Hatano Y., Katsumura Y., Mozumder A. (Eds.) Charged Particle and Photon Interactions with Matter - Recent Advances, Applications, and Interfaces
Подождите немного. Документ загружается.

840 Charged Particle and Photon Interactions with Matter
Roser, J. E., Manico, G., Pirronello, V., and Vidali, G. 2002. Formation of molecular hydrogen on amorphous
water
ice: Inuence of morphology and ultraviolet exposure. Astrophys. J. 581: 276–284.
Roser,
J. E., Swords, S., Vidali, G., Manico, G., and Pirronello, V. 2003. Measurement of the kinetic energy of
hydrogen
molecules desorbing from amorphous water ice. Astrophys. J. Lett. 596: L55-L58.
Schutte,
W. A., Allamandola, L. J., and Sandford, S. A. 1993. An experimental study of the organic mol-
ecules produced in cometary and interstellar ice analogs by thermal formaldehyde reactions. Icarus 104:
118–137.
Sen, A. D., Anicich, V. G., and Federman, S. R. 1992. Formaldehyde reactions in dark clouds. Astrophys.
J. 391:
141–143.
Shalabiea,
O. M. and Greenberg,J. M. 1994.Two key processes in dust/gas chemical modelling: Photoprocessing
of
grain mantles and explosive desorption. Astron. Astrophys. 290: 266–278.
Snyder,
R. J. and Hollis, J. M. 1976. HCN, X-ogen /HCO
+
/, and U90.66 emission spectra from L134 (interstel-
lar
molecules). Astrophys. J. Lett. 204: L139-L142.
Sosa,
C. and Schlegel, H. B. 1986. Ab initio calculations on the barrier height for the hydrogen addition to
ethylene
and formaldehyde.
The
importance of spin projection. Int. J. Quantum Chem. 29: 1001–1015.
Takahashi,
J., Masuda, K., and Nagaoka, M. 1999. Product energy distribution of molecular hydrogen formed
on
icy mantles of interstellar dust. Astrophys. J. 520: 724–731.
Takayanagi,
T. and Sato, S. 1990. The bending-corrected-rotating-linear-model calculations of the rate con-
stants for the H+H
2
reaction and its isotopic variants at low temperatures: The effect of van der Waals
well.
J. Chem. Phys. 92: 2862–2868.
Taketsugu,
T., Tajima, A., Ishii, K., and Hirano, T. 2004. Ab initio direct trajectory simulation with nonadiabatic
transitions of the dissociative recombination reaction HCNH
+
+ e
−
→ HNC/HCN + H. Astrophys. J. 608:
323–329.
Talbi, D., Ellinger, Y., and Herbst, E. 1996. On the HCN/HNC abundance ratio: A theoretical study of the H +
CNH↔ HCN + H exchange reactions. Astron. Astrophys. 314: 688–692.
Tielens, A. G. G. M. 1989. Dust in dense clouds. In IAU Symposium 135, Interstellar Dust, L. A. Allamandola
and A.
G. G. M.
Tielens
(eds.), Santa Clara, CA, pp. 239–262. Dordrecht, the Netherlands: Kluwer.
Tielens,
A. G. G. M., Tokunaga, A. T., Geballe, T. R., and Baas, F. 1991. Interstellar solid CO: Polar and
nonpolar
interstellar ices. Astrophys. J. 381: 181–199.
Van
Ijzendoorn, L. J., Allamandola, L. J., Baas, F., and Greenberg, J. M. 1983. Visible spectroscopy of matrix
isolated
HCO:
The
2
A″(Π)←X
2
A’ transition. J. Chem. Phys. 78: 7019–7028.
Villa, J., Corchado, J. C., Gonzalez-Lafont, A., Lluch, J. M., and Truhlar, D. G. 1998a. Explanation of deute-
rium and muonium kinetic isotope effects for hydrogen atom addition to an olen. J. Am. Chem. Soc.
120:
12141–12142.
Villa,
J., Gonzalez-Lafont, A., Lluch, J. M., and Truhlar, D. G. 1998b. Entropic effects on the dynamical bottle-
neck
location and tunneling contributions for C
2
H
4
+ H → C
2
H
5
: Variable scaling of external correlation
energy
for association reactions. J. Am. Chem. Soc. 120: 5559–5567.
Wada,
A., Mochizuki, N., and Hiraoka, K. 2006. Methanol formation from electron-irradiated mixed H
2
O/CH
4
ice
at 10
K.
Astrophys. J. 644: 300–306.
