Haug H., Koch S. Quantum theory of the optical and electronic properties of semiconductors
Подождите немного. Документ загружается.

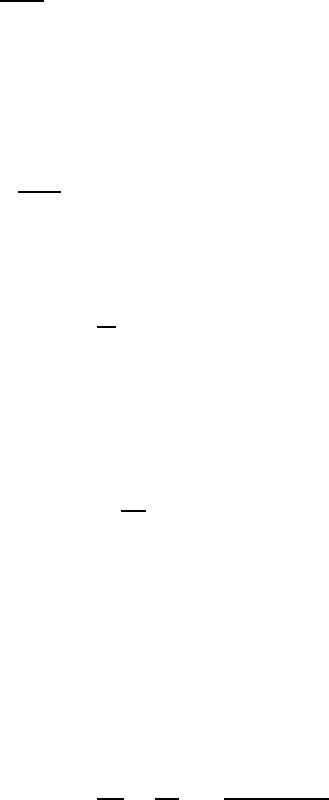
January 26, 2004 16:26 WSPC/Book Trim Size for 9in x 6in b ook2
Atoms in a Classical Light Field 19
Inserting (2.10) and (2.11) into Eq. (2.6), we obtain in order ∆
0
da
(0)
n
dt
=0 , (2.12)
which is satisfied by
a
(0)
n
= δ
n,l
. (2.13)
In first order of ∆,wehave
i
da
(1)
n
dt
= −E(t)d
nl
e
−i
ln
t
. (2.14)
For n = l there is no field-dependent contribution, i.e., a
(i)
l
≡ 0 for i ≥ 1,
since d
ll
=0. Integrating Eq. (2.14) for n = l from −∞ to t yields
a
(1)
n
(t)=−
1
i
t
−∞
dt
E(t
)d
nl
e
−i
ln
t
, (2.15)
where a
(1)
n
(t = −∞)=0has been used. This condition is valid since we
assumed that the electron is in state l without the field, Eq. (2.9).
To solve the integral in Eq. (2.15), we express the field through its
Fourier transform
E(t) = lim
γ→0
dω
2π
E(ω)e
−iωt
e
γt
. (2.16)
Here, we introduced the adiabatic switch-on factor exp(γt), to assure that
E(t) → 0 when t →−∞. We will see below that the switch-on parameter γ
plays the same role as the infinitesimal damping parameter of Chap. 1. The
existence of γ makes sure that the resulting optical susceptibility has poles
only in the lower half of the complex plane, i.e., causality is obeyed. For
notational simplicity, we will drop the lim
γ→0
in front of the expressions,
but it is understood that this limit is always implied. Inserting Eq. (2.16)
into Eq. (2.15) we obtain
a
(1)
n
(t)=−
d
nl
dω
2π
E(ω)
e
−i(ω+
ln
)t
ω +
ln
+ iγ
, (2.17)
whereweletγ → 0 in the exponent after the integration.
If we want to generate results in higher-order perturbation theory, we
have to continue the iteration by inserting the first-order result into the
RHS of (2.6) and calculate this way a
(2)
etc. These higher-order terms
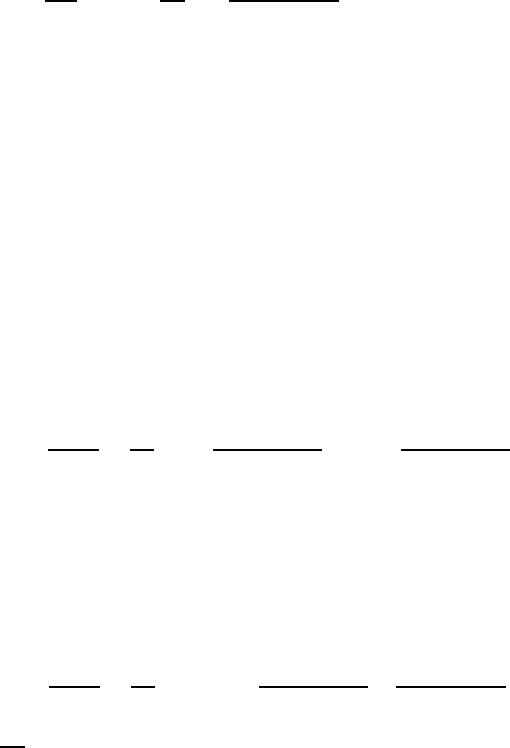
January 26, 2004 16:26 WSPC/Book Trim Size for 9in x 6in b ook2
20 Quantum Theory of the Optical and Electronic Properties of Semiconductors
contain quadratic and higher powers of the electric field. However, we are
limiting ourselves to the terms linear in the field, i.e. we employ linear
response theory.
