Heard D.E. (editor) Analytical Techniques for Atmospheric Measurement
Подождите немного. Документ загружается.


202 Analytical Techniques for Atmospheric Measurement
with the instrument walls. The air flow was slowed from the aircraft velocity of 200 to
100 m/s in a large bypass duct. A sub-sample of air in this duct entered the detection
duct where flow speeds were regulated between 10 and 100 m/s. Observations showed
that turbulence in the flow at speeds below 40 m/s resulted in loss of OH. The gas passed
by three detection cells used for measurement of OH, HO
2
, and H
2
O (Section 4.3.4).
NO was injected after the first cell to allow for detection of HO
2
in the second cell. An
NO injector was also positioned before the first cell as a diagnostic for the HO
2
-to-OH
conversion efficiency.
The aircraft platform flew dozens of times in the first deployment of this HO
x
instrument and hundreds since. Diagnostics showed [OH] was linear in laser power,
confirming non-quadratic behaviour, and showing that retrieved [OH] was independent
of laser pulse energy. Also [OH] was observed to be zero at night, further confirming the
absence of laser-generated OH. Finally, titration of ambient OH with perfluoropropene
C
3
F
6
was also used to confirm the absence of laser-generated OH. Perfluoropropene
is the compound of choice for chemical modulation as it contains no hydrogen atoms
that could possibly re-form OH while titrating ambient OH (Dubey et al., 1996). Intro-
duction of perfluoropropene to the inlet only removes ambient OH. Laser-generated OH
molecules are detected in nanoseconds after their creation by the same laser pulse, and
their reaction with perfluoropropene is too slow for removal prior to detection.
The background of OH LIF instruments is routinely measured by tuning the laser on
and off the chosen rovibronic absorption feature in the OH absorption spectrum. An
OH ‘reference’ chamber, in which high concentrations of OH are produced, is used to
lock the laser frequency. Ideally all components of the background are invariant over
this small shift in excitation wavelength. Most OH LIF instruments continuously employ
spectral modulation during operation and occasionally use chemical modulation to test
for interferences.
In principle, the calibration of the balloon and aircraft-borne instruments could be
calculated from detailed knowledge of the transmission of the optical system and the
detailed temperature-dependent state-to-state quenching parameters (Smith & Crosley,
1990; Wennberg et al., 1994a). In practice, the optical transmission is not known well
enough and a direct calibration is necessary. Such calculations do, however, provide a
useful check on the signal and magnitude of the observed pressure and temperature
dependence. The balloon-borne and aircraft-borne instruments were calibrated using a
known quantity of OH that was produced by reacting an NO
2
reference standard with
excess H atoms:
H +NO
2
→ OH +NO (4.15)
These calibrations were performed over a range of temperatures and pressures and using
N
2
and N
2
/O
2
/Ar mixtures (Anderson, 1976; Stimpfle and Anderson, 1988; Stimpfle
et al., 1990; Wennberg et al., 1994a).
For the ER-2 instrument, the calibration was checked against absorption at very high
mixing ratios of OH to test the assumption that the OH delivered to the detection region
could be calculated from the concentration of the NO
2
standard. These two methods
agreed to within 10%. The ER-2 instrument utilized the N
2
Stokes Raman scattering at
302 nm as its transfer standard. This was more reliable than the Rayleigh transfer standard

Fluorescence Methods 203
used in the balloon instrument because the laser scatter from the detection chamber is
much lower at red-shifted wavelengths than at the Rayleigh lines.
The uncertainty in the ER-2 OH measurements (Perkins et al., 2001) was ±13%1,
which is a combination of the uncertainty of the laboratory calibration and the uncertainty
in the inference of the sensitivity from in-flight diagnostics. The precision was 0.03 ppt in a
two-second integration. For HO
2
, additional uncertainty in the chemical conversion leads
to a slightly greater uncertainty of ±15%, with a precision of 0.15 ppt in a two-second
integration. Typical detection limits were 0.05 ppt.
4.3.2 Instruments for lower tropospheric OH measurements
The central role of OH in tropospheric chemistry was recognized in the late 1960s and
early 1970s (Weinstock, 1969). In the troposphere, OH removes VOCs such as methane
and isoprene by initiating oxidation. In the presence of NO
x
and sunlight, the oxidation
results in catalytic production of ozone. Secondary aerosols may also be produced. In the
lower troposphere, ozone is an irritant and the principal component of photochemical
smog. In the upper troposphere, ozone is a potent greenhouse gas.
