Heitjans P., Karger J. (Eds.). Diffusion in Condensed Matter: Methods, Materials, Models
Подождите немного. Документ загружается.

17 Field-Assisted Diffusion Studied by Electrophoretic NMR 731
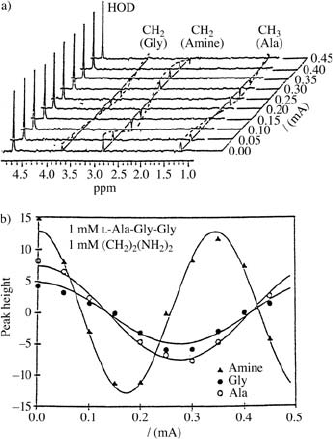
Fig. 17.8. (a)
1
H ENMR (250 MHz)
spectraofamixtureof1mMethylene
diamine with an amino acid (1 mM L-
Ala-Gly-Gly) in D
2
O [10].
(b) The intensity of the
1
H NMR lines
in Fig. 17.8a versus current I.From
the different cosine modulations of the
amine line and the amino acid lines,
the different mobilities can be derived
[10].
technique, which is also independent of optical properties, is a unique tool for
the investigation of the transport of ionic charge carriers in disordered media.
Thus, it is not surprising that this field of ENMR application developed
comparatively fast in the last years. The first successful measurements of
ionic mobilities in porous systems, namely in gels, have been performed in
the author’s laboratory [26, 27]. It could be demonstrated that in the fluid-
filled pore space both the observed ionic self-diffusion coefficient D
±
and
the ionic mobility u
±
in an external electric field show a dependence on the
observation time ∆ (see Fig. 17.9). As previously observed for liquid molecules
confined in porous media the phenomenon of “anomalous diffusion” occurs
(see Chaps. 10, 19 and 22). Now, this phenomenon has been also observed
for self-diffusion of charged species and for the first time it has been shown
that also the analogous phenomenon of “anomalous field-assisted diffusion”
or “anomalous electric mobility” exists [26], which means that there is a
time regime, where the average displacement z(∆) of an ion by migration
along the electric field is a non-linear function of time. At long observation
times ∆ the quantities D
+
and u
+
become independent of ∆ and reach their
effective values D
eff
+
and u
eff
+
in the porous system, the quantities D
+
/D
0
+
and u
+
/u
0
+
in Fig. 17.9 thus reach plateau values. (The index “0” refers to
quantities in the non-confined state.) The inverse plateau value T (D
+
)=
D
0
+
/D
eff
+
delivers an important characteristic value of the porous medium,
namely its tortuosity T (see, e. g., [28]; cf also Fig. 10.13 in Chap. 10). T of a
porous medium is usually defined as the squared ratio of the true migration
path length of a fluid particle and the straight-line distance between the
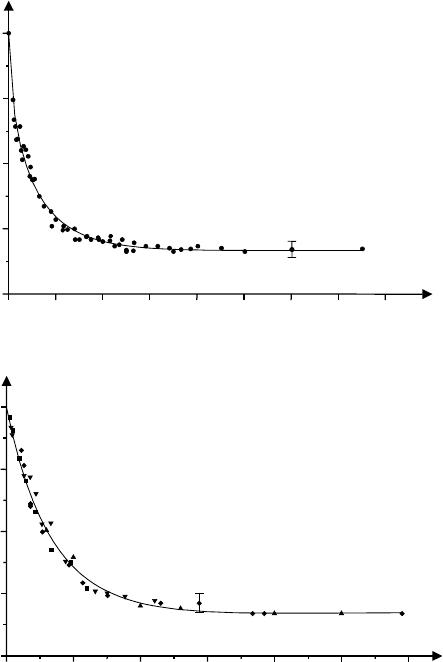
732 Manfred Holz
(a)
0
0.2 0.4 0.6 0.8 1.0 1.2 1.4 1.6
0.2
0.4
0.6
0.8
1.0
[s]
D
+
/D
0
+
(b)
0 200 400 600 800 1000 1200
0.2
0.4
0.6
0.8
1.0
u
+
/u
+
0
"'[Vm
-1
s]
Fig. 17.9. (a) The relative ionic self-diffusion coefficient D
+
/D
0
+
of the (C
4
H
9
)
+
4
cation in aqueous solution confined in the gel Sephadex LH-20 as a function of the
observation time ∆. D
0
+
=0.49 × 10
−9
m
2
s
−1
is the ionic self-diffusion coefficient
in the non-confined bulk solution [26].