The total wave function (2.5) is now
ψ(r,t)=e
−i
l
t
ψ
l
(r)
−
m=l
d
lm
ψ
m
(r)
dω
2π
E(ω)
e
−iωt
ω +
lm
+ iγ
+ O(E
2
) . (2.18)
The field-induced polarization is given as the expectation value of the
dipole operator
P(t)=n
0
d
3
rψ
∗
(r,t)dψ(r,t) , (2.19)
where n
0
is the density of the mutually independent (not interacting) atoms
in the system. Inserting the wave function (2.18) into Eq. (2.19), and keep-
ing only terms which are first order in the field, we obtain the polarization
as
P(t)=−n
0
m
|d
lm
|
2
dω
2π
E(ω)
e
−iωt
ω +
lm
+ iγ
+ E
∗
(ω)
e
iωt
ω +
lm
− iγ
.
(2.20)
In the integral over the last term, we substitute ω →−ω and use E
∗
(−ω)=
E(ω), which is valid since E(t) is real. This way we get
P(t)=−n
0
m
|d
lm
|
2
dω
2π
E(ω)e
−iωt
1
ω +
lm
+ iγ
−
1
ω −
lm
+ iγ
=
dω
2π
P(ω)e
−iωt
. (2.21)
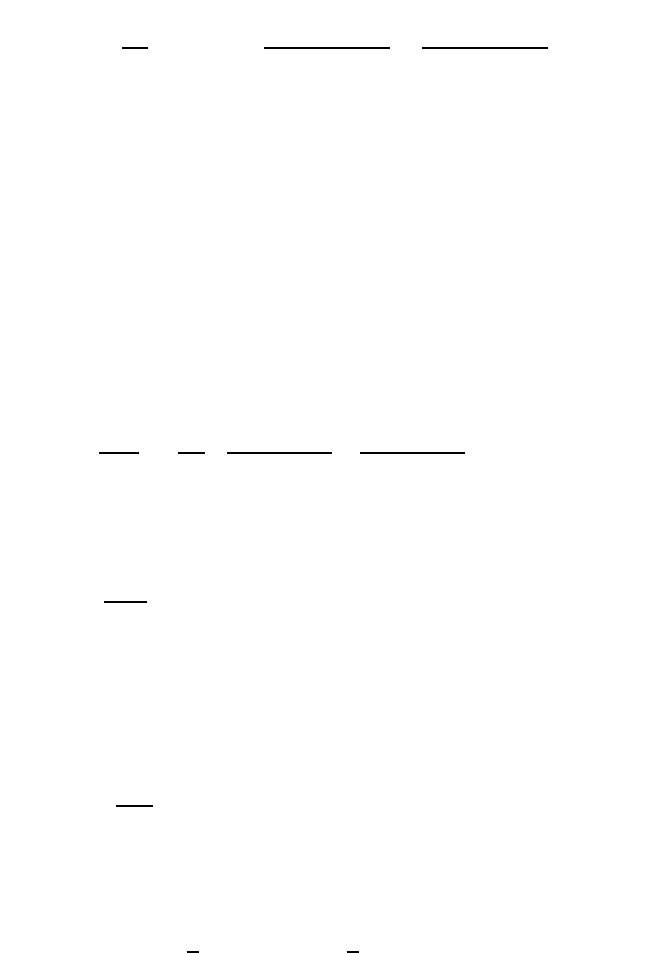
January 26, 2004 16:26 WSPC/Book Trim Size for 9in x 6in b ook2
Atoms in a Classical Light Field 21
This equation yields P(ω)=χ(ω)E(ω) with the optical susceptibility
χ(ω)=−
n
0
m
|d
lm
|
2
1
ω +
lm
+ iγ
−
1
ω −
lm
+ iγ
. (2.22)
atomic optical susceptibility
2.2 Oscillator Strength
If we compare the atomic optical susceptibility, Eq. (2.22), with the result
of the oscillator model, Eq. (1.7), we see that both expressions have similar
structures. However, in comparison with the oscillator model the atom is
represented not by one but by many oscillators with different transition
frequencies
ln
. To see this, we rewrite the expression (2.22), pulling out
the same factors which appear in the oscillator result, Eq. (1.7),
χ(ω)=
n
0
e
2
2m
0
n
f
nl
