Heitjans P., Karger J. (Eds.). Diffusion in Condensed Matter: Methods, Materials, Models
Подождите немного. Документ загружается.

17 Field-Assisted Diffusion Studied by Electrophoretic NMR 721
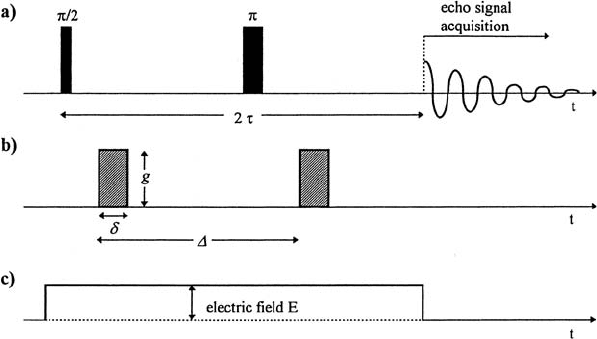
Fig. 17.1. Basic pulsed field gradient (PFG) spin-echo experiment in elec-
trophoretic NMR. The diagram of the pulse program shows the radiofrequency
pulses (π/2- and π-pulse) for the generation of the spin-echo signal at 2τ (a). Pulsed
field gradients with a strength g, a duration δ and a distance ∆ are applied for flow
(and diffusion) measurements (b). In an ENMR experiment the electric field E is
switched on in the sample cell during the spin-echo sequence (c). The acquisition
of the second half of the spin-echo signal starts at 2τ .
we show the effects of diffusion and flow in a magnetic field gradient g.We
see that in the case of diffusion the spins, which were at t = 0 in the plane
0, show at times t>0 a frequency distribution, whereas in the case of flow
(Fig. 17.2(b)) the frequency of all those species is regularly increasing with
t. Diffusion as an incoherent motion produces a damping of the spin-echo
signal due to random dephasing of the spin precession about the magnetic
field, whereas flow as a coherent motion produces a shift of the spin-echo
signal phase.
Since the NMR diffusion experiment is outlined in Chap. 10, we will here
deal only with the basics of NMR flow experiments. We consider a flow or
drift of particles in z-direction along a stationary gradient g = ∂B
z
/∂z.The
particles shall have a uniform velocity v and then they have at time t the
position z(t)=v · t. The nucleus residing in a particle experiences a time-
dependent magnetic field
B(z(t)) = B
0
+ z(t) · g, (17.9)
where B
0
represents the constant magnetic main field. The resonance fre-
quency ω(t) of such a nucleus is then
ω(t)=γB
0
+ γgvt. (17.10)
This means that at a time t a frequency difference with respect to the refer-
ence frequency ω
0
= γB
0
occurs. From (17.10) it follows
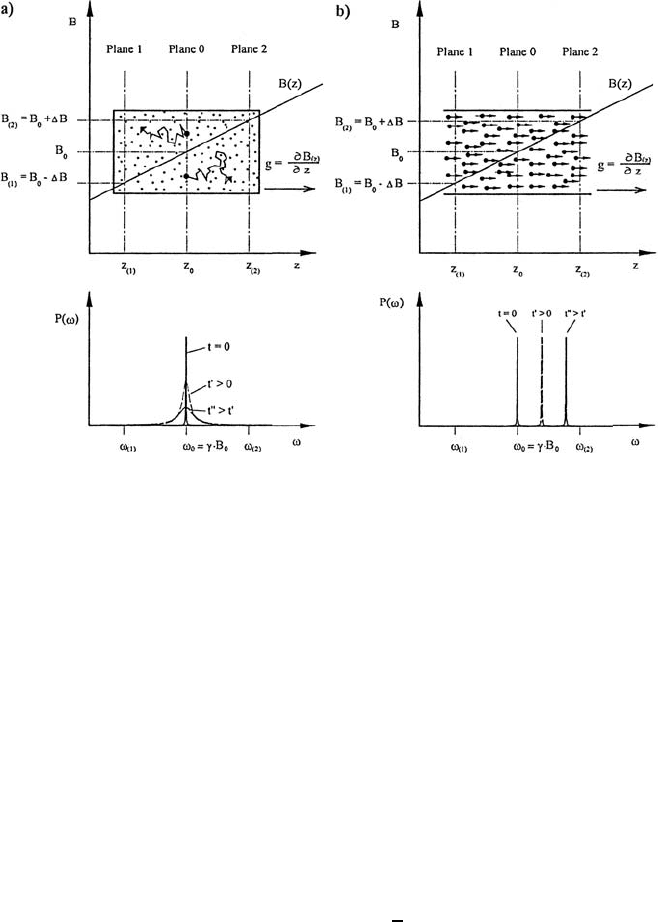
722 Manfred Holz
Fig. 17.2. The effect of diffusion (a), and flow (b) in the presence of a magnetic
field gradient g in z-direction. The magnetic field B(z) increases from left to right.