Watson,
W. D. 1974. Ion-molecule reactions, molecule formation, and hydrogen-isotope exchange in dense
interstellar
clouds. Astrophys. J. 188: 35–42.
Watson,
W.
D. 1977. Interstellar chemistry. Acc. Chem. Res. 10: 221–226.
Watson,
W. D. 1980. Molecule formation in cool, dense interstellar clouds. In Interstellar Molecules, IAU
Symposium 87,
B. H.
Andrew(ed.),
Quebec, Canada, pp. 341–350. Dordrecht, the Netherlands: Reidel.
Woon,
D. E. 2002. Modeling gas-grain chemistry with quantum chemical cluster calculations. I. Heterogeneous
hydrogenation
of CO and H
2
CO on icy grain mantles. Astrophys. J. 569: 541–548.
Yang, K. and Rabitz, H. 1994. Quantum effects in the surface penetration of energetic hydrogen atoms. J. Chem.
Phys. 101: 8205–8213.

841
30
Radiation Effects on
Semiconductors
and Polymers
for
Space Applications
Takeshi Ohshima
Japan Atomic Energy Agency
Takasaki,
Japan
Shinobu Onoda
Japan Atomic Energy Agency
Takasaki,
Japan
Yugo Kimoto
Japan Aerospace Exploration Agency
Tsukuba,
Japan
30.1 introduCtion
Technology for space exploration and development constantly progresses. Many space missions
have been completed and even more are planned. For example, the space station project has been
realized; as a result, one can stay beyond the bounds of the earth for long periods. The uses and
benets from man-made satellites are expansive, including weather forecast, broadcast, resource
Contents
30.1 Introduction .......................................................................................................................... 841
30.2 Space
Solar Cells ..................................................................................................................842
30.2.1
Radiation
Degradation of Solar Cells in Space ........................................................842
30.2.2
Basic
Mechanism of Radiation Degradation of Solar Cells .....................................844
30.2.3
Space
Solar Cells ......................................................................................................847
30.2.4
Radiation Degradation of Triple-Junction Solar Cells .............................................849
30.2.5 Prediction
Methodology of Triple-Junction Solar Cell Degradation........................ 851
30.3
Electronic
Devices................................................................................................................ 853
30.3.1
Overview
of Single-Event Effects in Space.............................................................. 853
30.3.2
Generation
of Charge and Ion Tracks in Semiconductors........................................854
30.3.3
Transient Currents Occurring Subsequent to Ion Track Generation........................ 857
30.3.4 Modeling
of Transient Voltage Pulse in Logic Circuits ...........................................859
30.4
Polymers ...............................................................................................................................863
30.4.1 General
Radiation Effects on Polymers....................................................................863
30.4.2
Ground
Evaluation Facility.......................................................................................868
30.4.3
Space Materials Exposure Experiment ....................................................................870
References...................................................................................................................................... 873
842 Charged Particle and Photon Interactions with Matter
exploration, broadband communication, global positioning, and so on. Missions for deep space as
well
as near the sun are also planned by the United States, Europe, and Japan.
Many
materials and devices that support missions with high reliability are used for space appli-
cations. Since the reliability and durability of such materials and devices directly relate to the life-
time of space applications, it is important to understand the characteristics of such materials and
devices before their launch. Ground testing is critical in the analysis and understanding of these
materials. In space, many kinds of high-energy radiations exist. For example, protons and heavy
ions with high energies come from the sun and from outside of our galaxy. High concentrations of
protons and electrons are trapped by the magnetic eld of the earth (Van Allen belts). Secondary
radiations such as x-rays and neutrons are also produced by the interaction between highly energetic
particles and space applications. In addition, highly reactive species such as atomic oxygen exist
and attack the surface of space-borne materials. Since the properties of materials and devices are
affected by their incidence, irradiation experiments on the ground must be carried out to predict
their lifetime in a space environment. In this chapter, radiation effects on semiconductor devices
and polymers are described from the point of view of space applications. First, the degradation of
the electrical performance of space solar cells is considered, and then, radiation effects on electronic
devices
are discussed. Finally, polymers for space applications are introduced.
30.2 spaCe solar Cells
30.2.1 radiation degradation of Solar cellS in Space
Three major effects, single-event effects (SEEs), total ionizing dose (TID) effects, and displacement
damage effects are observed in semiconductor devices by the incidence of charged particles. SEEs
are malfunctions of electronic devices such as large scale integration (LSIs) and power devices. There
are both nondestructive and destructive SEE failures. More comprehensive details of SEEs will be
described in Section 30.3. The TID effect only affects metal-insulator-semiconductor (MIS) struc-
tures, such as metal-oxide-semiconductor (MOS) devices, because of electron-hole pair generated
in insulator layers due to irradiation. If the TID effect occurs, the electrical characteristics of MIS
devices gradually degrade with increasing dose of radiations, until a destructive malfunction occurs.