The high water vapour concentrations in the troposphere have required instruments
developed for tropospheric OH measurement to use resonant excitation and detection
at 308 nm. This is superior to excitation at 282 nm because the product of the ozone
absorption cross section and the photolysis quantum yield of O
1
D is reduced by a factor
of ∼30 at 308 nm relative to 282 nm. Early instruments for tropospheric OH detection
(Davis et al., 1976; Wang and Davis, 1974; Wang et al., 1975, 1976) failed due to laser-
generated OH. Hard and co-workers (1984) reduced laser-generated OH using FAGE,
as described in Section 2.2.5, and their early success combining FAGE with excitation at
308 nm using high repetition–rate lasers has been adopted as a model by most research
groups using LIF to observe tropospheric OH (Creasey et al., 1997; Faloona et al., 2004;
Hard et al., 1995; Holland et al., 1995; Kanaya et al., 2001).
All of the current generation instruments operate at low pressure, pump optically
with 1–20 mW of 1–10 kHz tunable light at 308 nm, and detect resonance fluorescence
at 308 nm. Most use similar methods for calibration and background measurement and
have similar inlet and optical cell designs. Some of the reviews that have described these
instruments and scientific results in detail are Crosley, 1995, and Heard and Pilling,
2003.
Inlets consist of a critical orifice (∼1 mm diameter) centred on either a flat plate or a
cone. Inlet losses are typically minimized by use of unreactive coatings such as halocarbon
wax and by judicious design of the inlet (Faloona et al., 2004; Stevens et al., 1994). NO
is injected immediately downstream of the orifice, with the reaction time determined by
the volume of the inlet and by the instrument’s pumping speed. Most OH instruments
utilize a single-pass optical cell and detect fluorescence with a PMT. The Pennsylvania
State University design (Faloona et al., 2004) is the exception and uses a multi-pass White
Cell with a microchannel plate detector. This instrument has the best sensitivity reported
to date with a detection limit of 14 ×10
5
molecules/cm
3
in a 30-second integration, SNR
of 2, and an uncertainty of ±16% 1 for both OH and HO
2
. Most other instruments
have reported detection limits in the range of 28 ×10
5
to 10
6
molecules/cm
3
in the same
averaging times and SNR values (Heard & Pilling, 2003).

204 Analytical Techniques for Atmospheric Measurement
Tropospheric OH instruments are usually calibrated by photolysis of water in the
presence of O
2
to produce OH and HO
2
in a 1:1 ratio. The 184.9 nm line of a mercury
lamp is used to photolyze water:
H
2
O +h
=1849nm
−−−−−→
H +OH (4.16)
H +O
2
+M →HO
2
+M (4.17)
The OH production rate is given by
dOH
dt
= H
2
O
H
2
O
OH
F (4.18)
where
H
2
O
is the absorption cross section of water at 184.9 nm,
OH
is the photolysis
quantum yield of water to form OH, and F is the photon flux of the mercury lamp.
Integration yields
OH
t
= H
2
O
H
2
O
OH
Ft (4.19)
The absolute water vapour concentration is measured by use of chilled hygrometers or
by IR spectroscopy, F is measured with an absolutely calibrated photomultiplier tube,
and the residence time is determined using calibrated flow measurements. Alternately,
the product Ft, which is difficult to determine accurately, can be measured by an ‘O
2
chemical actinometer’ (Bloss et al., 2004; Holland et al., 1995; Kanaya et al., 2001; Schultz
et al., 1995). The same mercury lamp photolyzes oxygen:
O
2
1849nm
−−−−→
O +O (4.20)
2O +O
2
+M →O
3
+M (4.21)
creating ozone, which is measured with a commercial ozone analyzer and compared to
the calculated O
3
produced:
O
3
= O
2
O
2
O
3
Ft (4.22)
or Ft =
O
3
O
2
O
2
O
3
where
O
2
is the absorption cross section of molecular oxygen and is the quantum
yield for ozone production. Actinometry can also be based on photolysis of N
2
O instead
of ozone (Faloona et al., 2004). The O(
1
D) photolysis product reacts with N
2
O to form
2NO molecules, which can be detected by chemiluminescence (see Chapter 7).
