Hatano Y., Katsumura Y., Mozumder A. (Eds.) Charged Particle and Photon Interactions with Matter - Recent Advances, Applications, and Interfaces
Подождите немного. Документ загружается.

820 Charged Particle and Photon Interactions with Matter
Figure 29.8a shows the TDS spectra for a 20ML H
2
CO lm being sprayed by H atoms for
300 min at 10 K. The formation of CO and CH
3
OH are clearly discernible from peaks with m/z
12 appearing at ∼35K and with m/z 31 at ∼140K, respectively. The CO was monitored by tracing
C
+
(m/z 12) but not CO
+
(m/z 28). This is because in the thermal desorption mass spectrum, CO
+
overlaps with
N
2
+
originating from the background impurity N
2
gas. The C
+
peaks appearing at
∼75 K and ∼97 K are due to the desorption of CO
2
(residual gas) and H
2
CO (reagent), respectively.
Without spraying the H atoms, no trace amounts of CO and CH
3
OH were detected in the TDS
spectra.
Figure 29.9 represents the temperature dependence on the yields (%) of CO and CH
3
OH formed
from the reaction of H with 20 ML thick solid H
2
CO. As seen in the gure, the yields of CH
3
OH
and CO increase steeply with decrease in temperature. It should be noted that the yields at 10 K
(∼1%) are much lower than those of C
2
H
6
from reactions H + C
2
H
2
(23%) and H + C
2
H
4
(33%)
(Hiraoka et al., 2000) and that of solid product (amorphous silicon) from H + SiH
4
(56%) (Hiraoka
et al., 2001a). It is likely that the rate constant for reaction H + H
2
CO is much smaller than those
for H + C
2
H
4
, H + C
2
H
2
, and H + SiH
4
at ∼10 K.
The reaction of H with the solid sample is affected by many factors, for example, the mor-
phology of the lm, substrate temperature, sample deposition rate, the ux of H atoms, H-atom
adsorption and desorption, H-atom surface and internal diffusion, the local heating of the lm by
the dissipation of the excess energy of the excited molecules, the buildup of the reaction products
near the surface of the lm, etc. For further investigation, it would be protable to study the low-
temperature solid-phase reactions by deconvoluting these factors independently. Recently, Roser
etal. (2002, 2003) measured the kinetic energy of hydrogen molecules formed by the recombina-
tion of hydrogen atoms desorbing from amorphous ice at 10 K. They found that the effective kinetic
temperature is ∼16K. They suggested that the internal energy of the newly formed H
2
molecule is
thermally accommodated to the ice at 10 K. It is likely that the nascent excited H
2
molecule suffers
from multiple collisions on the surface of the amorphous ice and dissipates its energy to the ice
2200
0.000
0.002
0.004
0.006
0.008
0.010
2100
H
2
CO
1246
H
2
CO
1182
230 K
210 K
190 K
170 K
150 K
130 K
110 K
90 K
70 K
50 K
30 K
10 K
After H
2
spray
1300 1200
Wavenumbers (cm
–1
)(c)
Absorbance
1100 1000 900
Figure 29.7 (continued) (c) Temperature dependence of FT-IR spectra for the 20ML thick H
2
CO lm
after the lm was sprayed with H
2
molecules without generating the plasma for 300min at 10K. Absence
of peaks for paraformaldehyde at ∼1126 and ∼951cm
−1
above ∼70K indicates that spontaneous polymeriza-
tion reaction does not take place in the neat H
2
CO lm. The sharpening of the peaks at 1246 and 1182cm
−1
for H
2
CO at ∼70K may be due to the change of morphology of the H
2
CO lm (probably from amorphous to
crystalline). (From Hiraoka, K. et al., Astrophys. J., 620, 542, 2005. Reproduced with permission of the AAS.)

Chemical Evolution onInterstellar Grains atLow Temperatures 821
matrix. Their nding clearly indicates that the morphology of the solid lm is a crucial factor for
the dissipation of the nascent excited product molecules. Hornekaer et al. (2003) performed detailed
laboratory experiments on the formation of HD from atom recombination on amorphous solid water
lms. They found that the recombination process is extremely efcient in the temperature range of
8–20K, implying fast mobility of H and D atoms at this temperature. These ndings implicitly sug-
gest that the H-atom ux should be suppressed as low as possible to investigate the low-temperature
solid-phase reactions in order to suppress the local heating of the lm. Otherwise, the reaction
temperature
cannot be dened precisely.