When charged particles are introduced into semiconductors, atoms at lattice sites are scattered
into non-lattice sites (knock-on effects); as a result, vacancies are created in semiconductors. In
fact, many kinds of residual defects such as divacancies, vacancy clusters, and vacancy-impurity
complexes are created by irradiation because generated vacancies thermally migrate and transform
into more stable defects. Since such defects act as scattering/recombination centers to free carriers,
electrical properties of semiconductors are degraded by damage generation due to charged particle
irradiation. This degradation is called the displacement damage effect. Similar to the TID effect,
the degradation behavior of semiconductors by displacement damage effect worsens with increasing
charged
particle uence until the fatal malfunction nally occurs.
Displacement
damage is the most dominant effect for space solar cells since the electrical perfor-
mance is degraded by crystal damage due to proton and electron irradiations. Therefore, electron/
proton irradiation experiments using accelerators are conducted on the ground to understand the
decrease of power generation of solar cells during missions. For the evaluation on the ground, the
electrical performance of solar cells is generally measured at a separate facility before/after pro-
ton irradiation experiments (sequential technique). Additional photovoltaic characterization such as
spectral response and photo luminescence are also performed because these measurements can be
done independently from irradiation experiments. However, it is important to note that solar cells
often become radioactive after high-energy (such as 10MeV) proton irradiation. In this case, it is
difcult to handle irradiated solar cells for the measurement of the post-irradiation electrical per-
formance. Thus, we must wait for radioactivity
of
the solar cells to subside before the evaluation of
Radiation Effects on Semiconductors and Polymers for Space Applications 843
radiation degradation can be completed. In an effort to increase the turnaround of samples, evalua-
tion techniques that can measure the electrical performance of solar cells during irradiation experi-
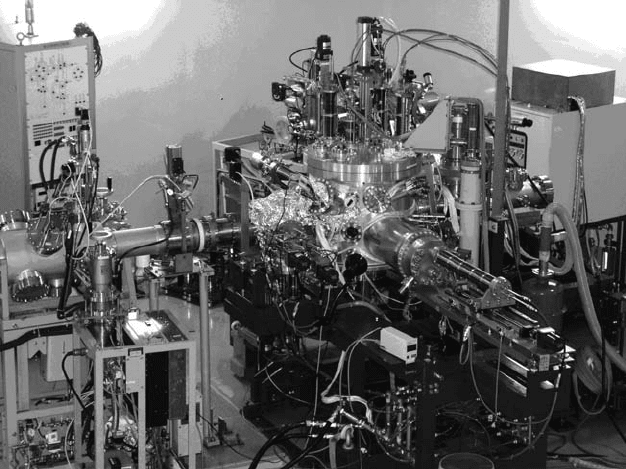
ments have been developed (Ohshima et al., 1996). Figure 30.1 shows a vacuum chamber installed at
Japan Atomic Energy Agency (JAEA), Takasaki, for space solar cell experiments. Since the irradia-
tion chamber has an AM0 solar simulator, the degradation behavior of the electrical performance
of solar cells can be evaluated during proton irradiation experiments (simultaneous technique). In
addition, the degradation behavior of solar cells under low temperature irradiation has become a
topic of interest, specically for missions to Saturn and Jupiter. To tackle such varied missions,
a sample holder with a temperature control system was installed in the chamber (Ohshima et al.,
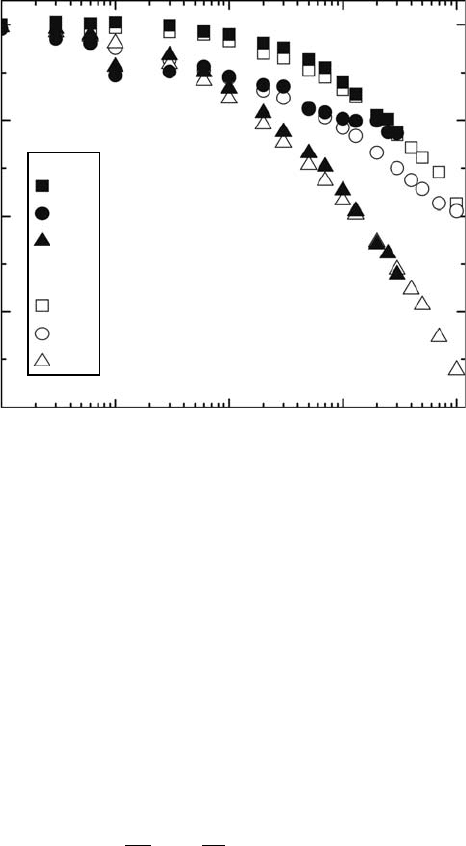
2005; Harris et al., 2008). Figure 30.2 shows the comparison of remaining factors of the electrical
performance (short current circuit, I
SC
, open circuit voltage, V
OC
, and maximum power, P
MAX
) of
space
triple-junction solar cells between RT and 175
K
irradiations (Ohshima et al., 2005).