Alternate calibration methods include monitoring the decay of a hydrocarbon by gas
chromatography in an irradiated, continuously stirred reactor in which OH is produced
by photolysis of water vapour (Hard et al., 1984), production of a steady state OH
concentration by alkene ozonolysis (Hard et al., 2002), and absorption of super-ambient
OH concentrations (Kanaya et al., 2001). Typically, these calibration strategies are used

Fluorescence Methods 205
at a single value (often close to zero) of the water vapour concentration. Ambient
measurements require accounting for water vapour quenching in the sample because it
quenches OH 22 times faster than nitrogen (Bailey et al., 1999). Additionally, losses of
radicals due to clustering, which is very dependent upon water vapour concentation, have
been observed in some instruments (Heard & Pilling, 2003), and thus calibrations are
ideally performed at ambient water vapour concentrations.
HO
2
is detected after chemical conversion to OH by reaction with NO. In tropospheric
HO
x
instruments, NO is added in the low pressure region downstream of the pressure-
reducing orifice. HO
2
-to-OH conversion efficiencies range from 30 to 95% (Creasey et al.,
2003; Kanaya et al., 2001; Stevens et al., 1994). In addition to the formation of HONO
(Reaction 4.14), a further complication encountered in the troposphere is conversion of
organic peroxy radicals RO
2
to OH by the reaction scheme
RO
2
+NO →RO +NO
2
(4.23)
RO +O
2
→ R
CHO +HO
2
(4.24)
HO
2
+NO →OH +NO
2
(4.13)
where R
CHO represents an aldehyde or ketone. Conversion rates of 5% for CH
3
O
2
and
C
2
H
5
O
2
have been measured (Kanaya et al., 2001; Holland et al., 2003). Ren et al. (2004)
report negligible interference from up to 100 ppt of methane-, ethane-, propane-, and
n-butane-derived RO
2
species.
The accuracy of LIF HO
x
measurements has also been assessed by comparison
to measurements using different techniques and therefore presumably subject to
different interferences, sampling artefacts, and calibration errors. Intercomparisons of
OH measurements have been reviewed in detail by Heard and Pilling (2003). For
example, OH measurements by LIF and by folded-path differential optical absorption
spectrometry (DOAS) were obtained in rural north-eastern Germany in an exper-
iment during the summer of 1994. A linear fit to the data gives DOAS measurement
of 109 ×LIF +028 ×10
6
molecules cm
−3
with an R
2
correlation coefficient of 0.8
(Hofzumahaus et al., 1998). Most of the scatter is accounted for by the imprecision of
the measurements. A comparison study of Penn State’s GTHOS (Ground-based Tropo-
spheric Hydrogen Oxides Sensor) LIF detector with NCAR’s perCIMS (peroxy radical
chemical ionization mass spectrometer) detector in HO
2
mode was conducted at a rural
site in Pennsylvania, USA, in 2002 (Ren et al., 2003a). Measurements of ambient HO
2
and both instruments’ calibration sources were compared. The comparison showed the
calibration sources were identical to within 2%. The accuracy of perCIMS in HO
2
mode
was estimated to be 41% at the 95% confidence interval, with 15% of the measured
HO
2
in perCIMS stemming from CH
3
O
2
. The accuracy of GTHOS was estimated to
be 32% at the 95% confidence level. Figure 4.5 shows a time series of ambient HO
2
measurements. A linear regression of the comparison data, with an integration period
of 140 seconds, was described by PerCIMS HO
2
= 096×GTHOS +06 ppt, with an R
2
value of 0.85.
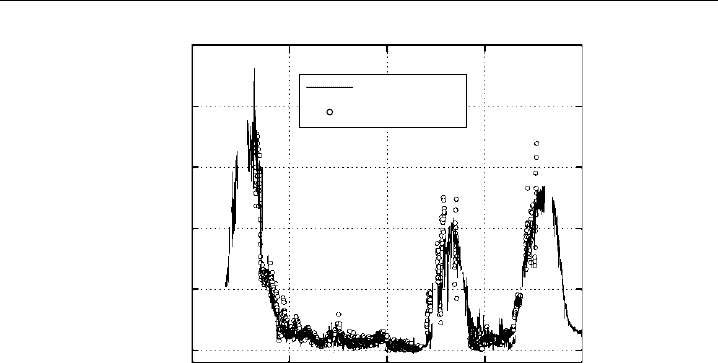
206 Analytical Techniques for Atmospheric Measurement
50
40
30
20
10
0
June 5 June 6 June 7
Date of 2002 (EDT)
HO
2
(pptv)
June 8
GTHOS HO
2
PerCIMS HO
2
Figure 4.5 Time series of ambient HO
2
measurements by LIF (GTHOS, solid line) and perCIMS (circles).