(b) The relative ionic mobility u
+
/u
0
+
as a function of the product of observation
time ∆
and the externally applied electric field E. u
0
+
=1.22 × 10
−8
m
2
s
−1
V
−1
.
All other details as in (a).
starting and finishing point of the particle’s motion in this medium. Since,
in general, the translational motion can have its origin in different processes
as flow, diffusion or electric field-assisted diffusion, one sometimes speaks
of “hydraulic”, “diffusional” or “electric” tortuosity, respectively [29]. From
the plateau value T (u
+
)=u
0
+
/u
eff
+
again the tortuosity of the system is
17 Field-Assisted Diffusion Studied by Electrophoretic NMR 733
available, now measured via the electric mobility. The comparison of the
behaviour of the ionic self-diffusion coefficient and the electric mobility of
the same ion in the medium with internal boundaries allows a first direct
experimental check of the validity of a very important relation in porous
media, namely the Einstein relation (D
±
∝ u
±
, see (17.1)). Fig. 17.9 reveals
that T (u
+
)=T (D
+
) is found, which means that indeed in the porous gel
system under investigation the Einstein relation remains valid [26,27]. In the
paper by Holz et al. [27], also a first attempt has been undertaken to derive
the specific surface S/V
p
of the porous medium from the time-dependence
of the field-assisted diffusion, similarly, as it has been performed, e. g., by
means of the observation time-dependence of the self-diffusion coefficient in
Sect. 10.5.3 of Chap. 10 [30–32]. The present author and his co-workers also
discussed basic advantages of time-dependent electric mobility measurements
for morphology studies of porous media [27].
Very important special porous media in life science and in technical appli-
cations are membranes, and it has been pointed out that ENMR might be a
powerful technique for the study of ion transport in those media [9]. Polymer
electrolyte membranes are of outstanding interest in connection with fuel
cells. Ise et al. [33] succeeded in measuring by ENMR the electro-osmotic
drag coefficient K
drag
in polymer membranes. This drag coefficient is defined
as the number of water molecules transferred through the membrane per H
+
ion, and the authors could determine K
drag
as a function of water content
and temperature for different membrane materials as Nafion and sulfonated
polyetherketones.
Anion and cation (Li
+
) transference numbers have been studied in com-
posite polymer electrolytes with fumed silica as an inorganic filler by
19
Fand
7
Li ENMR, respectively, [34, 35]. In this way the ionic transport properties
could be investigated as a function of filler content and Li salt concentration
without the usual need of assumptions. The power and validity of ENMR
could be demonstrated by the independent measurement of the anion and
cation transference numbers for different salts, where within the experimen-
tal error these transference numbers sum to unity. Also in lithium polymer
gel electrolytes, used in lithium secondary batteries, the individual ionic mo-
bilities of cations and anions could be measured by
7
Li and
19
FENMRexper-
iments [36]. The authors also observed by
1
H ENMR a solvent drift in the gel
electrolyte when the strength of the applied electric field exceeds a distinct
value, where also an anomalous change of the Li
+
mobility occurs. They dis-
cuss as a possible reason a flow of lithium, compensating lithium deposition
at the electrode surface, possibly causing a counter flow of solvent.
