Hermanson G. Bioconjugate Techniques, Second Edition
Подождите немного. Документ загружается.

730 18. Discrete PEG Reagents
5. Purify the biotinylated protein from excess reagent and reaction by-products using dialysis
or gel fi ltration (desalting resin).
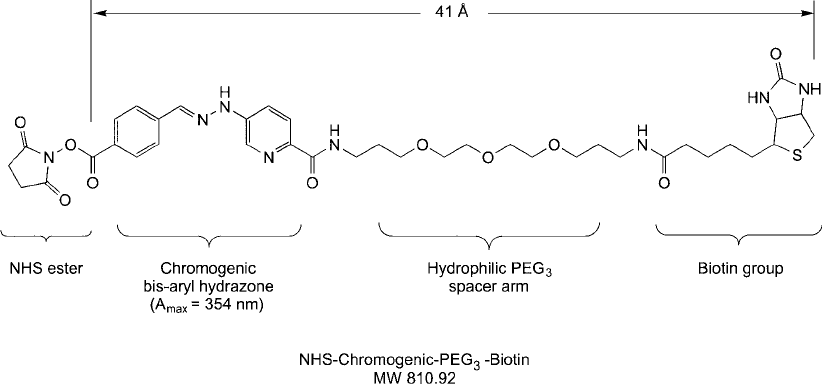
3.2. NHS-Chromogenic-PEG
3
-Biotin
A novel detectable biotinylation reagent containing a hydrophilic PEG spacer is NHS-
chromogenic-PEG
3
-biotin (also called chromogenic biotin; Thermo Fisher, Solulink; Figure 18.15 ).
Next to the terminal NHS ester of this compound is a bis-aryl hydrazone group created from
the reaction of a 6-hydrazinium nicotinate derivative and a benzaldehyde group to form the
chromogen having an absorbance at 354 nm ( 29,000 M
1
cm
1
). The NHS ester end can
be used to modify amine-containing molecules and form a stable amide linkage ( Figure 18.16 ).
The spacer arm contains a hydrophilic spacer made from three ethylene oxide units, which
provide water solubility for the compound.
Proteins biotinylated with this reagent will have a characteristic absorbance band at 354 nm,
which can be used to determine accurately the number of biotin groups per molecule. No other
biotinylation compound has such built-in quantifi cation capability. This feature eliminates the
need to consume conjugate by doing a HABA assay to test for the level of biotin incorporation
(Chapter 23, Section 7).
The following protocol is adapted from the manufacturers ’ recommendations.
Protocol
1. Dissolve a protein or other amine-containing molecule to be biotinylated in 0.1 M
sodium phosphate, 0.15 M NaCl, pH 7.2–7.5, at a concentration of 1–10 mg/ml. Note
Figure 18.15 NHS-chromogenic-PEG
3
-biotin contains an amine-reactive NHS ester that can be used to label
biomolecules through an amide linkage. The chromogenic bis-aryl hydrazone group within the spacer arm of
the reagent allows the degree of biotinylation to be quantifi ed by measuring its absorbance at 354 nm. The
compound also contains a hydrophilic PEG spacer, which provides greater water solubility.