(From Ren et al., 2003a.)
4.3.3 OH fluorescence as an indirect detection strategy
In addition to HO
2
, other species can be measured indirectly by OH fluorescence. Water is
measured by the stratospheric hygrometer described by Weinstock et al. (1994). Photolysis
of water vapour by Lyman radiation at 121.6 nm produces electronically excited OH:
H
2
O +h1216nm →OHA
2
+
+H (4.25)
A fraction of the OH produced is highly rotationally excited in the
= 1 level of the
A
2
+
electronic state. Following vibrational relaxation, emission from highly excited
rotational levels of the
˜
A
2
+
= 0 state is detected at 305–325 nm in an optical cell
similar to that of the stratospheric HO
x
instrument aboard the ER-2.
Ambient detection of nitrous acid (HONO) by photo-fragmentation–LIF should also be
feasible and prototypes have been described (Liao et al., 2003; Rodgers and Davis, 1989).
4.3.4 Naphthalene
Martinez et al. (2004) discovered that naphthalene could be measured by their HO
x
detector thanks to a fortuitous overlap between the absorption spectra of naphthalene
and OH. Naphthalene has a sharp absorption peak at 308.0026 nm, slightly to the red of
the Q
1
2 rotational line of OH at 307.9951 nm. The naphthalene absorption is negligible
to the blue of the Q
1
2 line which was used as the off-resonance wavelength for spectral
modulation of OH. Since the Penn State group alternated their offline measurement to
high and low frequencies of the OH peak, they were able to produce accurate OH and
naphthalene observations after the interference was identified as a useful signal.

Fluorescence Methods 207
4.3.5 Measurement of the OH lifetime
Most of the techniques described in this chapter are used to measure the concentration
of a particular atmospheric species. However, the concentration is just one of many
parameters that are useful for testing our understanding of atmospheric chemistry. A few
LIF instruments have been constructed which measure the total reactivity of OH k
total
or equivalently its lifetime
OH
defined as:
k
total
=
−1
OH
=
i
k
i
C
i
= k
CO
CO +k
NO
2
NO
2
+k
CH
4
CH
4
+··· (4.26)
where k
i
C
i
is the product of an individual reactant concentration and its second order
rate constant with OH. The advantage of measuring the total loss rate is that it is not
possible to measure every compound with which OH reacts; indeed the identity of every
compound that reacts with OH is not known. The two methods of measuring the total
reactivity of OH combine LIF detection of OH with standard techniques borrowed from
laboratory kinetics.
Kovacs and Brune (2001) demonstrated measurement of the OH lifetime in 2001. Their
method, TOHLM (Total OH Loss Measurement), also used by Heard and co-workers at
Leeds (Johnson, 2004), is based on the decay of OH concentrations when mixed with
ambient air in a flow tube as depicted in Figure 4.6. Approximately 500 ppt of OH and
HO
2
in approximately a 1:1 ratio, produced by photolysis of water vapour by a mercury
lamp, is released into a 5 cm diameter, glass flow tube by a moveable injector. Ambient
air flows into the flow tube and the relative OH concentration is measured downstream
by LIF. As the total flow rate and reaction distance are known, the reaction time between
the injected OH and the ambient air is known for any injector tube position. Plotting OH
concentration versus reaction time yields decay profiles that are fit to single exponentials
to extract the first order rate constant. Injection of a known and high concentration of
a compound with a well-quantified reaction rate with OH, such as carbon monoxide, is
used to ‘calibrate’ the flow conditions, and injection of zero air is used to quantify wall
losses of OH which must be subtracted from the total measured loss. The measurement is
simplified by the fact that the OH concentration does not have to be quantified absolutely.
Rather, only the relative decay of the OH concentration over time is measured.