Detection and Identification of Charged Species by ENMR Phase
Difference Spectroscopy
We saw that for resonance lines of coherently moving particles a typical phase
shift ∆φ occurs in the spectrum (Sect. 17.2.2). If a mixture contains both
charged and uncharged species, in an ENMR experiment only the lines of
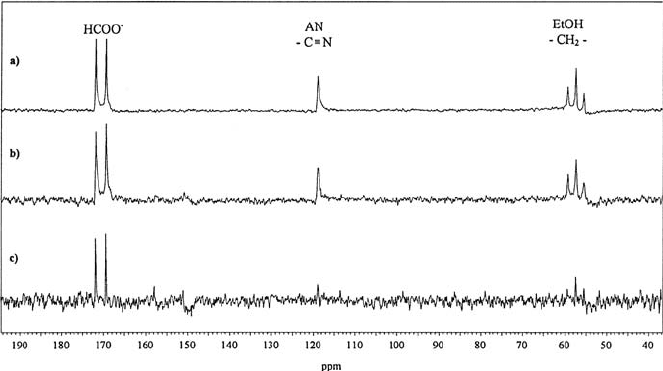
734 Manfred Holz
Fig. 17.10. “Mobility filter” ENMR experiment:
13
CNMRspectraofanaqueous
mixture of ethanol (EtOH), acetonitrile (AN) and sodium formate [40]. (a) Normal
(undecoupled)
13
CNMRspectrum(I = 0); (b)
13
CENMRspectrum(I =0);
(c) Difference spectrum of (a) and (b), where only the lines of charged species
(HCOO
−
)remain.
charged species are phase-shifted while the lines of uncharged particles are
not affected. This fact can be utilized for the detection and identification
of charged species in multi-component mixtures, provided that these species
contain the nucleus under observation. As an example of such a procedure,
we show first in Fig. 17.10 (a) the normal
13
C spectrum (I =0)ofanaque-
ous mixture of ethanol (EtOH), acetonitrile (AN) and sodium formate [40].
When the same spectrum is then taken in presence of an electric field (I =0)
(Fig. 17.10(b)) only the formate ion lines are phase-shifted and in the differ-
ence spectrum of the spectra with I =0andI = 0 the lines without a phase
shift disappear and only the lines of the ion remain (see Fig. 17.10 (c)).
In this way, in a simple 1D ENMR experiment a “mobility filter” can
be generated, which is able to filter from a complex spectrum only the lines
of migrating species, which in this way can be detected and simultaneously
identified via their NMR lines [40].
17.5.2 2D and 3D Experiments
In 2D NMR a two-dimensional data set is generated and the signal intensities
S(F
1
,F
2
) are given as a function of two variables F
1
and F
2
. One advantage
of 2D NMR is the fact that the spectrum is stretched in two dimensions and
much more lines can be resolved. However, the main advantage is that in
the 2D spectrum “correlations” between certain values of F
1
and F
2
can be
detected and assigned.
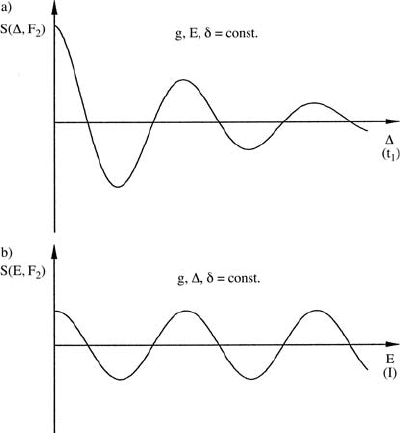
17 Field-Assisted Diffusion Studied by Electrophoretic NMR 735
Fig. 17.11. ENMR signal intensity S at a given frequency F
2
,(a)when∆ = t
1
is
(stepwise) varied, (b) when the electric field E (current I)isvaried.
The 2D spectrum is obtained by recording the NMR signals in the time
domain as a function of the acquisition time t
2
,asinnormal1DNMR,
however, this is performed k times, whereby another time t
1
(or another
variable) is stepwise increased in k increments resulting in k signals with 2N
data points:
S
1
(t
(1)
1
,t
2
),S
2
(t
(2)
1
,t
2
), ··· ,S
k
(t
(k)
1
,t
2
).
Thereafter, a first Fourier transformation with respect to t
2
is performed,
followed by a second Fourier transformation with respect to t
1
, yielding the
2D spectrum S(F
1
,F
2
)withk × N data points [2, 16].