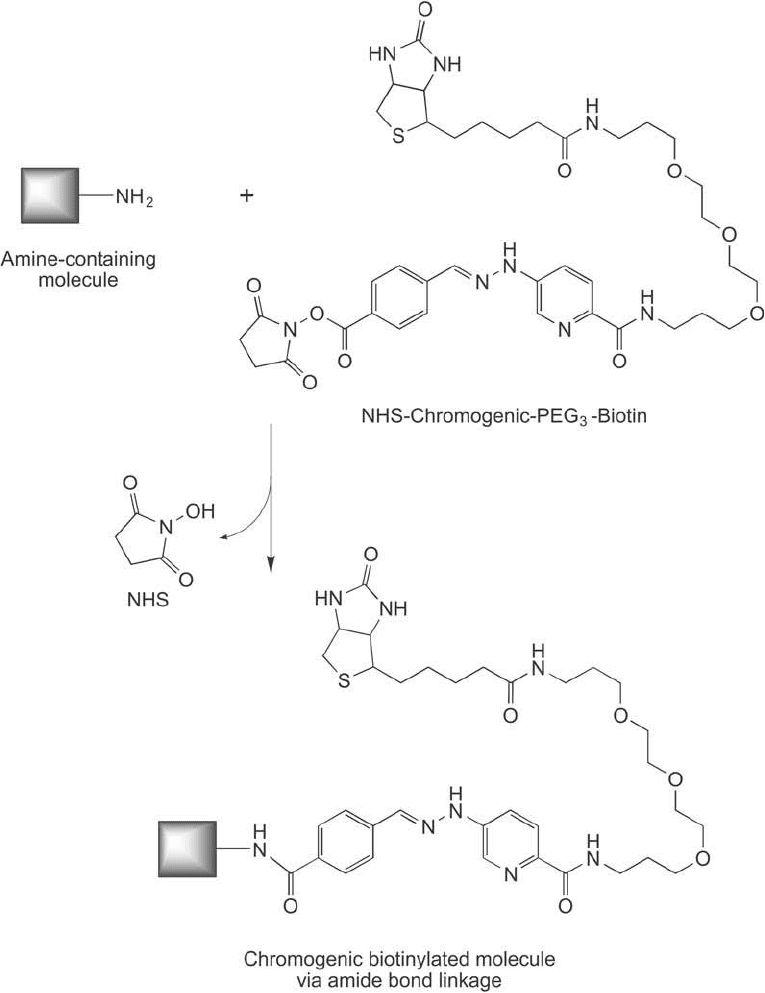
3. Biotinylation Reagents Containing Discrete PEG Linkers 731
Figure 18.16 NHS-chromogenic-PEG
3
-biotin reacts with amine groups in proteins or other molecules to form
amide bond derivatives.
732 18. Discrete PEG Reagents
that protein solutions that are more dilute than this may require higher levels of bioti-
nylation reagent addition to achieve the same yield of modifi cation.
2. In a fume hood, dissolve NHS-chromogenic-PEG
3
-biotin in DMF at a concentration of
12.33 mM (2 mg/200 l DMF). With mixing, add a quantity of the reagent to the protein
solution to provide the desired molar excess (i.e., 10- to -20 fold excess).
3. React for 30–60 minutes at room temperature or 2 hours at 4°C.
4. Purify the modifi ed protein from unreacted biotinylation reagent and reaction by-prod-
ucts using dialysis or gel fi ltration. Complete removal of the excess reagent is necessary
to provide accurate measurement of the biotin incorporation level by absorptivity.
5. Measure the absorbance of the biotinylated protein solution at 354 nm. Use the molar
extinction coeffi cient for the chromogenic group ( 29,000 M
1
cm
1
) to determine the
concentration of biotin present. To determine the molar ratio of biotin-to-protein, divide
the molar concentration of biotin by the molar concentration of protein present (which
may be determined by using the Coomassie assay or the BCA assay methods).
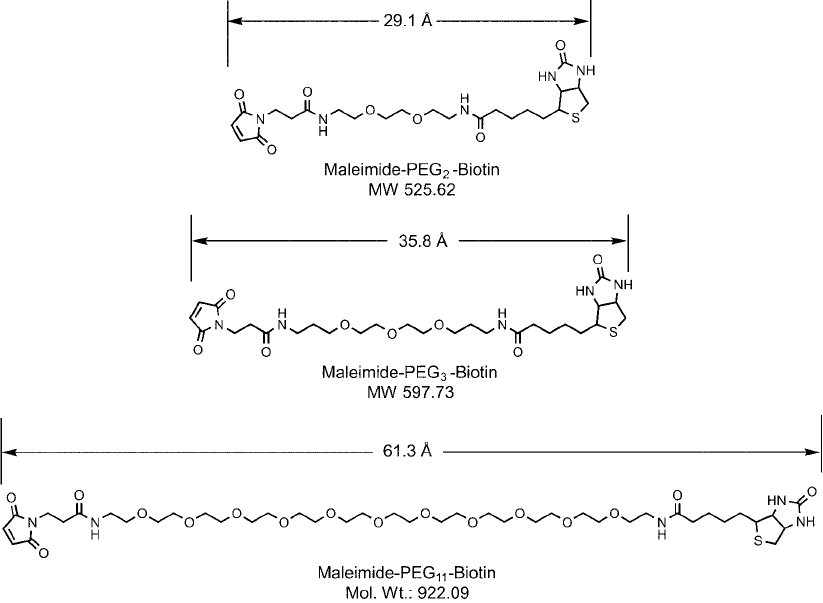
3.3. Maleimide–PEG
n
–Biotin Compounds
Discrete PEG–biotin compounds containing a terminal maleimide group may be used to label sulf-
hydryl-containing proteins and other molecules through thioether bond formation ( Figure 18.17 ).