In
additiontogroundexperiments,on-orbit ight demonstrationshavealsobeenconducted(Imaizumi
et al., 2005). The satellite named MDS-1 (Mission Demonstration-test Satellite No. 1) was launched in
2002. The MDS-1 was placed in a geosynchronous transfer orbit (GTO) with apogee of 36,000km and
perigee of 500km. Typical solar cells designed for terrestrial applications were mounted on the MDS-1,
and their on-orbit electrical performance was measured. A GTO was selected for this ight experiment
because it crosses the Van Allen radiation belts which consist of high-energy charged particles, mainly
electrons and protons. Thus, solar cells were subjected to high concentrations of electrons and protons
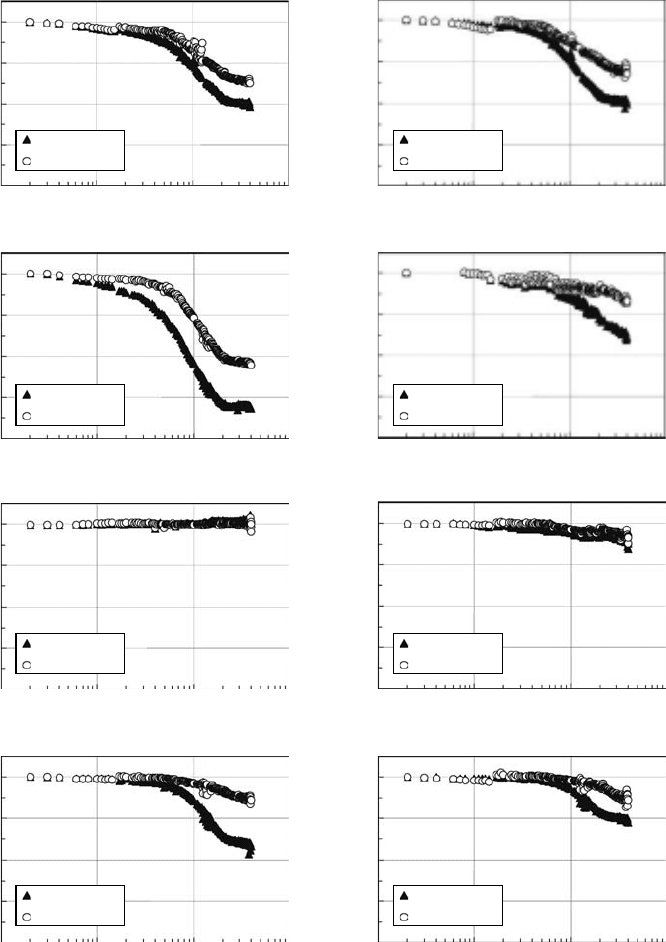
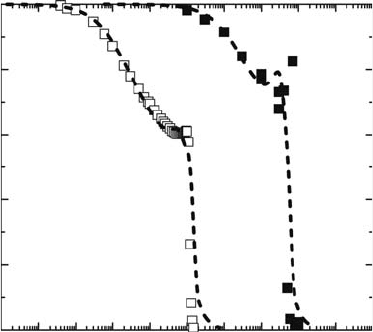
for a short time. Figure 30.3 shows the remaining factor of I
SC
for solar cells on the MDS-1 after launch
(Imaizumi et al., 2005). The I
SC
for all cell types, except Cu(In,Ga)Se
2
(CIGS), decreased within days
after launch as shown in Figure 30.3. Since MDS-1 cuts across the Van Allen belts, the degradation
behavior for solar cells without CIGS can be explained in terms of heavy damage created by proton and
electron irradiations. For CIGS solar cells, it has been reported that electrical performance recovers due
to the annealing of defects even at room temperature (Kawakita et al., 2002). Therefore, the apparent
lack of degradation for CIGS solar cells on MDS-1 is attributed to on-orbit defect annealing.