nl
1
ω −
ln
+ iγ
−
1
ω +
ln
+ iγ
. (2.23)
Hence, each partial oscillator has the strength of
f
nl
=
2m
0
|x
nl
|
2
nl
. (2.24)
oscillator strength
Here, we used |d
nl
|
2
= e
2
|x
nl
|
2
. Adding the strengths of all oscillators by
summing over all the final states n, we find
n
f
nl
=
2m
0
n
n|x|ll|x|n(
n
−
l
) . (2.25)
Using the Schrödinger equation H
0
|n =
n
|n,wecanwrite
l|x|n(
n
−
l
)=
1
l|[x, H
0
]|n = −
1
l|[H
0
,x]|n , (2.26)
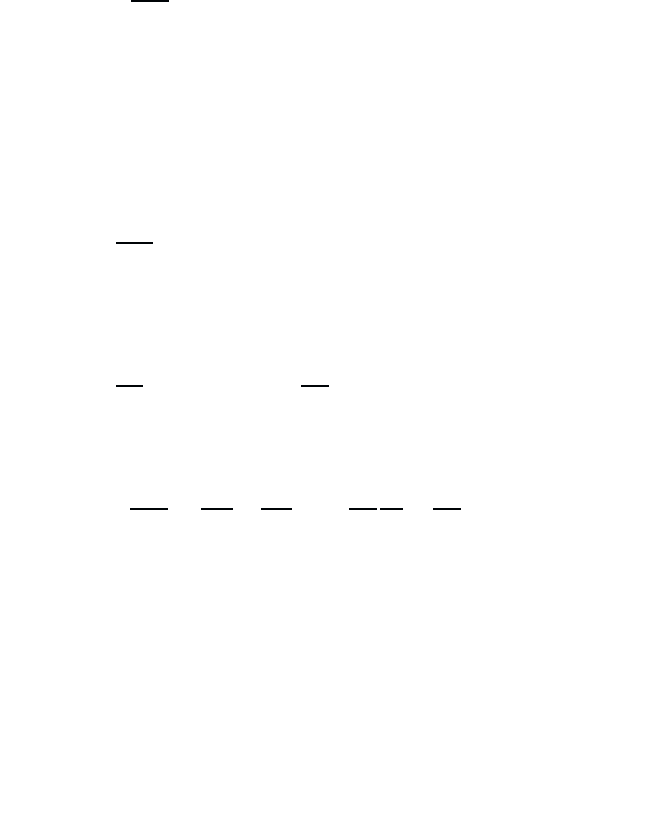
January 26, 2004 16:26 WSPC/Book Trim Size for 9in x 6in b ook2
22 Quantum Theory of the Optical and Electronic Properties of Semiconductors
where [H
0
,x]=xH
0
−H
0
x is the commutator of x and H
0
. Inserting (2.26)
into (2.25) and using the completeness relation
n
|nn| =1we get
n
f
nl
= −
2m
0
2
l|[H
0
,x]x|l . (2.27)
Alternatively to (2.26), we can also manipulate the first term in Eq. (2.25)
by writing
n|x|l(
n
−
l
)=n|[H
0
,x]|l , (2.28)
so that
n
f
nl
=
2m
0
2
l|x[H
0
,x]|l . (2.29)
Adding Eqs. (2.27) and (2.29) and dividing by two shows that the sum over
the oscillator strength is given by a double commutator
n
f
nl
=
m
0
2
l|[x, [H
0
,x]]|l =
m
0
2
l|[[x, H
0
],x]|l . (2.30)
The double commutator can be evaluated easily using
[x, H
0
]=−
2
2m
0
x
d
2
dx
2
−
d
2
dx
2
x
=
2
m
0
d
dx
=
i
m
0
p
x
(2.31)
and
[p
x
,x]=−i (2.32)
to get
n
f
nl
=1 . (2.33)
oscillator strength sum rule
Eq. (2.33) is the oscillator strength sum rule showing that the total tran-
sition strength in an atom can be viewed as that of one oscillator which is
distributed over many partial oscillators, each having the strength f
nl
.