The targeting of thiol groups in proteins often is used to direct the modifi cation reaction away
from binding sites or active centers in proteins, thus preserving activity. Maleimide reagents in
general are the second most-popular reactive group used for bioconjugation purposes, second only
to NHS esters. Unlike biotinylation compounds containing a hydrophobic hydrocarbon chain
(Chapter 11), the discrete maleimide–PEG-based reagents provide increased hydrophilicity for
modifi ed molecules and maintain solution stability even at high substitution levels.
The maleimide group reacts with thiols in the pH range of 6.5–7.5 to form a stable thioether
linkage with very little cross-reactivity with amines at this pH ( Figure 18.18 ). However, the
maleimide ring is subject to hydrolysis in aqueous solution, and since it is next to an extremely
hydrophilic PEG chain in these reagents, this factor may increase the hydrolysis rate beyond
that typically observed with hydrocarbon-based spacers (Chapter 2, Section 2.2). For this rea-
son, stock solutions of a maleimide–PEG
n
–biotin compound should be made in highly pure and
dry organic solvent, which then can be added to an aqueous reaction medium to commence the
biotinylation process.
The three maleimide–PEG
n
–biotin compounds illustrated in this section provide short,
medium, and very long chain spacer options, with the longer chains resulting in the greatest
degree of hydrophilicity of modifi ed molecules. The following protocol is adapted from general
maleimide-based biotinylation methods, as discussed in Chapter 11, Section 2.
Protocol
1. Dissolve a sulfhydryl-containing protein or other thiol-molecule in a thiol-free buffer
within a pH range of 6.5–7.5. The use of 20 mM sodium phosphate, 150 mM NaCl,
pH 7.2, works well for this reaction. The concentration of protein should be in the range
of 1–10 mg/ml. Lower concentrations of protein may result in the need to increase the
molar excess of biotinylation reagent to obtain an acceptable level of modifi cation. If a
thiol is not present on the molecule to be biotinylated, one may be created by disulfi de
reduction or through the use of a thiolation reagent (Chapter 1, Section 4.1).
2. Prepare a stock solution of the maleimide–PEG
n
–biotin compound in DMAC, DMSO, or
DMF (pure and dry solvents only) at a concentration of 10–20 mM.
3. With mixing, add an aliquot of the biotin solution to the protein solution to obtain at
least a 10-fold molar excess over the quantity of protein present. As thiols typically are
present in limiting amounts on proteins, the use of a high-molar reagent ratio is not
required to achieve acceptable yields of biotinylation.
4. React with gentle mixing for 2 hours at room temperature or 4 hours at 4°C.
5. Purify the biotinylated protein by dialysis or gel fi ltration using a desalting resin.
3.4. Hydrazide-PEG
4
-Biotin
Hydrazide-containing PEG-biotinylation reagents provide reactivity with carbonyl groups
(e.g., aldehydes) to label carbohydrates or glycoproteins via hydrazone bond formation ( Figures
18.19 and 18.20 ). The hydrazide group also may be coupled with carboxylate-containing
3. Biotinylation Reagents Containing Discrete PEG Linkers 733
Figure 18.17 Maleimide–PEG
n
–biotin compounds of three different discrete PEG sizes are available, including
a PEG
11
chain that provides a molecular length of over 60 Å.