The nuclear spins in planes 0, 1 and 2 have different NMR resonance frequencies ω
0
,
ω
(1)
and ω
(2)
, respectively. In the lower diagrams the frequency distribution P (ω)
is given for the nuclear spins, which were at time t = 0 in the plane 0. In the case
of diffusion (a) the random walk of the spin-carrying particles results in a Gaussian
frequency distribution at times t>0, which is the origin of the echo damping. In
the case of plug flow (b) the frequency distribution is a δ-function, which is shifted
to higher frequencies ω with increasing t, resulting in a phase shift of the spin-echo
signal.
∆ω(t)=γgvt. (17.11)
Since ∆ω = ∆φ/t is valid, also a phase angle difference increasing with t,
with respect to the reference phase, appears. At time t = τ ,wheretheradio
frequency π-pulse in the spin-echo pulse sequence is applied (see Fig. 17.1),
we have a phase difference
∆φ(τ)=
τ
0
γgvtdt =
1
2
γgvτ
2
. (17.12)
The π-pulse has the effect (see also Fig. 13.2 of Chap. 13) that it inverts
all nuclear spin precession phases and thus it makes ∆φ(τ)to−∆φ(τ). At
the time 2τ, where the spin echo maximum appears and where the signal is
measured, we finally have a phase difference

17 Field-Assisted Diffusion Studied by Electrophoretic NMR 723
∆φ(2τ)=−∆φ(τ)+
2τ
τ
γgvtdt = γgvτ
2
. (17.13)
In an experiment with two pulsed field gradients of length δ and distance ∆
(see Fig. 17.1) (pulsed gradients have some important advantages over the
application of stationary gradients [4, 5, 7]), the phase shift at 2τ is simply
∆φ(2τ)=γgvδ∆. (17.14)
The measurement of ∆φ with known g, δ and ∆ then allows the determination
of the velocity v.
In phase sensitive signal detection, the phase shift ∆φ due to the flow
leads to a cosine modulation of the signal S
v
(2τ) (the subscript v stands for
presence of flow) as a function of g, v, δ or ∆. The total spin-echo signal
intensity in presence of diffusion, flow and T
2
relaxation is then [9]:
S
v
= S
0
cos(γgvδ∆) · exp
−
2τ
T
2
− γ
2
Dg
2
δ
2
∆ −
1
3
δ
. (17.15)
Here we see the three influences on the spin-echo signal, namely the flow
(cosine term), T
2
relaxation (first term in the exponent) and diffusion (second
term in the exponent). If we measure S
v
(2τ) and then switch off the flow (in
electrophoretic NMR one can switch off the electric current) and measure
S
v=0
(2τ), the simple intensity ratio
S
v
(2τ)
S
v=0
(2τ)
=cos(γgvδ∆) (17.16)
yields the influence of flow alone and represents the first simple method for
the actual measurement of v.
How is ∆φ, the quantity of interest, obtained in practice? In modern NMR
spectrometers the resonance signals are first excited and recorded in the time
domain and then transformed mathematically into the frequency domain by
a Fourier transformation (FT) yielding the NMR spectrum. In principle ∆φ
can be measured in the time domain [8, 15] or after FT in the frequency
domain. The latter is today the method of choice. For the introduction and
propagation of this principle, Richard Ernst has been awarded by the 1991
Nobel prize in chemistry [16]. In particular in ENMR there are different
possibilities for the practical procedure. ∆φ or cos(∆φ)ismeasuredasa
function of one of the variables g, δ, ∆ or v,wherev canbevariedwiththe
applied electric field E and thus by a variation of the electric current I in
the sample cell. As shown in Fig. 17.3 it is possible to observe and evaluate
the cos(γgvδ∆) modulation of the NMR line intensity as a function of E
(or I). By determination of the electric field E
1
, which is required for just
one modulation period, one can directly obtain the mobility by means of
u
±
=2π/γgδ∆E
1
.