At a few sites, the total OH reactivity measured by TOHLM was compared with the
total OH reactivity calculated by summing the product of the individually measured OH
sinks (e.g. CO, CH
4
,NO
2
, VOCs) with their respective reaction rate coefficients with
OH (i.e. by explicit evaluation of the right hand side of Equation 4.26). In Nashville
during the SOS ’99 study, observed OH reactivities were on average 40% higher than the
calculated reactivities (Kovacs et al., 2003), whereas during the summer of 2001 in the
PMTACS-NY field study in New York City, the calculated and observed OH reactivities
agreed to within 10% (Ren et al., 2003b). In a forest in northern Michigan, the difference
between the observed and the calculated reactivities values was found to be exponentially
dependent on temperature and to closely resemble the temperature profile of terpene
emissions, suggesting the existence of significant concentrations of unmeasured biogenic
VOCs (Di Carlo et al., 2004).
In an alternative strategy described by Calpini and co-workers (Calpini et al., 1999;
Jeanneret et al., 2001) and successfully implemented by Sadanaga and co-workers (2004b),
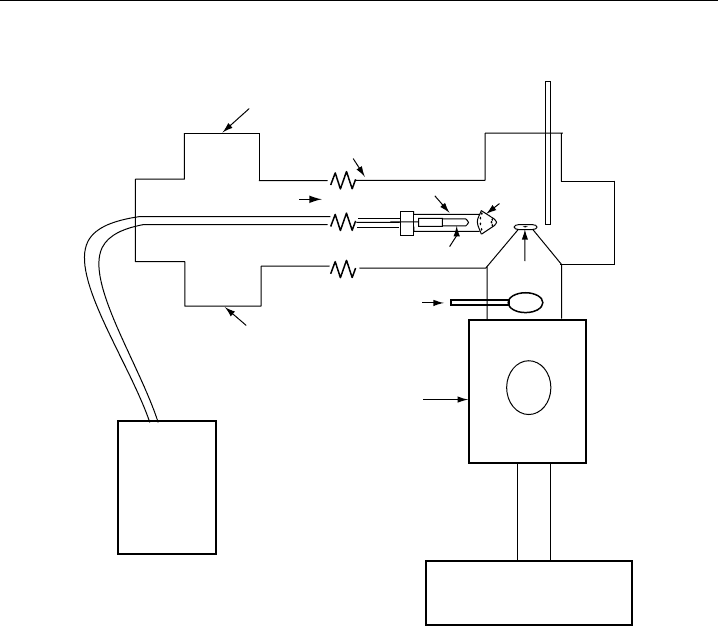
208 Analytical Techniques for Atmospheric Measurement
FLOW TUBE
N
2
and H
2
O
Bubbler
Diffuser
MERCURY LAMP
SHIELD
Detection
cell
MCP
Laser
in
Blower
Hot wire
anemometer
FLOW
Ambient
air in
Calibration
gas in
Vacuum pump
NO
Addition for
HO
2
detection
Detection
inlet
Figure 4.6 The OH lifetime instrument of Kovacs and Brune. Ambient air enters the flow tube from the
left and mixes with high concentrations of OH released at a series of distances from the LIF inlet. (From
Kovacs and Brune, 2001, with kind permission of Kluwer Academic Publishers.)
OH is produced by flash photolysis of ambient ozone using the fourth harmonic of an
Nd:YAG laser at 266 nm (80 mJ/pulse, 10 Hz) followed by reaction of the O(
1
D) photolysis
product with water vapour. The subsequent decay of the high OH concentration produced
(up to 10
11
molecules/cm
3
) is measured by LIF in real time, thus indicating the OH
reactivity.
