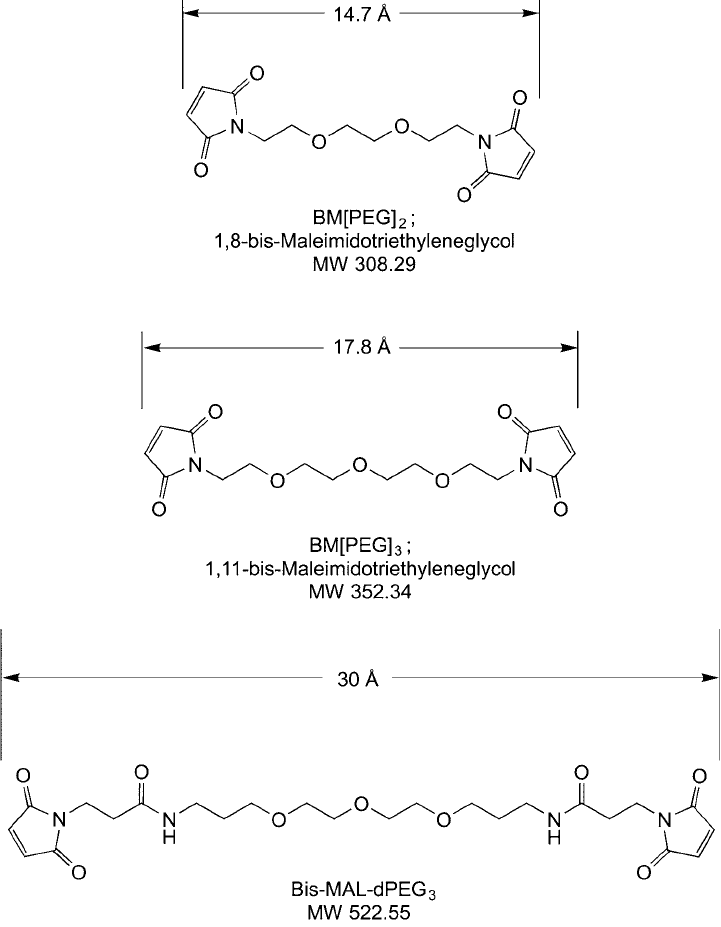
BM(PEG)
2
, BM(PEG)
3
, and Bis-MAL–dPEG
3
BM(PEG)
2
is 1,8-bis-maleimidodithyleneglycol, a hydrophilic reagent containing a 14.7 Å spacer
arm. This compound also is called BM(PEO)
2
by Thermo Fisher, wherein PEO is the acronym
1. Homobifunctional PEG Crosslinkers 715
Figure 18.5 The modifi cation of an APTS-modifi ed surface containing amines with bis-NHS–PEG
5
yields
hydrophilic spacers containing terminal NHS esters for coupling proteins.
716 18. Discrete PEG Reagents
for poly(ethylene oxide), but to maintain a consistent nomenclature throughout this book,
it is referred to under the PEG name. Another pair of crosslinkers, BM(PEG)
3
and bis-MAL–
dPEG
3
have one additional PEG unit and provide similar bifunctional maleimides, but differ
only in their structures leading from the PEG spacer to the maleimides ( Figure 18.6 ). BM(PEG)
5
,
Figure 18.6 The structures of BM(PEG)
2
, BM(PEG)
3
, and bis-MAL–dPEG
3
contain thiol-reactive maleimide
groups on the ends of a discrete PEG spacer arm.
has a 17.8 Å spacer, while bis-MAL–dPEG
3
has a longer 30 Å length. These dimensions are
measured between the outer hydrogens on the maleimide groups using linear three-dimensional
structures after energy minimization. The actual structural confi gurations in aqueous solution
almost certainly will not be linear and thus the molecular dimensions will differ from these
values.