Figure 30.1 Photograph of a vacuum chamber for space solar cell evaluation installed at Japan Atomic
Energy
Agency (JAEA), Takasaki. The chamber is connected with a beam line of the AVF Cyclotron.
844 Charged Particle and Photon Interactions with Matter
30.2.2 baSic MechaniSM of radiation degradation of Solar cellS
Solar cells generate electric power when photo-induced minority carriers in a base layer migrate
and reach a top layer before recombination. Thus, the diffusion length (lifetime) of minority car-
riers is one of the most important parameters for the solar cell performance. As mentioned above,
charged particles create defects in semiconductors. Since some of the radiation-induced defects act
as recombination centers, a decrease in diffusion length (lifetime) of minority carriers due to irra-
diation is the most dominant degradation effect in solar cells. In this region, experimental results
can
be described by the following semi-empirical equation (Tada et al., 1982),
1
L L
K
Φ
= − Φ
2
0
2
L
1
,
(30.1)
where
L
Φ
and L
0
are the minority carrier diffusion length after and before irradiation, respectively
K
L
and Φ are the damage coefcient of minority carrier and uence
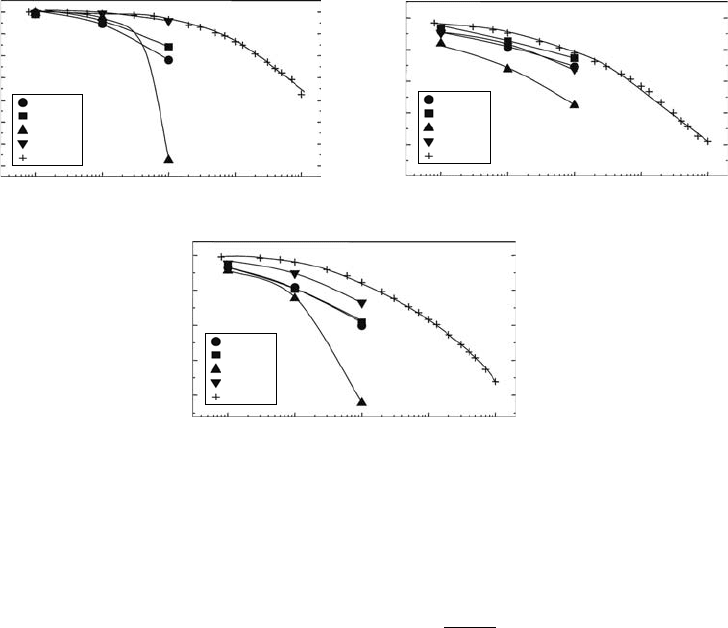
Figure 30.4 shows the remaining factors of triple-junction solar cells irradiated with protons of vari-
ous energies (Sumita et al., 2003). As shown in the gure, the tting lines calculated using the semi-
empirical equation are in agreement with experimental results. From the fundamental point of view,
radiation-induced defects in new materials are intensively investigated because many candidates for
high efciency solar cells have been proposed and properties of defects in such new materials are
not
yet fully understood (Walters et al., 2001; Dharmarasu et al., 2002; Soga et al., 2003).
As
the second irradiation effect, majority carrier removal occurs in high-uence regions, and the
reduction
of majority carriers can be empirically expressed as
1.0
0.8
175 K
I
SC
V
OC
P
MAX
I
SC
V
OC
P
MAX
RT
0.6
0.4
0.2
10
10
10
11
10
12
10 MeV proton fluence (cm
–2
)
Remaining factors
InGaP/GaAs/Ge triple-junction solar cells
10
13
10
14
Figure 30.2 Comparison of remaining factors of the electrical performance (short current circuit, I
SC
,
open circuit voltage, V
OC
, and maximum power, P
MAX
) of space InGaP/GaAs/Ge triple-junction solar cells
between RT and 175K irradiations. (Reprinted from Ohshima, T. et al., Evaluation of the electrical charac-
teristics of III-V compounds solar cells irradiated with proton at low temperature, Proceedings of 31st IEEE
Photovoltaic Specialists Conference,
Lake Buena Vista, FL, 2005, pp. 806–809. With permission.)