January 26, 2004 16:26 WSPC/Book Trim Size for 9in x 6in b ook2
Atoms in a Classical Light Field 23
Writing the imaginary part of the dielectric function of the atom as
(ω)=4πχ
(ω),usingχ(ω) from Eq. (2.23) and employing the Dirac
identity, Eq. (1.69), we obtain
(ω)=ω
2
pl
π
2
n
f
nl
nl
[δ(ω −
nl
) − δ(ω −
ln
)] , (2.34)
with ω
2
pl
=4πn
0
e
2
/m
0
.Since|l is the occupied initial state and |n are
the final states, we see that the first term in Eq. (2.34) describes light
absorption. Energy conservation requires
n
= ω +
l
, (2.35)
i.e., an optical transition from the lower state |l to the energetically higher
state |n takes place if the energy difference
nl
is equal to the energy ω
of a light quantum, called a photon. In other words, a photon is absorbed
and the atom is excited from the initial state |l to the final state |n.This
interpretation of our result is the correct one, but to be fully appreciated it
actually requires also the quantum mechanical treatment of the light field.
The second term on the RHS of Eq. (2.34) describes negative absorption
causing amplification of the light field, i.e., optical gain. This is the basis of
laser action. In order to produce optical gain, the system has to be prepared
in a state |l which has a higher energy than the final state |n, because the
energy conservation expressed by the delta function in the second term on
the RHS of (2.34) requires
l
= ω +
n
. (2.36)
If the energy of a light quantum equals the energy difference
ln
, stimulated
emission occurs. In order to obtain stimulated emission in a real system,
one has to invert the system so that it is initially in an excited state rather
than in the ground state.
2.3 Optical Stark Shift
Until now we have only calculated and discussed the linear response of an
atom to a weak light field. For the case of two atomic levels interacting
with the light field, we will now determine the response at arbitrary field
intensities. Calling these two levels n =1, 2 with
2
>
1
, (2.37)
January 26, 2004 16:26 WSPC/Book Trim Size for 9in x 6in b ook2
24 Quantum Theory of the Optical and Electronic Properties of Semiconductors
we get from Eq. (2.6) the following two coupled differential equations:
i
da
1
dt
= −E(t)e
−i
21
t
d
12
a
2
, (2.38)
i
da
2
dt
= −E(t)e
−i
21
t
d
21
a
1
, (2.39)
where we used d
ii
=0. Assuming a simple monochromatic field of the form
E(t)=
1
2
E(ω)(e
−iωt
+ c.c.) (2.40)
yields
i
da
1
dt
= −d
12
E(ω)
2
e
−i(ω+
21
)t
+ e
i(ω−
21
)t
a
2
, (2.41)
i
da
2
dt
= −d
21
E(ω)
2
e
−i(ω−
21
)t
+ e
i(ω+
21
)t
a
1
, (2.42)
where
12
= −
21
has been employed. These two coupled differential equa-
tions are often called the optical Bloch equations. If we are interested only
in the light-induced changes around the resonance,
ω
2
−
1
, (2.43)
we see that the exponential factor exp[i(ω −
21
)t] is almost time-
independent, whereas the second exponential exp[i(ω +
21
)t] oscillates very
rapidly. If we keep both terms, we would find that exp[i(ω −
21
)t] leads to
the resonant term proportional to
1
(ω −
21
)+iγ
→ P
1
ω −
21
− iπδ(ω −
21
) (2.44)
in the susceptibility, whereas exp[i(ω +
21
)t] leads to the nonresonant term
proportional to
1
(ω +
21
)+iγ
→ P
1
ω +
21
− iπδ(ω +
21
) . (2.45)
For optical frequencies satisfying (2.43), the δ-function in (2.45) cannot be
satisfied since
2
>
1
, and the principal value gives only a weak contribution
January 26, 2004 16:26 WSPC/Book Trim Size for 9in x 6in b ook2
Atoms in a Classical Light Field 25
to the real part. Hence, one often completely ignores the nonresonant parts
so that Eqs. (2.41) and (2.42) simplify to
i
da
1
dt
= −
d
12
E(ω)
2
e
i(ω−
21
)t
a
2
, (2.46)
i
da
2
dt
= −
d
21
E(ω)
2
e
−i(ω−
21
)t
a
1
. (2.47)
This approximation is also called the rotating wave approximation (RWA).