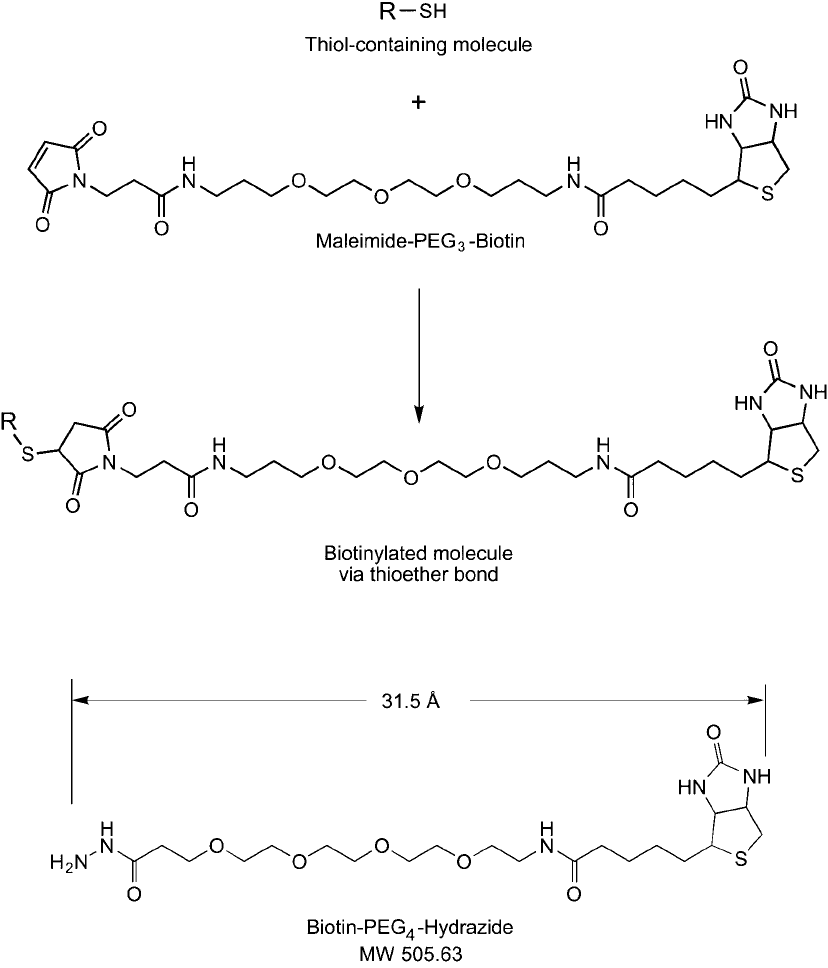
734 18. Discrete PEG Reagents
Figure 18.18
Maleimide–PEG
n
–biotin compounds react with thiol-containing molecules to form thioether linkages.
Figure 18.19 Biotin-PEG
4
-hydrazide is a hydrophilic biotinylation reagent that can be used to modify glycans
or carbohydrates at their reducing end or after periodate oxidation to create aldehydes.
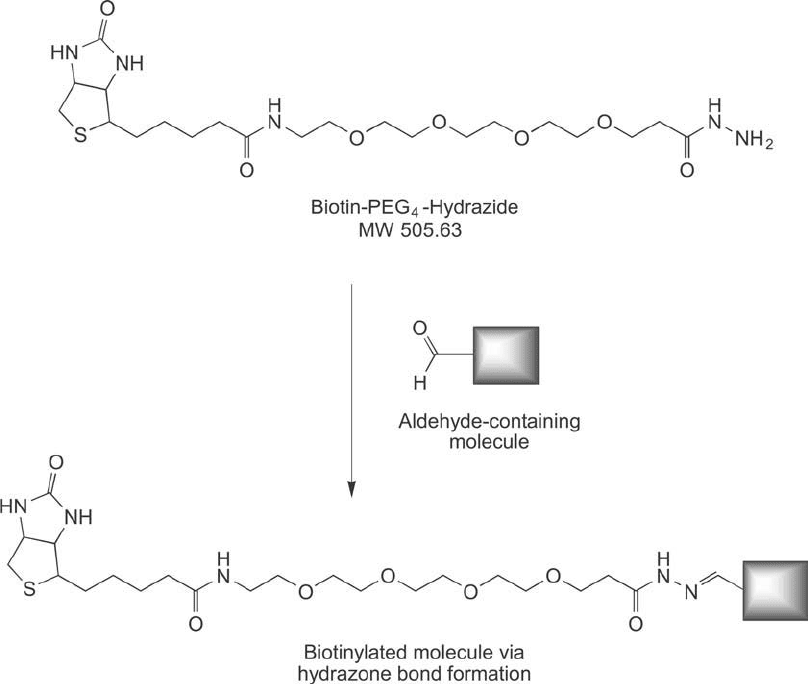
3. Biotinylation Reagents Containing Discrete PEG Linkers 735
Figure 18.20 Biotin-PEG
4
-hydrazide reacts with aldehyde-containing molecules to form a hydrazone linkage.