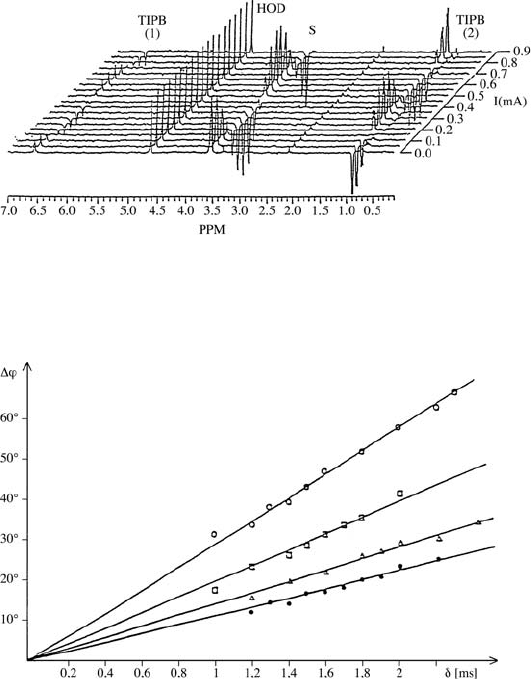
724 Manfred Holz
Fig. 17.3.
1
H ENMR spectra of an “oil-in water” microemulsion as a function of the
electric current I [10], showing the cos(∆φ) modulation of the lines of electrically
charged species, whereas the line of the solvent water (HOD) is unaffected. (S =
ionic surfactant, TIPB = triisopropylbenzene).
Fig. 17.4.
7
Li
+
spin-echo phase shifts ∆φ as a function of the PFG duration δ.
The different slopes correspond to different Li
+
mobilities in various aqueous LiCl
solutions [15].
With modern NMR instruments a signal phase shift of a line in the spec-
trum can be directly observed and printed out. Thus one can, e.g., vary δ,
resulting in the linear dependence ∆φ(δ)=Kδ with K = γgv∆.Fromthe
slope of this straight line the velocity v is derived (see Fig. 17.4). Since the
really acting magnetic field gradient g is the variable, which is less accurately
known compared with the quartz controlled times δ and ∆, often, similar
as in NMR self-diffusion studies [5], measurements are performed relative to
a reference system, here with known v. This procedure then allows velocity
measurements by a simple comparison of slopes, without the need of knowing
the actual g values.
17 Field-Assisted Diffusion Studied by Electrophoretic NMR 725
17.3 NMR in Presence of an Electric Direct Current.
Technical Requirements, Problems and Solutions
The DCNMR experiments are performed in common NMR spectrometers,
normally equipped with a superconducting magnet. For the PFG experiments
a probe head with gradient coils (actively shielded coils are recommended)
is required together with the corresponding gateable high-current power sup-
ply. Such units are commercially available. Special accessories for DCNMR
are the probe cells with two electrodes, where the electric field E can be
applied (see Fig. 17.5) and a gateable constant DC power supply (typically
0 –250 mA, 0 –1 000 V) for the electrophoretic cell. The electric field is applied
as a pulsed field (see Fig. 17.1) where the gate pulses are usually derived from
the computer system of the spectrometer and are thus synchronized with the
rf excitation pulses and the magnetic field gradient pulses. The electrodes
used are Pt or Ag/AgX (X=Cl,Br,J) electrodes. More technical details can
be found elsewhere [8–11].
The electric current flowing during the NMR experiment in the sample
cell is the origin of a number of experimental problems, which shall be briefly
discussed together with the solutions found so far:
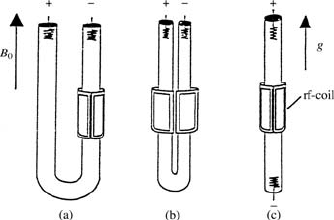
Magnetic Fields Induced by the Electric Current
The electric current produces a magnetic field in the plane perpendicular to
its direction of flow. This field can interfere with the Zeeman field B
0
and
results in undesired B
0
field inhomogeneities and instabilities. However, there
is a relatively easy way to overcome this problem by choosing as the current
direction that of the magnetic field B
0
(z-direction) (see Fig. 17.5). The ad-
ditional magnetic fields then lie in the x, y-plane and have no components
in the z-direction, which is the relevant direction in the NMR experiment
and thus no signal disturbances are produced. This z-direction for the elec-
tric current is the “natural” direction in superconducting magnets since it is
also the axis of the magnet bore, and cylindrical sample cells are commonly
mounted parallel to this axis.