In 2D ENMR, F
2
is the chemical shift axis, which allows the identifi-
cation of the chemical species and F
1
is the mobility axis. In this way the
observed species are “correlated” with their mobility in the electric field. This
kind of experiments, developed by Johnson and co-workers [10, 11], is called
“mobility-ordered spectroscopy” (MOSY) and it should be mentioned that
also very interesting analogous 2D “diffusion-ordered spectroscopy” (DOSY)
experiments have been developed, where the second axis is the diffusion
axis [11, 41].
In practical 2D ENMR two quantities can be incremented, namely ∆ in
the PFG experiment (Fig. 17.11) or the velocity v, via a stepwise increase of
the electric field E and thus of the current I.Ifwechooset
1
= ∆ and stepwise
increase ∆, the influence of normal diffusion on the decay of the transverse
magnetization S also increases (see (17.15) and Fig. 17.11 (a)), leading to a
736 Manfred Holz
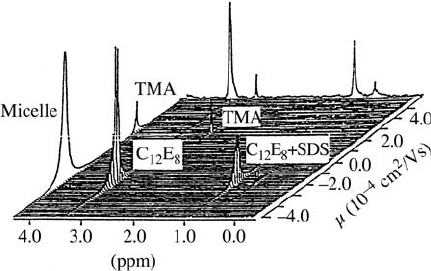
Fig. 17.12. Mobility-ordered (MOSY) 2D ENMR spectrum (stacked plot) of
(CH
3
)
+
4
(TMA) in the presence of mixed micelles [11]. [Sodium dodecyl sulfate
(SDS) and octaethylene glycol dodecyl ether (C
12
E
8
)]. Note the different sign for
the mobilities of TMA and C
12
E
8
.
line broadening in the 2D spectrum. Thus, it proved to be more favourable
to hold constant all the timing parameters and to increment the field E since
in this case the intensity oscillations are not damped (Fig. 17.11 (b)) [10,11].
2D ENMR experiments require the observation (and Fourier transformation)
of several intensity oscillations as a function of ∆ or E, that means in these
experiments high ∆φ-values must be achieved. Since ∆φ is proportional to
the magnetogyric ratio γ of the nucleus, 2D ENMR is much easier performed
with high-γ nuclides as
1
H,
19
F than with nuclides having a lower γ as
7
Li or
13
C [40]. So far, 2D experiments have only been performed with
1
HENMR.
In Figs. 17.12 and 17.13 two examples of MOSY experiments are given,
where in Fig. 17.12 the 2D spectrum is shown as a stacked plot and in
Fig. 17.13 as a contour map, which is the mostly used representation of
spectra in 2D NMR. The two examples show the most interesting field of
application of 2D ENMR, namely mobility measurement of molecular aggre-
gates, as micelles and vesicles. In Fig. 17.12 the
1
HMOSYspectrumofa
mixture of (CH
3
)
4
N
+
ions and micelles is shown. This spectrum also demon-
strates the important fact that in ENMR not only the amplitude but also
the sign of the mobility is obtained, allowing the determination of the sign of
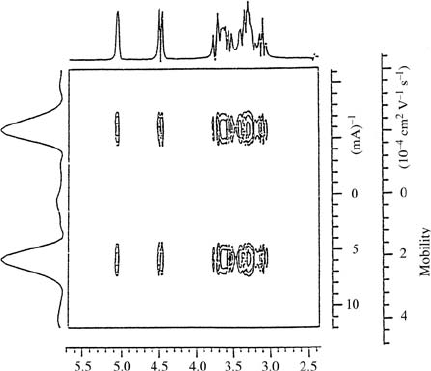
charge of the species of interest. In the second example (Fig. 17.13) glucose,
as a good NMR active label, has been entrapped inside vesicles, for which in
this way the vesicle mobilty in water is found to be u =2×10
−8
m
2
V
−1
s
−1
.
A very interesting extension of ENMR, namely 3D ENMR for the study
of protein mixtures, has been demonstrated by He and co-workers [37, 38].
With two frequency axes a 2D spectrum of the protein mixture is generated.