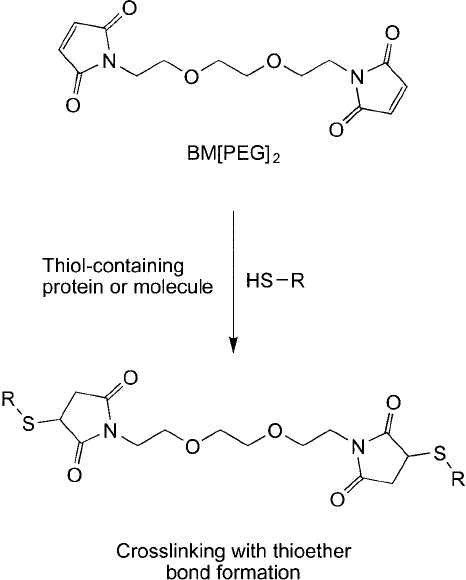
All of these bifunctional maleimides can be used to conjugate together two proteins con-
taining available thiol groups ( Figure 18.7 ). For instance, artifi cial bispecifi c antibodies have
been created by coupling two disulfi de-reduced antibodies together having different antigenic
specifi cities using the thiol-reactive crosslinker SPDP (Chapter 5, Section 1.1). This was done
by reducing the disulfi des in the hinge region of antibodies to produce two heavy/light chain
complexes containing one antigen binding site each and then conjugating the fragments from
both antibodies together with SPDP (Foglesong et al., 1989). Coupling one reduced antibody
with another of a different specifi city using a hydrophilic reagent such as BM(PEG)
2
will form
a conjugate having two different antigen binding capabilities. The advantage of using a PEG-
based compound over SPDP is greater water solubility of the resultant conjugate.
BM(PEG)
2
also can be used to determine protein–protein interactions or subunit interac-
tions if there are available free thiol groups on each protein or subunit. The following protocol
can be used to crosslink two thiol-containing proteins.
1. Homobifunctional PEG Crosslinkers 717
Figure 18.7 BM(PEG)
2
reaction with thiol-containing proteins forms crosslinks via thioether linkages.
718 18. Discrete PEG Reagents
Protocol
1. Dissolve the thiol-containing proteins to be crosslinked in 50 mM sodium phosphate,
pH 6.5–7.5, containing 10 mM EDTA to prevent metal-catalyzed sulfhydryl oxidation.
2. Dissolve BM(PEG)
2
in DMSO or DMF at a concentration of 10–20 mM (3.1 mg/ml to
make a 10 mM solution).
3. Add a quantity of the crosslinker solution to the protein solution to provide a 1- to 10-
fold molar excess of crosslinker to protein. Lower molar ratios will help prevent olig-
omerization of protein if the proteins contain more than one available thiol group. A
series of different molar ratios may be studied to optimize the level of reagent addition.
4. React for 1–2 hours at room temperature.
5. Purify the conjugate by dialysis or gel fi ltration.
2. Heterobifunctional PEG Reagents
Heterobifunctional crosslinkers contain different reactive groups or functionalities at each end of a
spacer arm. Traditional reagents of this type are discussed in Chapter 5, but the PEG-based com-
pounds are reviewed exclusively here, because of their unique water-soluble characteristics. The
most popular aliphatic heterobifunctional compound is SMCC (or sulfo-SMCC), which contains
an NHS ester on one end and a maleimide group on the other end. However, this compound suf-
fers from a cross-bridge that is both water-insoluble and immunogenic. Redesigning this crosslinker
to have a PEG cross-bridge provides enhanced water solubility for modifi ed proteins or other mol-
ecules as well as displaying very low immunogenicity. These benefi ts of discrete PEG spacers create
a new generation of heterobifunctional compounds, which dramatically improve the performance
of conjugates made from them. The following sections describe these reagents in more detail.
2.1. Maleimide–PEG
n
–NHS Ester Compounds
One of the most useful types of crosslinkers ever invented is a heterobifunctional compound
containing an NHS ester on one end and a maleimide group on the other end. The ability to
link an amine-containing molecule to another molecule containing a thiol group provides con-
trol over the conjugation process, which avoids the potential for oligomerization that often
occurs when using homobifunctional crosslinkers. Probably the most signifi cant recent develop-
ment in this type of reagent is the introduction of PEG spacers in their cross-bridge construc-
tion. Unlike the previous iteration of these compounds that all used hydrophobic spacers
(Chapter 5, Section 1), the PEG-based reagents provide water solubility both of the initial com-
pound and of the crosslink itself after the reaction has taken place.