Fig. 17.5. Three basic DCNMR cell
geometries (a), (b), (c), with the cor-
responding electrode arrangements.
The radio frequency (rf) transmit-
ter/receiver coil is a saddle coil. The
direction of the Zeeman field B
0
and
of the imposed field gradient g =
∂B
z
/∂z is also shown.
726 Manfred Holz
Resistive Heating by the Current in the Sample Cell
More serious problems in ENMR can arise from resistive heating. The un-
desired heat production by the current in the electrolyte solution can result
in a temperature increase, unstable temperature conditions and finally in a
macroscopic convection in a liquid sample. Any macroscopic motion in a liq-
uid system like convection, vibrations or shock waves can be the source of
dramatic measuring errors in NMR mobility or diffusion measurements since
we have to measure small displacements in the micrometer or sub-micrometer
region.
The first step to solve the problem of resistive heating is the application of
pulsed electric fields, so that a current flows only during the relatively short
time of the spin-echo sequence, and the use of effective cooling systems. As
a second step there is the possibility of using stabilizers against convection
as agar-agar [17] or some other gelling agents. In particular agar-agar proved
to be a suitable stabilizer for aqueous solutions without affecting markedly
the diffusion and mobility of ions and the diffusivity of the solvent water.
However, the experience shows that with low electric currents of 10 mA
also measurement in the free solution is possible. A third possibility to over-
come heat-induced convection is the application of special pulse sequences,
including rf, magnetic gradient and electric field pulses, which remove spec-
tral artifacts arising from convection. This novel method is called “Convection
compensated ENMR” [18].
Electro-Osmotic Flow
When ions migrate in the electric field there are always collisions with the
neutral solvent molecules which are connected with a transfer of momen-
tum in the direction of the ion flow. However, this effect is cancelled by the
counter-ions, which move in the opposite direction, but the cancellation acts
only under the condition that there is a homogeneous charge distribution,
which means local charge neutrality [19]. On the other hand, an inhomoge-
neous contribution of mobile charges occurs on charged surfaces, since only
the screening counter-ions are mobile. This fact results in a solvent flow at
the surface in the direction of the electrophoretic migration, and in a vertical
sample tube a counterflow appears in the centre. In this way electro-osmosis
produces macroscopic flow in the sample, which disturbs the ENMR experi-
ment, resulting in an undesired damping of the NMR signal intensity [10,20].
The solution of this problem is not so easy, however also here a gelling agent
proved to be successful. Johnson and coworkers [10] proposed a coating of
the surface by methylcellulose to reduce electro-osmosis.
Mechanical Disturbances
Due to the fact that in DCNMR experiments electric direct currents are
switched in a strong external magnetic field B
0
, strong forces are acting on
all electrical connections (gradient coils, wires, electrodes etc.) in which the
17 Field-Assisted Diffusion Studied by Electrophoretic NMR 727
current does not flow parallel to B
0
. In this case during switching shock
waves may be generated, which can lead to displacements in the electrolyte
solution. This problem must be solved by a very careful rigid fixing of all
electrical connections and electrodes within the probe head.
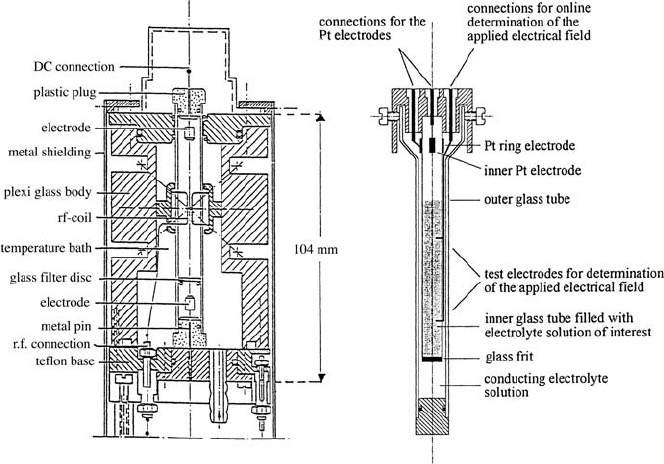
17.4 ENMR Sample Cells
We will give two examples of electrolytic cells in use in our laboratory.