4.4 The nitrogen oxides
The nitrogen oxide radicals NO
x
≡ NO +NO
2
are central to the production of ozone
in the troposphere and the destruction of ozone in the stratosphere. Accurate and precise
measurements of the atmospheric abundance of NO
x
and their oxidation products –
peroxy nitrates, alkyl nitrates and nitric acid – are thus important to understanding and
modeling air quality (Liang et al., 1998; Russell & Dennis, 2000; Thornton et al., 2002),
acid rain and nitrogen deposition (Galloway, 2001; Munger et al., 1998), greenhouse
forcing (Mickley et al., 1999), the carbon cycle (Lerdau et al., 2000; Holland et al.,
1997), and stratospheric ozone (Wennberg et al., 1994b). Measurements of NO
x
are
Fluorescence Methods 209
also important to combustion science (Docquier & Candel, 2002) and are used in some
strategies for detection of explosives (Steinfeld & Wormhoudt, 1998). Detection of NO
2
is difficult because high concentrations of other NO
y
species represent interferences for
many techniques. Additionally, concentrations vary by 1–2 orders of magnitude at a
single location and by several orders of magnitude between the combustion sources and
the remote atmosphere, where mixing ratios are as small as 1–2 ppt. Till the time of
writing, no technique has become the singular method of choice. Instruments based
on IR absorption (Chapter 2), visible absorption (Chapter 3), LIF (this chapter) and
chemiluminescence (Chapter 7) are all in active use.
Although NO is routinely measured accurately by chemiluminescence in scientific and
a wide variety of other settings, high quality measurements of NO
2
by chemiluminescence
are relatively rare and have been limited to scientific instruments. The most sensitive,
in situ instruments for observing NO
2
are photofragmentation – chemiluminescence
and direct LIF. The latter is emerging as competitive with the former in both cost and
sensitivity. In many monitoring networks, surface catalysis is used to convert NO
2
to NO
prior to chemiluminescence detection. These measurements virtually always overestimate
NO
2
because of the conversion of nitric acid, alkyl nitrates and peroxy-nitrates into NO
2
.
As a result, the data from such networks is ignored by the scientific community, although
it serves some useful purposes for development of public policy.
4.4.1 Photophysics of NO
2
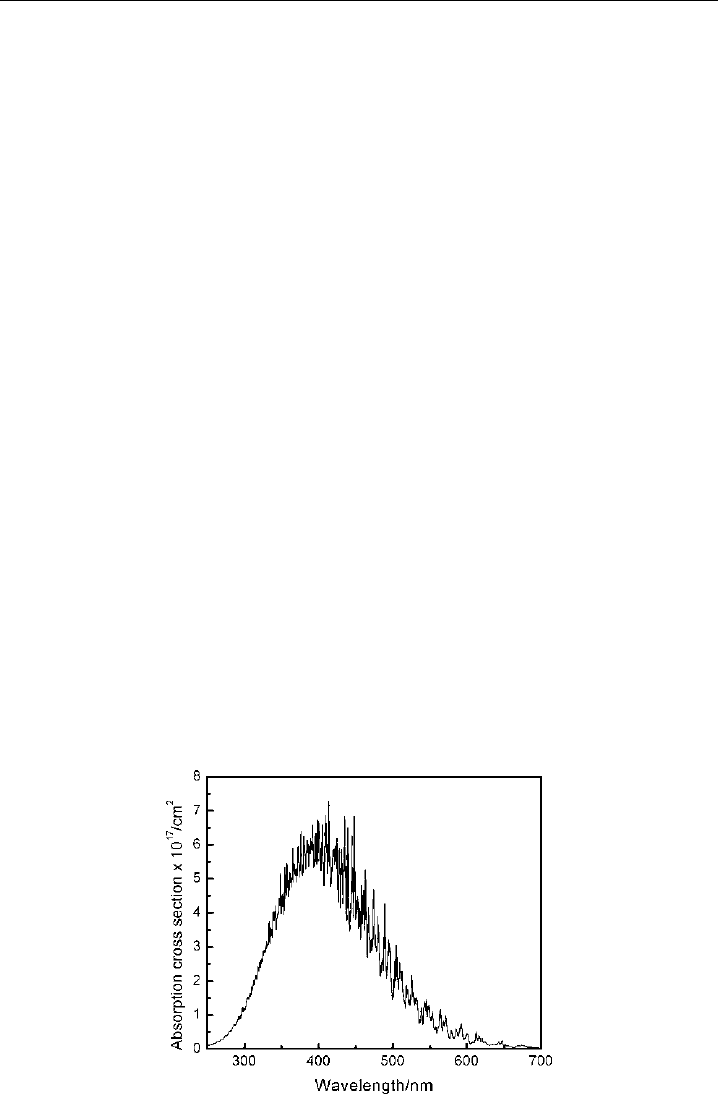
Figure 4.7 depicts the absorption spectrum of NO
2
. It is highly congested due to the
presence of numerous overlapping ro-vibronic transitions involving the X
2
A
1
,A
2
B
2
,B
2
B
1
,
and the C
2
A
2
electronic states and as a result of large differences in the geometries
of the ground and the excited states. The rotational structure is embedded in a broad
continuum, which peaks at 413 nm. Photodissociation to NO +O
3
P with near-unity
quantum yield occurs for excitation wavelengths shorter than 398 nm. At short times
Figure 4.7 NO
2
absorption spectrum at 293 K. (From Burrows et al., 1998.)