As
shown in Figure 29.7a, the absorption of paraformaldehyde appears during the H-atom spray
at 10 K. This suggests that the polymerization reaction involving the matrix H
2
CO molecules pro-
ceeds even at 10K. When the 20ML H
2
CO lm was warmed from 10 to 250K without being
sprayed by H atoms, no absorption of paraformaldehyde appeared (Figure 29.7c). This indicates that
polymerization is initiated by residual radicals such as HCO, CH
3
O, CH
2
OH, etc., formed in the
H
2
CO matrix. The steady-state concentration of radicals must be kept low because these radicals
should be annihilated efciently by the reactions with H atoms. In fact, we could not detect any
intermediate
radicals such as HCO, CH
2
OH, CH
3
O, etc., in the infrared spectra.
table 29.5
possible
r
eactions
t
aking
p
lace
in the r
eactions
of h and d
with
h
2
Co and Ch
3
oh
reaction (ΔH°) type of reaction
H
+ H
2
CO → CH
3
O (29.3a)
(−94) Addition
→
CH
2
OH (29.3b)
(−135) Addition
H
+ CH
3
O → CH
3
OH (29.4)
(−435) Recombination
H
+ CH
2
OH → CH
3
OH (29.5)
(−393) Recombination
H
+ H
2
CO → HCO + H
2
(29.6)
(−64) Abstraction
H
+ HCO → CO + H
2
(29.7)
(−373) Abstraction
H
+ HCO → [H
2
CO]* → CO + H
2
(29.8a)
(−373) Unimolecular dissociation
H
+ HCO → [H
2
CO]* → HCO + H (29.8b)
Back dissociation
H
+ HCO → [H
2
CO]* → H
2
CO (29.9)
(−371) Recombination
D
+ H
2
CO → CH
2
DO (29.10a)
Addition
→
CH
2
OD (29.10b)
Addition
D
+ CH
2
DO → CH
2
DOD (29.11)
Recombination
D
+ CH
2
OD → CH
2
DOD (29.12)
Recombination
D
+ H
2
CO → HCO + HD (29.13)
Abstraction
D
+ HCO → CO + HD (29.14)
Abstraction
D
+ HCO → [HDCO]* → CO + HD (29.15a)
Unimolecular
dissociation
→HCO (or DCO) + D (or H) (29.15b)
Back dissociation
D
+ HCO → [HDCO]* → HDCO (29.16)
Recombination
H + CH
3
OH → CH
2
OH + H
2
(29.17)
(−42) Abstraction
H
+ CH
3
OH → CH
3
O + H
2
(29.18)
(+2.5) Abstraction
H
+ CH
2
OH → H
2
CO + H
2
(29.19)
(−301) Abstraction
H
+ CH
2
OH → CH
3
OH (29.20)
(−393) Recombination
D
+ CH
3
OH → CH
2
OH + HD (29.21)
Abstraction
D
+ CH
2
OH → CH
2
DOH (29.22)
Recombination
Source: From Hiraoka, K. et al., Astrophys. J., 620, 542, 2005. Reproduced with permission of
the AAS.
Note: ΔH°
denotes the enthalpy change of reaction in kJ mol
−1
.

822 Charged Particle and Photon Interactions with Matter
Figure 29.7b shows the temperature dependence of the FT-IR spectra when the temperature
of the lm, having been sprayed by H atoms for 300 min at 10K, was increased up to 230K. The
absorption of paraformaldehyde at 1232, 1126, and 951 cm
−1
starts to grow steeply with increasing
temperature above ∼70K. This temperature just corresponds to that for the start of rapid decrease
of absorption of H
2
CO at ∼1182 and ∼1246cm
−1
. This coincidence clearly indicates that the self-
diffusion
of H
2
CO molecules in solid promotes the polymerization of H
2
CO.