molecules using a carbodiimide reaction with EDC (Chapter 3, Section 1) or an active
ester derivative (Chapter 2, Section 1). Like the other discrete PEG reagents, the hydrazide-
PEG
4
-biotin compound is very hydrophilic and won ’t promote aggregation or precipitation of
labeled proteins. Aldehyde functionalities may be created on glycoproteins or other carbohy-
drates by oxidation using sodium periodate (Chapter 1, Section 2.2, 4.4–4.6) or by modifi -
cation with SFB (Chapter 17, Section 2). The reducing end of sugars or glycans also may be
labeled with these hydrazide reagents to produce a biotin–carbohydrate that is modifi ed at only
a single site.
Hydrazide-PEG
4
-biotin can be used to label specifi cally glycoproteins on cell surfaces after
mild periodate oxidation of the glycan structures (Wilchek and Bayer, 1987). The hydrophilic
nature of the PEG spacer will prevent the biotinylation reagent from easily penetrating cell
membranes, thus the labeling reaction is restricted to outer membrane glycoproteins. After
biotinylation and cell lysis, the labeled proteins may be detected or isolated using (strept)avidin
reagents (Jang and Hanash, 2003; Ding et al ., 2005; Handlogten et al ., 2005).
736 18. Discrete PEG Reagents
Hydrazide-PEG
4
-biotin contains a 31.5 Å spacer consisting of four ethylene oxide units,
which effectively imparts water solubility to the reagent. Other hydrazide biotinylation rea-
gents that contain no spacer or a hydrocarbon spacer, such as biotin-hydrazide and biotin-
LC-hydrazide, are water-insoluble and actually will lower the solubility of modifi ed molecules.
Hydrazide-PEG
4
-biotin can be used to modify molecules without the tendency for aggregation
or precipitation. In addition, the compound is stable in aqueous environments, as it contains
no groups that are easily hydrolysable. A stock solution may be prepared in a water-miscible
organic solvent such as DMAC, DMSO, or DMF to facilitate transfer of a small amount to an
aqueous reaction.
The following protocol describes a method for the periodate oxidation of a glycoprotein
followed by biotinylation of the resultant aldehydes using hydrazide-PEG
4
-biotin. Chapter 1,
Section 4.6 describes an alternative protocol for the modifi cation of glycans at their reducing
ends with hydrazide compounds.
Protocol
1. Dissolve a glycoprotein to be oxidized in 0.1 M sodium acetate, pH 5.5 (oxidation
buffer), at a concentration of 2–10 mg/ml. PBS at physiological pH may be used for this
reaction, as well. The use of cold buffers for the oxidation step will limit the extent of
carbohydrate oxidation and the potential for protein oxidation.
2. Dissolve sodium meta-periodate in oxidation buffer at a concentration of 20 mM. Protect
from light.
3. Add an equal volume of the glycoprotein solution to the periodate solution with mixing.
4. React for 10–20 minutes with gentle mixing and protected from light.
5. Quench the oxidation reaction by the addition of at least a 4-fold molar excess of
N-acetylmethionine or sodium sulfi te over the concentration of periodate in the reaction
mixture (e.g., 40 mM). Pre-dissolve the quencher in buffer at a higher concentration prior
to adding an aliquot of it to the reaction solution. React for 10 minutes. Alternatively,
the oxidation reaction may be stopped by the removal of excess periodate by gel fi ltra-
tion using a desalting column.
6. Prepare a 50 mM solution of hydrazide-PEG
4
-biotin in DMAC, DMSO, or DMF. Add
a quantity of this solution to the purifi ed, oxidized protein to provide at least a 10-fold
molar excess of biotinylation reagent over the concentration of protein present.
7. React with mixing for 2 hours at room temperature.
8. The hydrazone bond can be reduced to stabilize the linkage by the addition of sodium
cyanoborohydride to a fi nal concentration of 50 mM. React for 30 minutes at room tem-
perature with mixing. All operations with cyanoborohydride should be done in a fume
hood. If the glycoprotein being modifi ed is sensitive to disulfi de reduction and potential
denaturation, then this step should be avoided.