They are both cylindrical cells oriented in z-direction (B
0
direction). The
first one [9, 21] is simply cylindric with an upper and a lower electrode (see
Fig. 17.6 (a). The advantage of this cell is a good filling factor, giving a good
signal-to-noise ratio, which is important for nuclei other than
1
H, the so-
called heteronuclei. The disadvantage comes from a possible gassing of the
lower electrode, which is not vented to the atmosphere. The second one (see
Fig. 17.6 (b) is a concentric cylindric chamber, where in the inner cylinder
the sample of interest is located. The outer cylinder is filled with an elec-
trolyte solution as a conductor. In order to be able to use as conductor in
Fig. 17.6. Two examples of ENMR cells. (a) probe head insert consisting of a plexi
glass body on which the gradient coils are mounted. The electrophoresis cell in the
centre is surrounded by a temperature bath liquid [21]. (b) Concentric cylindrical
ENMR cell, where the two electrodes are located in the upper part of the cell.
728 Manfred Holz
the outer cell always the same kind of electrolyte solution as it is observed in
the inner cylinder, in the author’s laboratory a very successful procedure has
been developed, which eliminates the undesired NMR signal contributions
from the ions in the outer cylinder. For this purpose in the outer cylinder
suited small beads are added, which due to magnetic susceptibility and/or
surface relaxation effects extremely broaden NMR signals coming from the
outer cylinder. Thus the undesired signals are suppressed by a “T
2
-filter”. The
advantage of this cell is that both electrodes are vented to the atmosphere,
the disadvantage is the lower filling factor.
17.5 ENMR Experiments (1D, 2D and 3D) and
Application Examples
First we ask the question: which nuclei can be used in ENMR? Whereas
in ordinary NMR more than 100 nuclides are accessible to the experiment,
the number of nuclides, which are suited for ENMR and NMR diffusion ex-
periments, is in the range of 10 to 30. The reason for that lies in the PFG
spin-echo experiment, which is applied and where the particles move during
a defined time (∆) and after that time the displacement is measured via
the spin-echo signal at 2τ. Thus, in particular for slowly moving particles,
∆ cannot be chosen too short, since a measurable displacement ∆z has
to be achieved. On the other hand, as can be seen from (17.15), the spin-
echo signal decreases with the nuclear magnetic relaxation (time constant
T
2
) and therefore the maximum “observation time” ∆ is limited by the re-
laxation influence. There are so-called “stimulated-echo” experiments [9–11],
which can be used in those cases where the transverse relaxation time T
2
is
much smaller than the longitudinal relaxation time T
1
and then commonly
T
1
is the limiting factor (see also Sect. 10.3.4 of Chap. 10). There is a large
amount of nuclides with nuclear spin I>1/2, which relax by the so-called
quadrupole mechanism and have often very short T
1
and T
2
values in the
µs range in liquid solutions so that they are not suited for ENMR measure-
ments. Generally, one can say that all spin-1/2 nuclides with a not too low
NMR receptivity should be usable, as, e.g.,
1
H,
13
C,
19
F,
29
Si,
31
P,
113
Cd,
119
Sn and
205
Tl. There are several quadrupolar nuclei, which are candidates
for ENMR, namely for example
2
H,
7
Li,
23
Na,
27
Al,
35
Cl,
51
Vand
133
Cs,
since they have under normal circumstances relaxation times, which are long
enough for the spin-echo experiment.
So far, in the comparatively young field of applied ENMR, the nuclei
1
H,
7
Li,
13
C,
19
Fand
133
Cs have actually been used in experiments, where
of course
1
H ENMR plays an outstanding role, owing to the high sensitiv-
ity of
1
H NMR and also as a consequence of the huge number of hydrogen
containing compounds of interest in pure and applied chemistry and in life
sciences.
17 Field-Assisted Diffusion Studied by Electrophoretic NMR 729
In NMR, in the last two decades a number of highly sophisticated tech-
niques have been developed allowing the representation of the spectra in a
multi-dimensional form [16]. Thus, we distinguish one- (1D), two- (2D) and
three-dimensional (3D) NMR experiments, corresponding to the representa-
tion of the line intensities as a function of one, two or three axes. In describing
typical ENMR experiments in the following, we will demonstrate their pos-
sible applications. We shall begin with common 1D ENMR.