The yield of paraformaldehyde was estimated from the TDS of H
2
CO recovered, compared to
that of the deposited H
2
CO not being sprayed by H atoms. About 10%–20% of deposited H
2
CO
50
CO
(CO
2
)
H
2
CO
CH
3
OH
m/z =12 (×100)
m/z =30
0.0
2.0×10
–10
4.0×10
–10
8.0×10
–10
6.0×10
–10
1.0×10
–9
1.2×10
–9
1.4×10
–9
1.6×10
–9
100
Temperature (K)(a)
Pressure (Torr)
150 200
m/z =31 (×100)
50
(CO
2
)
H
2
CO
m/z =12 (×100)
m/z =30
m/z =31 (×100)
0.0
2.0×10
–10
4.0×10
–10
6.0×10
–10
8.0×10
–10
1.0×10
–9
1.2×10
–9
1.4×10
–9
1.6×10
–9
100
Temperature (K)(b)
Pressure (Torr)
150 200
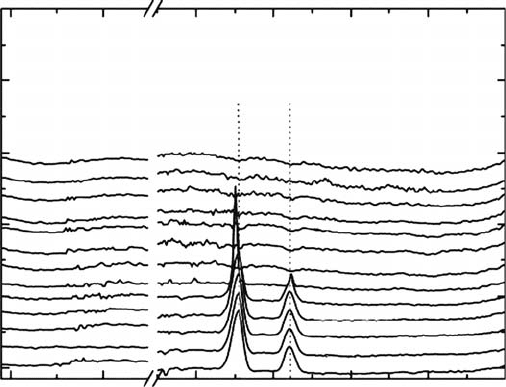
Figure 29.8 (a) TDS spectra for a 20ML dose H
2
CO lm sprayed by H atoms for 300 min at 10K. The
peak at m/z 12 at ∼35K is due to desorption of the reaction product CO. The peak at m/z 12 at ∼75K is probably
due to desorption of CO
2
(residual impurity gas in the vacuum chamber). The peaks with m/z 12, 30, and 31 at
∼97K are due to desorption of the reagent H
2
CO. The peak with m/z 31 at ∼97 K originates from the isotope
peaks of
H CO
2
13 +
and HD
12
CO
+
. The peak with m/z 31 at ∼140K is due to desorption of the reaction product
CH
3
OH (m/z 31 is the base peak for CH
3
OH). (b) TDS spectra for the 20ML dose H
2
CO lm sprayed by H
2
molecules for 300min at 10K without generating the plasma. The absence of peaks at m/z 12 at ∼35K and
m/z
31 at ∼140
K
indicates that the CO and CH
3
OH detected in (a) are products from the reaction H + H
2
CO.
Chemical Evolution onInterstellar Grains atLow Temperatures 823
(20ML) was found to be converted to paraformaldehyde at elevated temperatures. The observed
high yield suggests that the radical-initiated polymerization of H
2
CO is a very efcient process as
already
reported by Goldanskii et al. (1973).
Preliminary
results from the positive ion cluster composition analyzer (PICC) on the Giotto
spacecraft during the encounter with the comet Halley have been presented by Huebner et al. (1987).
160 180
m/z =91
m/z =75
m/z =61
m/z =45
0.0
2.0×10
–13
4.0×10
–13
6.0×10
–13
8.0×10
–13
200
Temperature (K)(c)
Pressure (Torr)
220 240
Figure 29.8 (continued) (c) TDS spectra for the 10ML thick H
2
CO lm reacted with H atoms for 300min
at 10K for higher masses. Peaks with m/z 45, 61, 75, and 91 start to grow at about ∼190K. This is likely due to
desorption of paraformaldehyde, CH
3
OCH
2
−O−CH
2
−O−CH
2
−O−
…
, formed by radical-induced polymerization
reactions in the solid H
2
CO lm. (From Hiraoka, K. et al., Astrophys. J., 620, 542, 2005. Reproduced with permis-
sion of the AAS.)
5
0.0
0.5
1.0
1.5
10
CO
CO
CH
3
OH
CH
3
OH
15 20
Temperature (K)
Yield (%)
25 30 35
Figure 29.9 Relationship between the percentage yields of CH
3
OH and CO and the reaction temperature
for the reaction H + H
2
CO. Film thickness of H
2
CO: 20ML. The data points in the gure were obtained by an
H-atom spray for 300 min over the H
2
CO lm at xed temperatures in the range of 10−30 K. After the H-atom
spray for 300min at the xed sample temperature shown in the gure, the sample temperature was decreased to
10K, and a TDS measurement was made for a quantitative analysis of the reaction products, CO and CH
3
OH.
(From Hiraoka, K. et al., Astrophys. J., 620, 542, 2005. Reproduced with permission of the AAS.)