9. Purify the biotinylated glycoprotein by gel fi ltration or dialysis.
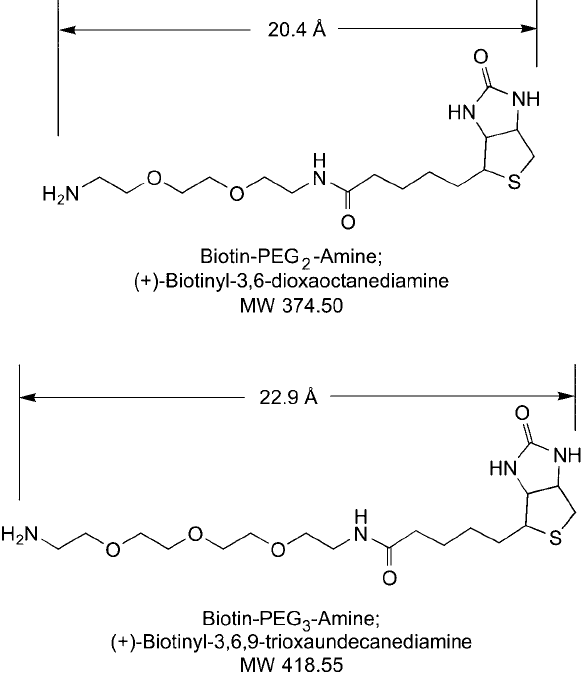
3.5. Biotin-PEG
n
-Amine Compounds
Biotin compounds containing a PEG spacer that terminates in a primary amine can be used
for the labeling of carboxylate molecules ( Figure 18.21 ). Activated carboxylates, such as those
containing an NHS ester, spontaneously react with the amines to give amide bond linkages. An
active ester also may be formed in situ by the activation of carboxylates with EDC in the pres-
ence of NHS or sulfo-NHS (Chapter 3, Section 1) ( Figure 18.22 ).
Biotin-PEG
2
-amine contains a short, two-unit ethylene oxide cross-bridge that provides
a 20.4 Å hydrophilic spacer, which has an amine on its end. Biotin-PEG
3
-amine is identical
except for one additional ethylene oxide unit. Both compounds are extremely water-soluble
and can be used to label organic molecules or biomolecules containing carboxylates. Bronfman
et al. (2003) used the biotin-PEG
n
-amine compounds to label nerve growth factor (NGF) pep-
tide on its carboxy lates using EDC-mediated amide bond formation. The biotinylated growth
factor then was used to study receptor internalization in live cells by probing with fl uorescent
streptavidin conjugates.
The following protocol can be used to biotinylate carboxylate-containing molecules in aque-
ous solution using the EDC/sulfo-NHS reaction.
3. Biotinylation Reagents Containing Discrete PEG Linkers 737
Figure 18.21 Biotin-PEG
n
-amine compounds can be used to modify carboxylate- or aldehyde-containing com-
pounds using a carbodiimide reaction.
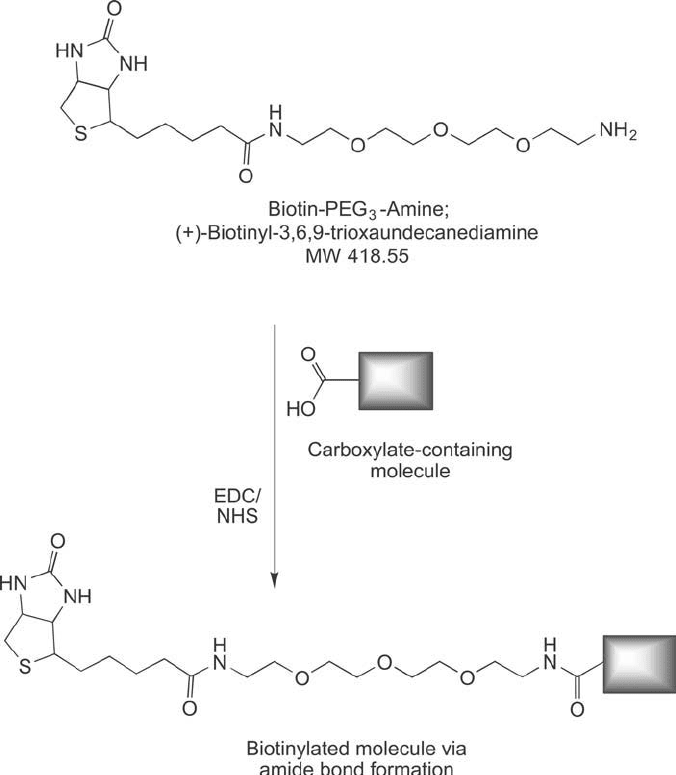
738 18. Discrete PEG Reagents
Figure 18.22
Biotin-PEG
n
-amine can be used to add a biotin label to carboxylate-containing molecules using
the EDC/(sulfo)NHS reaction, which forms a stable amide linkage.