This name originates from the fact that the periodic time development
in Eqs. (2.46) and (2.47) can be represented as a rotation of the Bloch
vector (see Chap. 5). If one transforms these simplified Bloch equations
into a time frame which rotates with the frequency difference ω −
21
,the
neglected term would be ω out of phase and more or less average to zero
for longer times.
To solve Eqs. (2.46) and (2.47), we first treat the case of exact resonance,
ω =
21
. Differentiating Eq. (2.47) and inserting (2.46) we get
d
2
a
2
dt
2
= i
d
21
E(ω)
2
da
1
dt
= −
d
12
E(ω)
2
2
a
2
= −
ω
2
R
4
a
2
, (2.48)
whereweusedd
21
= d
∗
12
and introduced the Rabi frequency as
ω
R
=
|d
21
E|
. (2.49)
Rabi frequency
The solution of (2.48) is of the form
a
2
(t)=a
2
(0)e
±iω
R
t/2
. (2.50)
For a
1
(t) we get the equivalent result. Inserting the solutions for a
1
and a
2
back into Eq. (2.5) yields
ψ(r,t)=a
1
(0)e
−i(
1
±ω
R
/2)t
ψ
1
(r)+a
2
(0)e
−i(
2
±ω
R
/2)t
ψ
2
(r) , (2.51)
showing that the original frequencies
1
and
2
have been changed to
1
±
ω
R
/2 and
2
± ω
R
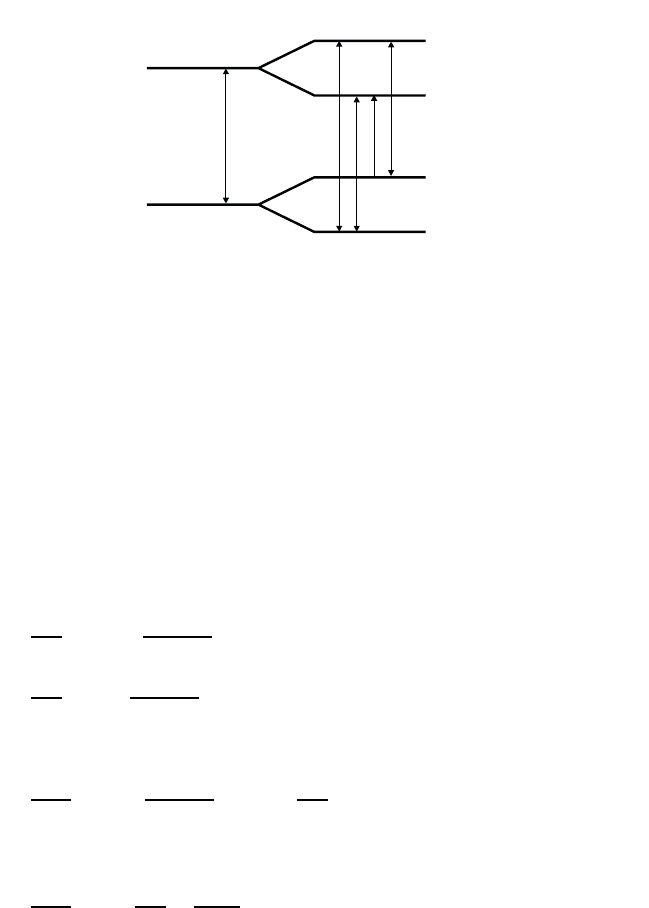
/2, respectively. Hence, as indicated in Fig. 2.1 one
has not just one but three optical transitions with the frequencies
21
,and
21
± ω
R
, respectively. In other words, under the influence of the light
January 26, 2004 16:26 WSPC/Book Trim Size for 9in x 6in b ook2
26 Quantum Theory of the Optical and Electronic Properties of Semiconductors
e
2
e
1
ew
2R
+2/
ew
1 R
+2/
ew
2R
-2/
ew
1 R
-2/
Fig. 2.1 Schematic drawing of the frequency scheme of a two-level system without
the light field (left part of Figure) and light-field induced level splitting (right part of
Figure) for the case of a resonant field, i.e., zero detuning. The vertical arrows indicate
the possible optical transitions between the levels.