824 Charged Particle and Photon Interactions with Matter
They pointed out that the sequence of proles with peaks at m/z 45, 61, 75, 91, and 105 has differ-
ences of 16, 14, 16, and 14 u, and the intensities decrease smoothly with increasing mass. These
peaks just correspond to the fragment ions from paraformaldehyde, CH
3
OCH
2
–O–CH
2
–O–CH
2
–
O–
…
. The formation of this type of paraformaldehyde may be initiated by the CH
3
O radical that
attacks the C atom of the H
2
CO molecule. Figure 29.8c shows the thermal desorption mass spectra
for the 10ML H
2
CO lm, reacted with H atoms for 300min. The peaks with m/z 45, 61, 75, and 105
start to grow at about ∼190 K. This is likely due to the desorption of paraformaldehyde formed by
the
CH
3
O-initiated polymerization.
Schutte et al. (1993) found that a trace amount of NH
3
in H
2
CO (NH
3
/H
2
CO = 0.05) is enough to
trigger the polymerization of H
2
CO to form paraformaldehyde spontaneously (without the presence
of radicals) at temperatures as low as 40K. As described, gaseous formaldehyde is readily formed
by heating paraformaldehyde above 70°C. Thus, the formaldehyde observed in the comae of comets
may
partly be due to the thermal decomposition of paraformaldehyde formed in the comet’s ice.
29.5.2 reaction of d with Solid h
2
co
As described in Section 29.3, the reactivity of D with C
2
H
2
was considerably lower than that of H.
Such a remarkable isotope effect was not found for C
2
H
4
. It would be interesting to examine the
reactivities of H and D atoms toward H
2
CO. In the reactions of D with H
2
CO, reactions (29.10)
through
(29.16) may take place.
In
reaction H + H
2
CO, the recombination reaction (29.9) regenerates H
2
CO that cannot be distin-
guished from the reagent H
2
CO. In contrast, reaction (29.16) forms HDCO that can be distinguished
from H
2
CO through mass spectrometry. Thus, the branching ratio of [reactions (29.14) and (29.15a)
for the formation of CO] to [reaction (29.16) for the formation of HDCO] can be determined in the
reaction D + H
2
CO. The quantitative analysis for HDCO was performed by measuring the m/z 31
ion
signal for DHCO
+
. The contribution from the isotope peak of
H CO
2
13
+
was corrected.
Figure 29.10 shows the temperature dependence on the yields (%) of CO, HDCO, and CH
2
DOD
formedfromthe reactionofD with solidH
2
CO(20ML) for300min ofD-atom irradiation. Anincrease
0.0
0.5
1.0
1.5
2.0
10
CO
CO
HDCO
HDCO
CH
2
DOD
CH
2
DOD
15 20
Temperature (K)
Yield (%)
25 30
Figure 29.10 Relationship between the percentage yields of CH
2
DOD, HDCO, and CO and the reaction
temperature for the reaction D + H
2
CO. Film thickness of H
2
CO, 20ML. The data points in the gure were
obtained by D-atom spray for 300 min over an H
2
CO lm at xed temperatures in the range of 10–30K. After
the D-atom spray for 300 min at the xed sample temperature shown in the gure, the sample temperature
was decreased to 10K, and a TDS measurement was made for a quantitative analysis of the reaction products.
(From
Hiraoka, K. et al., Astrophys. J., 620, 542, 2005. Reproduced
with permission of the AAS.)
Chemical Evolution onInterstellar Grains atLow Temperatures 825
of the product yields with decrease of temperature is reproduced as in the case of H+H
2
CO. While
the D-atom addition reactions (29.10a) and (29.10b) followed by reactions (29.11) and (29.12) form
CH
2
DOD, the H-atom abstraction reaction (29.13) leads to the formation of CO and HDCO. Thus, the
ratio of [the yield of CH
2
DOD (∼0.25%)] to [those of CO and HDCO (∼0.25+ ∼1.4≈∼1.7%)], that is,
∼0.25:∼1.7
≈ 1:7, will give an approximate branching ratio of [the D-atom addition reactions (29.10a)
and (29.10b)] to [the H-atom abstraction reaction (29.13)] (reaction temperature, 10K). It is apparent
that the abstraction reaction (29.14) is much more favorable than the addition reaction (29.10).
The intermediate radical HCO formed by reaction (29.13) has two chemical channels, that is, the
formation of CO by reactions (29.14) and (29.15a) and the formation of HDCO by reaction (29.16).
The much higher yield of HDCO (∼1.4%) than that of CO (∼0.25%) indicates that the recombination
reaction (29.16) is a main channel for reaction D + HCO. This is reasonable because reaction (29.16)
is a radical−radical recombination reaction whose energy barrier should be much smaller than that
of
an abstraction reaction (29.14).