210 Analytical Techniques for Atmospheric Measurement
<1 s following excitation, the emission spectrum of NO
2
exhibits vibrational structure
at pressures below ∼10 m torr (Donnelly et al., 1979). At longer times or higher pressures,
the emission spectrum resembles a broad peak centred in the near-IR due to efficient
stepwise vibrational relaxation of the excited electronic state (Clough & Thrush, 1967,
Mazely et al., 1994, Patten et al., 1990). The excited state lifetime of NO
2
∼100 sis
much longer than expected based on its integrated absorption cross section and is a
strong function of excitation wavelength, pressure, and spectral region observed during
measurement (Donnelly & Kaufman, 1978, Patten et al., 1990, Sackett & Yardley, 1972).
This long radiative lifetime is a result of strong interaction between the excited electronic
states and upper vibrational levels of the ground electronic state as first described by
Douglas (1966). Quenching of electronically excited NO
2
is rapid; the air-average value
with 532 nm excitation is 6 ×10
−11
cm
3
molecule
−1
s
−1
(Donnelly et al., 1979).
4.4.2 Single-photon NO
2
instrument design
In contrast to the design of tropospheric OH LIF instruments, which have for the most
part converged, the design and operation of NO
2
LIF instruments are widely varied
and evolving rapidly. There is no standard excitation wavelength or optical cell design.
Most instruments operate at reduced pressure and use pulsed lasers with time-gated
fluorescence detection. Early attempts at detection of tropospheric NO
2
via LIF were
conducted at atmospheric pressure but suffered from an aerosol interference (Tucker
et al., 1975). Interest was not renewed until work by Fong and Brune (1997), Perkins
et al. (2001), and Thornton et al. (2000) in the late 1990s.
The choice of excitation wavelength is a trade-off between specificity and sensitivity.
Specificity is maximized in the red end of the spectrum because of the high ratio of
on-resonance to off-resonance absorption, while sensitivity is maximized in the blue
end where the absolute absorption cross section is highest. Due to the greater ease in
producing tunable light and the specificity afforded at longer wavelengths, excitation at
all wavelengths is competitive. Current NO
2
LIF instruments use excitation wavelengths
ranging from the blue to the red region of the spectrum. Emission from excited NO
2
peaks in the near-IR, dictating the type of PMT to use. Multi-alkali or cooled GaAs
photocathodes, with quantum efficiencies of ∼8–15% in the range of 800–900 nm are
typically used.
Most NO
2
LIF instruments use a dual-wavelength technique to demonstrate specificity
to NO
2
. This spectral modulation is similar to the background measurement technique
used by OH LIF instruments except that for NO
2
there is still appreciable fluorescence
at the off-resonance frequency due to the continuum in the absorption spectrum. This
technique has been applied in both the stratosphere (Perkins et al., 2001) and the
troposphere (Cleary et al., 2002; Fong & Brune, 1997; Matsumoto and Kajii, 2003;
Matsumi et al., 2001; Matsumoto et al., 2001; Thornton et al., 2000). In the instrument
described in detail by Thornton et al. (2000), the output from a narrow linewidth dye laser
pumped by a 3 W, 8 kHz Nd:YAG laser excites a pair of overlapping rotational lines in the
(430) A
2
B
2
←000X
2
A
1
vibronic band of NO
2
at 170865cm
−1
(585.257 nm). The laser
frequency is repeatedly tuned on- and off-resonance with this peak, spectrally modulating
Fluorescence Methods 211
the fluorescence signal. The NO
2
concentration is proportional to the difference between
the ‘online’ and ‘offline’ signal.
The detection limit of the instrument described by Thornton et al. is 6 ppt for a
1-minute integration, with SNR = 2. The precision is shot-noise limited and at 1.5 ppb,
NO
2
equals 16% 1 in 1 second. If combined with the supersonic jet technology
described by Cleary et al. (Section 4.4.3), an enhancement of ∼20 in the signal rate could
be expected.