However, in protein mixtures severe signal overlapping occurs and conven-
tional NMR methods have difficulties in characterising structures or struc-
tural changes of multiple protein components in biochemical reactions. Now
17 Field-Assisted Diffusion Studied by Electrophoretic NMR 737
Fig. 17.13. Example of a MOSY contour map. 2D
1
H ENMR spectrum of glucose
entrapped inside vesicles [11]. The 1D NMR spectrum of glucose is shown as a
projection at the top. At the left the 1D mobility spectrum is given.
the overlapping signals can be separated in a 3D experiment, where the third
axis is the electrophoretic mobility, allowing the simultaneous measurement
of the different proteins due to their differing mobility. The otherwise needed
physical separation is no more necessary. “Capillary array ENMR”, devel-
oped by the same group [39], allows the study of protein mixtures at high
ionic strength in buffer solutions.
17.5.3 Mobility and Velocity Distributions. Polydispersity and
Electro-Osmotic Flow
In Fig. 17.11 (b) we saw that in an ENMR experiment the intensity oscil-
lations are not damped if we vary the electric field E. However, there it
was supposed that all observed particles have the same velocity. In polydis-
perse samples, on the other hand, we have a distribution of mobilities g(u)
of particles with the same chemical shift (of the same chemical kind) but
of different size and mass. In such a case, in (17.14), (17.15) we have to
replace cos(γgvδ∆)=cos(γguEδ∆)by
-
+∞
−∞
g(u)cos(γguEδ∆)du and this
results in a damping of the intensity oscillations as a function of E (or I)
(see Fig. 17.14(a). However, from an inverse FT of S(E,F
2
) with respect to
E (or I) the mobility distribution g(u) can be derived (Fig. 17.14 (b) [10,42].
Even for monodisperse solutions the presence of electro-osmotic flow leads
to mobility (velocity) distributions. Such a mobility distribution was studied
in the just described manner in an oil-in-water microemulsion [43]. The aim
was the study of the development of the electro-osmosis profile in a vertical
738 Manfred Holz
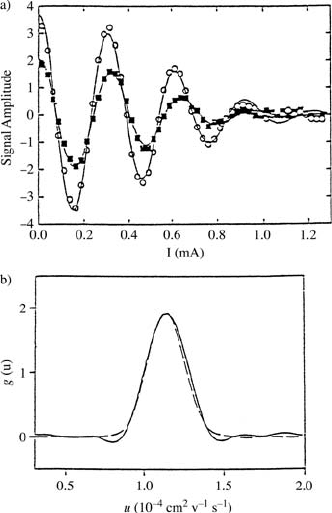
Fig. 17.14. (a)
1
HENMRsig-
nal intensity of sucrose entrapped in
charged phospholipid vesicles versus
current I in a polydisperse system,
showing the damping of S(E, F
2
) due
to a mobility distribution [42]. (b) The
mobility distribution g(u) obtained by
a Fourier transformation of the data in
(a) (solid line). The dashed line repre-
sents a fit curve [42].
cylindrical tube as a function of ∆ and as a function of t
el
,i.e.ofthetime
during which the current flowed before the PFG experiment was started.
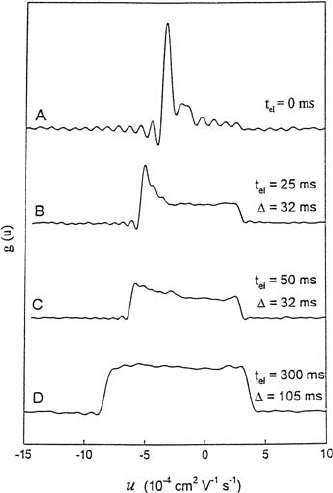
The result (Fig. 17.15) shows the development of the mobility distribution
g(u) with time, when both electrophoretic and electro-osmotic mobilities are
present. The electrophoretic migration velocity is then superimposed by an
opposite flow at the wall and a counterflow in the centre of the tube. After
a time of ca 400 ms in this example the system reached equilibrium and, as
theoretically expected, the flow profile, that is the mobility as a function of
thetuberadiusr
t
, becomes purely parabolic [42].