It
should be noted that the yield of CO from D + H
2
CO in Figure 29.10 is much lower than that
from H + H
2
CO in Figure 29.9. This suggests that reaction (29.13) followed by reactions (29.14)
and (29.15a) is less efcient than reaction (29.6) followed by reaction (29.7) and (29.8a). The yield
of deuterated methanol CH
2
DOD formed by addition reactions (29.10) through (29.12) is consider-
ably smaller than that of CH
3
OH formed from reactions (29.3) through (29.5). It is evident that both
D-atom
addition and abstraction reactions are less efcient than the H-atom reactions.
29.5.3 reactionS of h and d with Solid ch
3
oh
In the previous section, it was shown that the reaction H + H
2
CO leads to the formation of both CO
and CH
3
OH. We further studied whether reaction H + CH
3
OH produces H
2
CO by the consecutive
H-atom abstraction reactions, CH
3
OH → CH
2
OH → H
2
CO. In the reaction H + CH
3
OH, reactions
(29.17) through (29.20) may take place. In these reactions, abstraction reaction (29.18) may be neg-
ligible at cryogenic temperatures because it is slightly endothermic and has a high energy barrier
of 58.9kJ mol
−1
(theoretical value due to Jodkowski et al. (1999)). Despite the scrutiny of the FT-IR
spectra for the CH
3
OH lm that reacted with H atoms for 300min, no absorption of H
2
CO and CO
appeared. These compounds were not detected by the much more sensitive TDS technique either.
Therefore,
the generation of H
2
CO from reaction H + CH
3
OH is concluded to be negligible.
As mentioned above, the reaction of H with CH
3
OH does not produce any detectable reaction
products. However, this does not necessarily mean that abstraction reaction (29.17) does not take
place because the intermediate product CH
2
OH may regenerate the original CH
3
OH by reaction
(29.20). In order to examine whether or not reaction (29.17) followed by (29.20) takes place, the
reaction of D with CH
3
OH was examined. If CH
2
DOH is formed, this will be evidence for the
occurrence
of reaction (29.21) followed by reaction (29.22).
Figure
29.11 shows the TDS spectra for 20 ML CH
3
OH lm, reacted with D atoms for 300min
at 10 K. The appearance of the peak with m/z 33 due to CH
2
DOH was clearly observed at 140K.
This indicates the occurrence of reactions (29.21) and (29.22). The yield of CH
2
DOH was 1.6%
for a 20 ML thick CH
3
OH lm sprayed by D atoms for 300min. No reaction products other than
CH
2
DOH were detected by TDS. The low yield of CH
2
DOH is reasonable because the large energy
barrier of ∼38kJ mol
−1
for reaction (29.17) has been predicted theoretically (Blowers et al., 1998;
Jodkowski
et al., 1999).
The
yields of reaction products for the reactions of H or D with 20 ML thick H
2
CO and CH
3
OH
for the H- and D-atom spray of 300min at 10K are summarized in Figure 29.12. In Figure 29.12a
and b, the reactions of H and D with H
2
CO lead to the formation of CO and CH
3
OH, that is, both
addition and abstraction reactions take place. From Figure 29.12b for D + H
2
CO, the branching ratio
of the abstraction reaction to the addition one can be estimated to be about 7:1 (1.7:0.25), that is,
the abstraction reaction is the prevalent process over addition. For D + HCO, the branching ratio of
the reaction channel to form CO, to that to form HDCO is about 1:6 (0.25:1.4), indicating that the
826 Charged Particle and Photon Interactions with Matter
radical–radical recombination reaction (29.16) takes place more preferably than the H-atom abstrac-
tion reaction (29.14). In Figure 29.12a and b, the reactivity of H toward H
2
CO is higher than that
of D. In the reactions of H and D with CH
3
OH, no H
2
CO was detected as a reaction product. Thus
CH
3
OH may be regarded as the terminal product in the consecutive reactions of H with CO because
no
H
2
CO was detected for H + CH
3
OH.