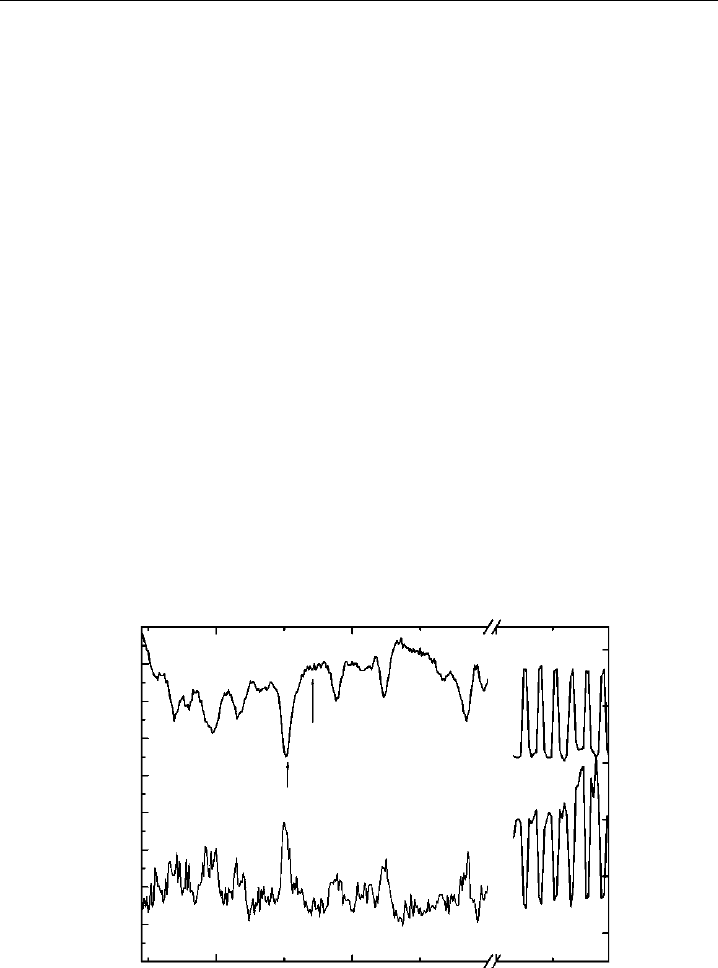
Figure 4.8 depicts NO
2
spectral modulation. The bottom trace is the fluorescence
excitation spectrum of ambient NO
2
. The top trace is the transmission through a 2.5 cm
reference cell containing 20 torr of pure NO
2
. After scanning the laser frequency in order
to locate the position of the online and offline peaks, the laser frequency alternates
between the two indicated frequencies. Use of spectral modulation conveys a spectroscopic
fingerprint of NO
2
in the fluorescence signal itself, greatly increasing the specificity of
the instrument. Any potential interference would only increase both the offline and the
online signals but would not affect the difference between the two, as it is unlikely that
any interference would respond to such a small change in excitation frequency. This
leaves ‘false NO
2
’ as the most likely potential interference, for example that produced
by the reaction of NO and O
3
or thermal decomposition of HO
2
NO
2
or N
2
O
5
in the
sampling lines. Such interferences can be quantified by calculation and by laboratory
experiment, and are usually insignificant.
Dye lasers (Fong & Brune, 1997; Perkins et al., 2001; Thornton et al., 2000), tunable
diode lasers (Cleary et al., 2002), and optical parametric oscillators (Matsumi et al.,
2001) have all been successfully used for NO
2
detection. The linewidth of dye lasers
450
400
350
300
250
200
150
100
50
0
2100 2200
Time (seconds)
Online
Offline
Signal (cps)
Transmittance
2400 2450
Figure 4.8 Spectral modulation of NO
2
. From time 2050 seconds to 2300 seconds, the laser frequency is
scanned. The bottom trace is fluorescence from ambient NO
2
and the top trace is the transmission through
a 2.5 cm reference cell containing 20 torr NO
2
. At time 2400 seconds, the laser frequency alternates
between the online and offline positions. The increase in fluorescence at the right of the figure is from
an increase in the ambient NO
2
mixing ratio.