Radiation Effects on Semiconductors and Polymers for Space Applications 845
1.0
0.9
0.8
0.7
0.6
1.0
0.9
0.8
0.7
0.6
1.0
0.9
0.8
0.7
0.6
1.0
0.9
0.8
0.7
0.6
Remaining factor of I
SC
1.0
0.9
0.8
0.7
0.6
Remaining factor of I
SC
1.0
0.9
0.8
0.7
0.6
Remaining factor of I
SC
1.0
0.9
0.8
0.7
0.6
Remaining factor of I
SC
1.0
0.9
0.8
0.7
0.6
Remaining factor of I
SC
Remaining factor of I
SC
Remaining factor of I
SC
Remaining factor of I
SC
1 10
(a)
(c)
(e)
(g) (h)
(f)
(d)
(b)
100
MET (days after launch)
MET (days after launch)
MET (days after launch)
MET (days after launch)
1000
1 10 100
MET (days after launch)
1000
1 10 100
MET (days after launch)
1000
1 10 100
MET (days after launch)
1000
1 10 100
MET (days after launch)
1000
1 10 100 1000
1 10 100 1000
1 10 100 1000
CG=500 μm
CG=100 μm
CG=500 μm
CG=100 μm
CG=500 μm
CG=100 μm
CG=500 μm
CG=100 μm
CG=500 μm
CG=100 μm
CG=500 μm
CG=100 μm
CG=500 μm
CG=100 μm
CG=500 μm
CG=100 μm
Figure 30.3 Remaining factor of I
SC
for various solar cells on the MDS-1 after launch. (a) U poly-crystalline
silicon cell. (b) Poly-crystalline silicon cell. (c) N-type base single crystal silicon cell. (d) InGaP/GaAs dual-
junction tandem cell. (e) Large-area CIGS cell. (f) High-efciency CIGS cell. (g) Single crystal silicon space
cell (2 Ω cm). (h) Single crystal silicon space cell (10 Ω cm). (Reprinted from Imaizumi, M. et al., Prog.
Photovolt: Res. Appl.,
13, 93, 2005. With permission. John Wiley & Sons, Ltd.)
846 Charged Particle and Photon Interactions with Matter
p p R p p
R
p
Φ
Φ
= −
−
0 C 0
or exp ,Φ =
Φ
C
0
(30.2)
where
p
Φ
and p
0
are majority carriers after and before irradiations, respectively
R
C
is a “carrier removal rate”
The carrier concentration in the base layer of solar cells decreases, when the carrier removal effect
appears. Since the depletion layer length in a solar cell depends on the difference of carrier concen-
tration between the top and base layers, the decrease in carrier concentration in the base layer causes
the depletion layer to extend. As a result, the number of minority carriers that can reach the top layer
from the base layer increases. Thus, this indicates that the I
SC
increases with increasing uence. Of
course, this very unique increase in I
SC
is a temporary behavior, and with further increase of uence,
the value of I
SC
suddenly drops to almost zero because the base layer becomes intrinsic or type con-
version occurs in the base layer; as a result, no pn junction exists (Matsuura et al., 2006). Figure 30.5
shows the remaining factor of I
SC
for Si solar cells irradiated with either 10MeV protons or 1 MeV
electrons as a function of uence. In a low uence region, the value of I
SC
gradually decreases with
increasing uence. This decrease suggests that the dominant effect is the decrease in the diffusion
length of minority carriers. On the other hand, in the higher uence region, the increase in I
SC
before
sudden dropping down is observed. This behavior can be explained in terms of majority carrier
removal. The excellent agreement of the above-mentioned mechanism with experimental results has
been
reported in the literature (Ohshima et al., 1996; Yamaguchi et al., 1996).
The
decreases in minority carrier diffusion length and majority carrier concentration are basi-
cally the main degradation effects of solar cells due to irradiation. However, in some cases, the
recovery of the electrical performance is also observed. For amorphous Si, the value of photocon-
ductivity becomes higher than the initial value by irradiation (Amekura et al., 2000). The electrical
1.0
0.9
0.8
0.7
0.6
0.5
Normalized I
SC
degradation
0.4
0.3
(a)
1.0
0.9
0.8
0.7
0.6
Normalized V
OC
degradation
(b)
0.03 MeV
0.10 MeV
I
SC
V
OC
0.25 MeV
0.2 MeV
10.0 MeV
0.03 MeV
0.10 MeV
0.25 MeV
0.2 MeV
10.0 MeV
1.0
0.8
0.6
0.4
0.2
Normalized P
MAX
degradation
(c)
P
MAX
10
10
10
11
Proton fluence (p/cm
2
)
10
12
10
13
10
14
10
10
10
11
Proton fluence (p/cm
2
)
10
12
10
13
10
14
10
10
10
11
Proton fluence (p/cm
2
)
10
12
10
13
10
14
0.03 MeV
0.10 MeV
0.25 MeV
0.2 MeV
10.0 MeV
Figure 30.4 Remaining factors of I
SC
, V
OC
, and P
MAX
for InGaP/GaAs/Ge triple-junction solar cells irradi-
ated with protons with various energies as a function of proton uence. Symbols and lines represent experi-
mental and tting results, respectively. (Reprinted from Sumita, T. et al., Nucl. Instrum. Methods B, 206, 448,
2003.