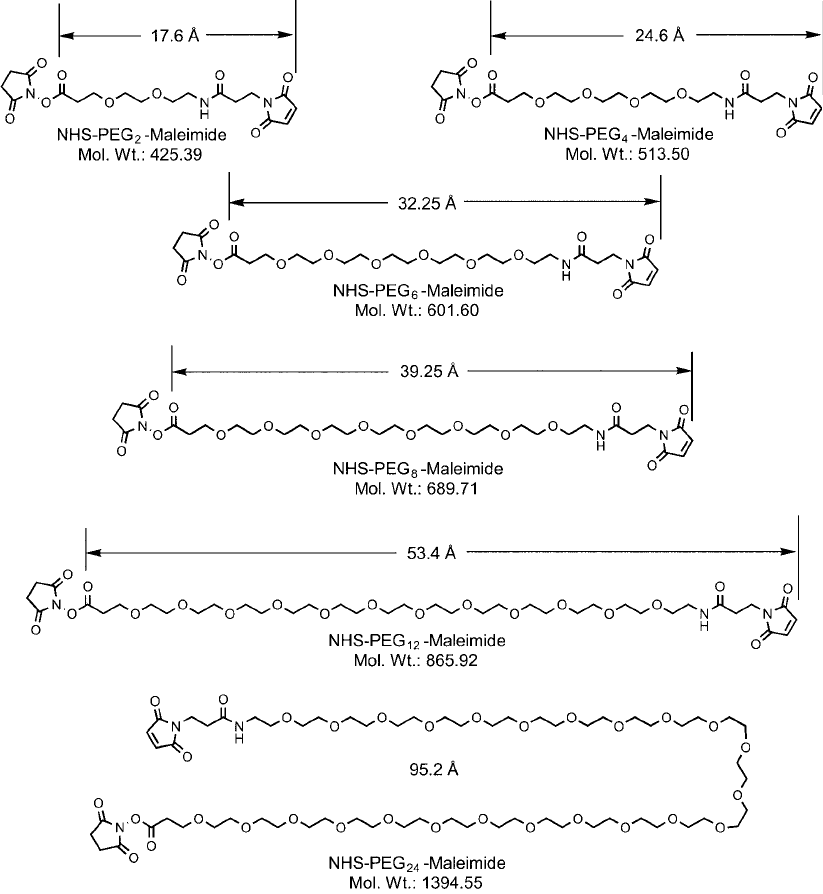
NHS–PEG
n
–maleimide crosslinkers now are available as a series of different chain lengths
of PEG within the cross-bridge, including repeating ethylene oxide units of 2, 4, 6, 8, 12, and
24 (Quanta BioDesign and Thermo Fisher). This series provides a range of molecular lengths
after conjugation from 17.6 to 95.2 Å, so that an optimal size can be determined for nearly any
application ( Figure 18.8 ). The longest crosslinker of the group, NHS–PEG
24
–maleimide, has a
total length that compares to almost the diameter of a typical immunoglobulin (IgG) antibody
molecule. Longer chain NHS–PEG–maleimide crosslinkers may be appropriate especially for
the masking of surfaces to permit coupling of affi nity ligands, while avoiding the potential for
nonspecifi c interactions with the surface structure. In addition, these heterobifunctional PEG
compounds are superior choices for linking haptens to carrier proteins to create immunogens.
In this application, the PEG chain provides a high degree of freedom of motion and does not
illicit an immune response itself, thus effectively generating antibodies in vivo with greater spec-
ifi city toward the hapten, not the carrier or the crosslinker.
2. Heterobifunctional PEG Reagents 719
Figure 18.8 Series of NHS–PEG–maleimide crosslinkers.