field the single transition possible in two-level atom splits into a triplet,
the main transition at
21
and the Rabi sidebands at
21
± ω
R
. Eq. (2.49)
shows that the splitting is proportional to the product of field strength and
electric dipole moment. Therefore, Rabi sidebands can only be observed
for reasonably strong fields, where the Rabi frequency is larger than the
line broadening, which is always present in real systems.
The two-level model can be solved also for the case of a finite detuning
ν =
21
− ω. In this situation, Eqs. (2.46) and (2.47) can be written as
da
1
dt
= ie
−iνt
d
12
E(ω)
2
a
2
(2.52)
da
2
dt
= ie
iνt
d
21
E(ω)
2
a
1
. (2.53)
Taking the time derivative of Eq. (2.52)
d
2
a
1
dt
2
= e
−iνt
d
21
E(ω)
2
a
2
ν + i
da
2
dt
, (2.54)
and expressing a
2
and da
2
/dt in terms of a
1
we get
d
2
a
1
dt
2
= −iν
da
1
dt
−
ω
2
R
a
1
4
(2.55)
with the solution
a
1
(t)=a
1
(0)e
iΩt
(or a
1
(t)=a
1
(0)e
−iΩt
) , (2.56)
January 26, 2004 16:26 WSPC/Book Trim Size for 9in x 6in b ook2
Atoms in a Classical Light Field 27
where
Ω=−
ν
2
±
1
2
ν
2
+ ω
2
R
. (2.57)
Similarly, we obtain
a
2
(t)=a
2
(0)e
−iΩt
(or a
2
(t)=a
2
(0)e
iΩt
) . (2.58)
Hence, we again get split and shifted levels
2
→ Ω
2
≡
2
+Ω=
2
−
ν
2
±
1
2
ν
2
+ ω
2
R
(2.59)
1
→ Ω
1
≡
1
− Ω=
1
+
ν
2
±
1
2
ν
2
+ ω
2
R
. (2.60)
The coherent modification of the atomic spectrum in the electric field of a
light field resembles the Stark splitting and shifting in a static electric field.
It is therefore called optical Stark effect. The modified or, as one also says,
the renormalized states of the atom in the intense light field are those of
a dressed atom. While the optical Stark effect has been well-known for a
long time in atoms, it has been seen relatively recently in semiconductors,
where the dephasing times are normally much shorter than in atoms, as
will be discussed in more detail in later chapters of this book.
REFERENCES
For the basic quantum mechanical theory used in this chapter we recom-
mend:
A.S. Davydov, Quantum Mechanics, Pergamon, New York (1965)
L.I. Schiff, Quantum Mechanics,3rded., McGraw–Hill, New York (1968)
The optical properties of two-level atoms are treated extensively in:
L. Allen and J.H. Eberly, Optical Resonance and Two-Level Atoms, Wiley
and Sons, New York (1975)
P. Meystre and M. Sargent III, Elements of Quantum Optics, Springer,
Berlin (1990)

January 26, 2004 16:26 WSPC/Book Trim Size for 9in x 6in b ook2
28 Quantum Theory of the Optical and Electronic Properties of Semiconductors
M.SargentIII,M.O.Scully,andW.E.Lamb,Jr.,Laser Physics, Addison–
Wesley, Reading, MA (1974)
PROBLEMS
Problem 2.1: To describe the dielectric relaxation in a dielectric medium,
one often uses the Debye model where the polarization obeys the equation
dP
dt
= −
1
τ
[P(t) − χ
0
E(t)] . (2.61)
Here, τ is the relaxation time and χ
0
is the static dielectric susceptibility.
The initial condition is
P(t = −∞)=0 .
Compute the optical susceptibility.
Problem 2.2: Compute the oscillator strength for the transitions between
the states of a quantum mechanical harmonic oscillator. Verify the sum
rule, Eq. (2.33).