With permission.)
Radiation Effects on Semiconductors and Polymers for Space Applications 847
properties of CIGS irradiated with charged particles recovered even close to room temperature
annealing (Kawakita et al., 2002). For InP and related materials, the recovery of the electrical prop-
erties by minority carrier injection has been reported (Yamaguchi et al., 1995, 1997). These are very
unique behaviors. However, the details of defect annealing/creation have not yet been fully claried
in
these materials. Further investigation by many researchers is currently underway.
30.2.3 Space Solar cellS
A brief history of space photovoltaics will be described before discussing the current status. Early
on, simple single pn junction solar cells based on crystalline Si were used. The efciency of Si
solar cells installed on Vanguard I, launched in 1954, was 7%–8% under AM0 illumination. With
improving device fabrication processes, the design of Si space solar cells was modied, resulting
in a conversion efciency increase. A back surface eld (BSF), which is a p
+
layer, was fabricated
behind a p-type base layer to reduce the surface recombination of photo-induced carriers. To avoid
heating solar cells under illumination at long wavelengths, a back surface reector (BSR) layer
which consists of a thin metal layer was applied to space solar cells. In addition to these modica-
tions, inverse pyramid shaped textures creating a non reective surface (NRS) were fabricated at
the surface of the Si solar cells (Katsu et al., 1994). Then, the conversion efciency of Si solar cells
increased up to 18% under AM0. Single-junction-GaAs-based solar cells were also applied to space
applications because of their relatively high conversion efciency (18%–20%) (Matsuda et al., 1994).
From the point of view of high radiation resistance, InP and related materials have been pro-
posed for space solar cells (Yamaguchi et al., 1984, 1995; Yamaguchi, 2001). Figure 30.6 shows the
remaining factor of P
MAX
for InP, GaAs, and Si solar cells as a function of 1MeV electron uence
(Yamaguchi, 2001). InP solar cells obviously have higher radiation resistance than GaAs and Si
solar cells. In addition, depending on irradiation conditions, InP solar cells show different radia-
tion degradation. From DLTS studies, the variety of the degradation behaviors for InP solar cells is
interpreted in terms of the reduction of a major radiation-induced center, which acts as a hole trap
by minority carrier injection (Yamaguchi et al., 1986). Although the reason of this reduction has
not yet been fully claried, a reformation of defects in InP by energy transfer from injected carriers
has been proposed, most likely because the migration energy of vacancies at both In and P lattice
sites are lower than another III-V compounds such as GaAs (Yamaguchi, 2001). Further investiga-
tion is necessary to more fully understand the detailed mechanism of this minority carrier injection
100
80
60
40
20
0
10
9
10
11
10
13
10 MeV-proton
1 MeV-electron
10
15
Fluence (cm
–2
)
Remaining factor of I
SC
10
17
10
19
Figure 30.5 Remaining factor of I
SC
for Si solar cells irradiated with either 10MeV protons or 1 MeV
electrons
as a function of uence. Symbols and lines represent experimental and tting results, respectively.

848 Charged Particle and Photon Interactions with Matter
effect. The radiation resistance of InP in space was observed with no signicant degradation of
I
SC
for about 1000 days after launch (in fact, a small increase by the injection effect was reported.)
(Weinberg,
1991).
Multi-junction
solar cells based on III-V compounds are the main streams for space applica-
tions nowadays because they combine high conversion efciency and high radiation resistance.
Figure 30.7 shows the schematic cross section of a typical InGaP/GaAs/Ge triple-junction solar
cell (3J solar cell). The 3J solar cells are grown on Ge substrates by an organometallic vapor phase
epitaxy (OMVPE). As shown in Figure 30.7, the 3J solar cells consist of three solar cells (InGaP,
GaAs, and Ge sub cells) with tunnel junction diodes located between each sub cell. Since each sub
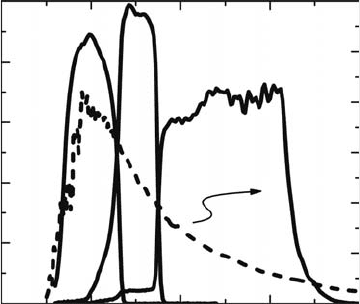
cell can absorb light at specic wavelengths as shown in Figure 30.8, very high efciency ∼28% can
be achieved with AM0 illumination. In addition, new compounds such as AlInGaP and AlGaAs are
utilized in the top and middle sub cells of state-of-the-art 3J solar cells, to further increase in the
conversion efciency (Takamoto et al., 2005). Nitride-based top cells have also been investigated in
an
attempt to improve conversion efciency (Dimroth et al., 2005). Furthermore, using an inverted
metamorphic (IMM) approach to overcome lattice mismatch issues, very high efciency 3J solar
cells
have been achieved (Geisz et al., 2008).