Protocol
1. Dissolve a carboxylate-containing peptide or other molecule in 0.1 M MES, pH 5.0 (reac-
tion buffer). Ideally, this molecule should contain only one carboxylate with no amines
to direct biotinylation to a single site and prevent polymerization of it during the conju-
gation process. However, if a peptide is to be biotinylated that also has amine groups,
then the use of a very high molar excess of the biotin-PEG
n
-amine reagent during the
reaction will limit the potential for peptide–peptide linking. The concentration of the car-
boxylate molecule should be low if it also has amines presence, but if it only has one or
more carboxylates, then it can be prepared at higher concentration. For example, to use
the biotin-PEG
n
-amine compounds to biotinylate a protein, the concentration should be
on the order of 1–2 mg/ml so that a large excess of biotinylation agent can be added. For
molecules that are sparingly soluble in aqueous solution, they may be dissolved fi rst in
ethanol and then added to the reaction buffer with mixing to make a fi nal ethanol con-
centration of not more than 50 percent.
2. Dissolve the biotin-PEG
n
-amine reagent in reaction buffer at a concentration of 25 mM.
3. Add a quantity of the biotin-PEG
n
-amine solution to the solution containing the car-
boxylate molecule to achieve the desired molar excess. For molecules containing a single
carboxylate to be modifi ed, a 1.5- to 2-fold molar excess may be suffi cient. However, for
proteins or peptides that also contain competing amines, a much larger excess of biotin
compound should be used (e.g., 100-fold excess). For instance, for protein biotinylation,
add 120 l of the biotin-PEG
n
-amine solution per ml of the solution prepared in Step 1.
4. Immediately before use, dissolve EDC in reaction buffer at a concentration of 25 mM.
Add 12 l of this solution per ml of the combined solution from Step 2. Mix well.
5. React for 2 hours at room temperature or 4 hours at 4°C with gentle mixing.
6. Purify the biotinylated protein or molecule using dialysis or gel fi ltration. For small mol-
ecule biotinylation where these separation methods may not be appropriate, other pro-
cedures may have to be developed, such as reverse-phase chromatography or organic
precipitation techniques.
3.6. Biotin–PEG
3
–Benzophenone
Biotin–PEG
3
–benzophenone is a biotinylation reagent with a hydrophilic spacer containing three
ethylene oxide units and a photoreactive group at its end (Quanta BioDesign). The benzophenone
is activated by UV light to an extremely reactive triplet-state ketone, which can insert into C H,
N H, and other structures, resulting in a covalent bond (Chapter 2, Section 7.2). The reac-
tion is one of the most effi cient photoreactive conjugation mechanisms available (Campbell and
Gioannini, 1979). Thus, this reagent provides a method of adding a biotin group to molecules that
don’t contain typical functionalities useful for bioconjugation. This may include polymeric sur-
faces or organic molecules lacking reactive targets ( Figure 18.23 ).
The presence of the PEG
3
spacer in this compound provides water solubility to the biotin
arm, whereas the benzophenone group should associate with more hydrophobic regions or sur-
faces, which may be ideal for the biotinylation photoreaction. The reagent can be used by dis-
solving it in an aqueous buffer suitable for use with whatever substance is to be biotinylated.
After mixing this solution with the target molecule or surface, exposure to UV light will initiate
the conjugation reaction. Unlike other photoreactive groups, a benzophenone doesn ’t undergo
decomposition to an inactive form if it doesn ’t couple to target molecules. Instead, it degrades
from the photo-excited state back to its initial state, so it can be once again photolyzed to an
active state. This process increases the likelihood that the benzophenone will couple to a target
molecule during the photoreaction. See Chapter 5, Section 4.3 for an illustration of the benzo-
phenone coupling reaction.
4. Discrete PEG Modifi cation Reagents
Large polymer PEG reagents having molecular weights 2,000 Da have been used for over 20
years as modifi cation agents for biological molecules (Chapter 25). These compounds often are
4. Discrete PEG Modifi cation Reagents 739