For the study of the electro-osmotic flow profile in capillaries also highly
sophisticated NMR micro-imaging techniques have been applied, which as
well deliver the velocity distributions and their time dependence [44].
The DCNMR studies described in this section might be a very interest-
ing tool for future developments in capillary electrophoresis (CE), where the
electro-osmotic flow is the driving force in these extremely important sepa-
ration techniques in biochemistry.
17.6 Conclusion
The possibility of performing NMR experiments in presence of an electric
current in the sample under investigation opens a novel way of using NMR
17 Field-Assisted Diffusion Studied by Electrophoretic NMR 739
Fig. 17.15. Mobility (velocity) dis-
tributions g(u) in presence of electro-
osmotic flow for various electric field
durations t
el
and flow times ∆,show-
ing the development of the electro-
osmotic flow profile in an “oil-in-
water” microemulsion [43].
techniques in the wide field of electrochemistry and its applications. The
electric charge or the electric current is thus introduced as a new parameter in
the NMR experiment. Electrophoretic NMR combines in-situ electrophoresis
with the selectivity of NMR, a fact, which is of particular importance for
the study of transport properties in complex multi-component fluid systems
with charge-carrying species, such as simple ions, charged macromolecules,
and charged supramolecular systems. In mixtures of organic compounds the
single components can be simultaneously observed, however, separated by
1
H
or
13
CNMR.
The pulsed field gradient NMR methods allow the non-invasive measure-
ment of electrophoretic mobilities u
±
and one always obtains the self-diffusion
coefficient D
±
of the distinct charged particles as a by-product. This offers
the possibility of a systematic qualitative comparison of transport diffusion
(here field-assisted diffusion) with self-diffusion, that means transport in the
non-equilibrium state with transport in the equilibrium state. Furthermore,
being independent of the optical properties of the sample, the ENMR meth-
ods may also be applied to porous systems. This field of application is just
in its beginning, but seems to be very promising for basic electrophoretic
mobility studies in systems with confined geometry.
Apart from mobility and diffusion studies, DCNMR also offers a new en-
trance to the investigation of electro-osmotic flow and phenomena related
with it. In particular in capillaries and porous systems those electro-osmotic
740 Manfred Holz
flow studies, also combined with NMR imaging techniques, represent a pow-
erful new tool.
On the other hand, ENMR has certain limitations owing to the compar-
atively low sensivity of radiofrequency spectroscopy and owing to problems
with nuclides or systems with short nuclear magnetic relaxation times T
1
and
T
2
. However, it should be pointed out that the low energies connected with
electromagnetic radiofrequency waves have the advantage that investigations
can be performed without any disturbance of thermal equilibrium.
ENMR is a comparatively young and novel technique and the required
accessory units are partly not yet commercially available and therefore so far
we cannot speak of an NMR “routine technique”. On the other hand, the
power of ENMR and DCNMR methods has been impressively demonstrated
[9–11] and it is clear that a further development of special techniques and a
further extension of applications will take place in the coming years.
Notation
A cross section of the electrophoretic cell
B magnetic field
B
0
magnetic field, corresponding to the NMR reference frequency ω
0
c concentration of an electrolyte in the solution
D self-diffusion coefficient
D
∗
±
ionic self-diffusion coefficient at c → 0
D
0
±
ionic self-diffusion coefficient in the non-confined state
DCNMR direct current NMR
DOSY diffusion ordered spectroscopy
E electric field
ENMR electrophoretic NMR
F Faraday constant
g magnetic field gradient
g(u) mobility distribution
I electric current
I
i
ionic strength
K
drag
electroosmotic drag coefficient
MOSY mobility ordered spectroscopy
PFG pulsed field gradient
P (ω) NMR frequency distribution
r radius of a particle
rf radio frequency
S NMR signal intensity
S
k
NMR signal intensity at the kth step in a 2D experiment
S
v
NMR signal intensity in presence of flow
S
0
initial NMR signal intensity
S/V
p
surface to pore volume ratio, also called “specific surface”