InGaP (top cell)
Tunnel junction
Anti-reflection coating
GaAs (middle cell)
Ge (substrate)
Figure 30.7 Schematic cross section of InGaP/GaAs/Ge 3 junction solar cells.
1
0.9
0.8
0.7
0.6
0.5
10
14
2 5 2 5
10
15
GaAs cell
InP cell
Si cell
Dark
Results obtained from GaAs
Results obtained from Si solar cells
Light illumination (70 mW/cm
2
)
Light illumination (10 mW/cm
2
)
Forward bias (5 mA/cm
2
)
InP cells
1 MeV electron fluence φ (cm
–2
)
Normalized maximum power P
φ
/P
0
10
16
Figure 30.6 Remaining factor of P
MAX
for InP, GaAs, Si solar cells as a function of 1MeV electron uence.
For InP solar cells, results obtained from irradiation under various conditions are depicted. (Reprinted from
Yamaguchi, M., Sol. Energy Mater. Sol. Cells, 68, 31, 2001. With permission.)
Radiation Effects on Semiconductors and Polymers for Space Applications 849
The thin-lm solar cells based on new materials such as CIGS and amorphous Si (s-Si) have been
studied for space applications (Kawakita et al., 2002; Granata et al., 2005). These solar cells are
thought to be very promising candidates because light weight and exible paddles can be designed
to take advantage of their unique characteristics. The technologies for these solar cells are improved
day-by-day, and mass production for terrestrial application has already started. However, as men-
tioned above, since these solar cells show unique recovery behaviors, the mechanism of radiation
effects should be revealed to predict accurate lifetime in a space environment before commercial
use
for space applications.
30.2.4 radiation degradation of triple-junction Solar cellS
As mentioned above, the degradation of solar cells in space is caused by the decreases in minority
carrier diffusion length and majority carrier concentration due to charged particle irradiation. The
decrease in these parameters can be scaled using K
L
and R
C
, respectively, and these values depend
on the material. Thus, in the case of multi-junction solar cells, each sub cell has its own K
L
as well
as R
C
values, and as a result, the degradation behavior of a sub cell is different from that of another.
This suggests that the degradation behavior of multi-junction solar cells is not simple like that of a
single
junction solar cell because the radiation resistance is different between component sub cells.
For V
OC
, the degradation behavior of multi-junction solar cells is the sum of the degradation of each
sub cell connected in series. On the other hand, the degradation behavior of I
SC
for multi-junction
solar cells is equal to the degradation behavior of a sub cell with the lowest I
SC
since the I
SC
for
multi-junction solar cells is limited by a sub cell with lowest I
SC
(current-limiting cell). Figure 30.9
shows the relative damage coefcient of V
OC
for InGaP/GaAs/Ge 3J solar cells as a function of the
energy of irradiated protons. The relative damage coefcient is dened as the degradation value
normalized by the degradation value due to 10MeV proton (or 1MeV electron) irradiation. For
example, the relative damage coefcient of 10 means that the decrease in the electrical performance
is 10 times larger than that in the case of 10MeV at the same uence. In Figure 30.9, the peaks
are observed around 0.03, 0.25, and 2MeV. This indicates that the degradation becomes worse by
proton irradiation at these energies. Calculations using a Monte Carlo code, SRIM (Ziegler et al.,
2008), show that the projection range (penetration depth) of protons with these energies corresponds
to the position of pn junction of sub cells. Since protons create heavy damage at the tail end of their
projection range, the three peaks can be interpreted in terms of the heavy damage creation in each
sub cell by protons with these energies.
100
80
60
40
20
0
0 500 1000
Wavelength (nm)
QE (%)
1500
Ge
GaAs
InGaP
2000
0
50
AM0 (μW/cm
2
/nm)
100
150
200
250
300
Figure 30.8 Quantum efciency (QE) of InGaP/GaAs/Ge 3J solar cells as a function of wave length.
Thespectrum
of AM0 is also shown in the gure.