17.5.1 1D ENMR Applications
The first ENMR experiments were performed on simple aqueous solutions
of tetraalkylammonium salts, where e.g. the concentration dependence of
the mobility of the hydrogen containing cations has been measured for the
purpose of demonstrating the agreement with results from classical methods
[8,17]. However, as pointed out above, the main advantage of ENMR lies in
the possibility of studying distinct species in multi-component mixtures.
Mobility Studies in Mixtures
Owing to difficulties with classical methods, there exist in the literature only
a few reliable mobility data of simple ions in electrolyte mixtures. Also for
such a simple system like LiX/CsX (X=Cl,Br,J) in aqueous solution there
were no data available.
7
Li
+
and
133
Cs
+
are excellently suited for ENMR and
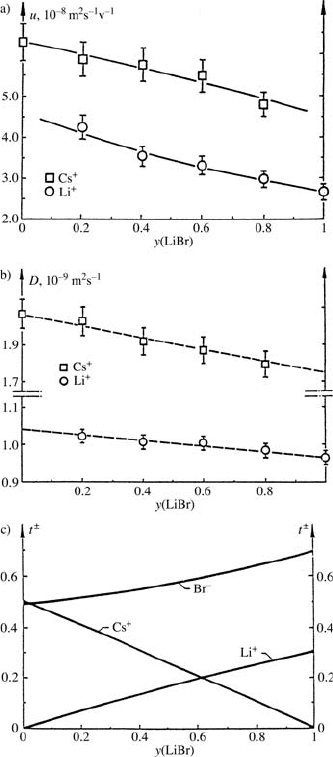
these two cations differ appreciably in their ionic radii. In Fig. 17.7 we see, as
an example for aqueous LiBr/CsBr mixtures at constant ionic strength, the
mobilities u
±
for both ions [21]. As a by-product one always obtains (with
electric current I = 0) the ionic self-diffusion coefficients D
±
,whicharealso
given in Fig. 17.7. It can be recognized that the mobilities show a curved
composition dependence, with opposite curvature for Li
+
and Cs
+
,which
means that the “fast” cation accelerates the migration of the “slow” cation
in the mixture. An opposite result represents the linear dependence of the
ionic diffusion coefficients. However, this is not surprising since, in contrast
to u
+
, D
+
is an equilibrium quantity. Having measured the mobilities of the
two cations, the transference numbers T
+
could be calculated. Since the sum
of all transference numbers must be equal to one, T
−
for the common anion
could also be determined (see Fig. 17.7).
Another example is shown in Fig. 17.8 where by high-resolution
1
HENMR
the electrophoretic mobilities of the amine and the amino acid in an aqueous
mixture could be measured simultaneously [10].
More recently, mixed anionic-nonionic surfactant micelles have been stud-
ied by the same experimental technique with the aim of characterizing the
surfaces of ionic micelles. New insight in counterion binding could be derived,
which cannot be easily gained by other methods. [23]
In the study and application of polyelectrolytes the quantitative determi-
nation of the effective charge density is an important but difficult task. Ap-
plying the combination of ENMR and PFG diffusion measurements, Wong
730 Manfred Holz
Fig. 17.7. Results of ENMR mea-
surements on a ternary aqueous mix-
ture of simple ions at constant ionic
strength [21]. System y ×0.5 m LiBr+
(1 − y) × 0.5mCsBrinH
2
O.
(a) The mobilities u
+
of Li
+
and Cs
+
as a function of mixture composition.
(b) The ionic self-diffusion coefficients
D
+
of Li
+
and Cs
+
.
(c) The transference numbers T
±
of
the three ionic constituents Li
+
,Cs
+
and Br
−
in the mixed electrolyte so-
lution.
and Scheler [24, 25], for the first time succeeded in measuring in this way the
electrophoretic mobility of polyelectrolytes in solution and they could de-
termine the effective charge density. The effect of addition of common salts
could also be analysed.
Diffusion and Field-Assisted Diffusion of Ions in Porous Media
In the literature it is often assumed, that the ionic mobility within a pore
can be approximated by its mobility in the bulk fluid and that therefore the
electric conduction of a porous system, filled with a liquid electrolyte, is only
restricted by geometrical factors [16]. The validity of this assumption could
not be experimentally checked by classical methods. ENMR, as a non-invasive