From the experimental results described above, we conjecture that the consecutive hydrogena-
tion reactions CO → HCO → H
2
CO → CH
3
CO(CH
2
COH) → CH
3
OH may not be sufcient to
explain the abundance of methanol found in the interstellar ices because the reactivities of H toward
CO and H
2
CO are found to be rather low. From the number density of H atoms (∼1cm
−3
), UV pho-
ton ux (∼10
3
photons cm
−2
s
−1
) (D’Hendecourt and Allamandola, 1986; Lanzerotti and Johnson,
1986; Allamandola et al., 1988; Grim et al., 1989; Johnson, 1990; Shalabiea and Greenberg, 1994;
Kaiser and Roessler, 1998), and cosmic-ray ux (∼10 particles cm
−2
s
−1
) (Lanzerotti and Johnson,
0.0
1.0×10
–11
2.0×10
–11
3.0×10
–11
4.0×10
–11
5.0×10
–11
6.0×10
–11
12010080
CH
2
DOH
m/z =33
CH
3
OH
m/z =31
140 160
Temperature (K)
Pressure (Torr)
180 200
Figure 29.11 TDS spectra for a 20 ML thick CH
3
OH lm reacted with D atoms for 300min at 10K. The
peak at m/z 33 appearing at ∼140 K is due to the formation of monodeuterated methanol, CH
2
DOH. (From
Hiraoka,
K. et al., Astrophys. J., 620, 542, 2005. Reproduced
with permission of the AAS.)
(a)
CH
2
OH
CH
3
O CH
3
OH
HCO
CO
H
2
CO
~1.3%~0.7%
(b)
CH
2
DODCH
2
DO
CH
2
ODHDCO
HCOCO
~0.25% ~1.7%
H
2
CO
~1.4%
~0.25%
(c)
H
2
CO CH
2
OH
CH
2
DOH
~1.6%
CH
3
OH
~0%
Figure 29.12 Reaction channels for reactions of (a) H-atom spray on H
2
CO (20ML), (b) D-atom spray on H
2
CO
(20ML), and (c) D-atom spray on CH
3
OH (20ML). The H/D-atom spray time was 300min, the lm thickness of
H
2
CO was 20ML, and the reaction temperature was 10K. The yields in the gure were determined by TDS. (From
Hiraoka, K. et al., Astrophys. J., 620, 542, 2005. Reproduced with permission of the AAS.)
Chemical Evolution onInterstellar Grains atLow Temperatures 827
1986; Johnson, 1990; Kaiser and Roessler, 1998) in the dense molecular cloud, the time interval for
irradiation by a hydrogen atom, an UV photon, and a cosmic particle on the dust grain (0.1μm in
diameter, cross section of ∼10
−10
cm
2
) can be crudely estimated to be once every day, once every
100 days, and once every 10,000 days, respectively. The contribution of a cosmic ray should be as
important as that of UV photons because a cosmic ray can generate about 100 suprathermal species
in solid phase (Kaiser and Roessler, 1998). Formaldehyde would be formed on the dust grains in the
dark clouds by the cooperative action of H-atom adsorption, UV photons, and cosmic rays: none of
these would be negligible because the reaction H + CO is quite inefcient.
Since the rate of reaction of H with CO (Hiraoka et al., 2002a) is much lower than those with
parafns (Hiraoka et al., 1992), olens (Hiraoka et al., 2000, 2002b; Hiraoka and Sato, 2001), and
SiH
4
(Hiraoka et al., 2001a), the chance for H atoms to react with CO will decrease drastically
when the mantle of the dust grain is being contaminated by other molecules, that is, the H atoms
adsorbed on the dust grains may well be annihilated by reactions with more reactive contaminants
and the less reactive CO will be left intact in the mantle. This may be the reason why the natal CO
is well preserved in the mantles of the dust grains and comets. We think that the role of CO to form
CH
3
OH becomes increasingly less important with the proceeding of the chemical evolution in the
dark clouds. Another source for the formation of CH
3
OH such as UV-photon and/or cosmic-ray
induced formation of CH
3
OH in the dirty H
2
O ice, containing some carbon source (e.g., CH
4
), must
be invoked (Allamandola et al., 1988; Kaiser and Roessler, 1998; Moore and Hudson, 1998; Hudson
and Moore, 1999). In Section 29.6, the interactions of low-energy electrons with CH
4
seeded in H
2
O
will be described in order to investigate the role of the cosmic rays on the formation of methanol on
the
dust grains.
29.6 methanol Formation From eleCtron-irradiated
mixed
h
2
o/Ch
4
iCe at 10 k
While the formation of many interstellar molecules are reasonably explained by the gas-phase
reactions, solid-phase reactions must be invoked for some molecules, such as saturated hydro-
carbons, H
2
CO, CH
3
OH, NH
3
, etc., which are of paramount importance for the evolution of life.
As described above, the origin of ubiquitous formaldehyde and methanol in interstellar objects
is controversial. Interstellar dust grains, comets, and icy satellites are subject to cosmic-ray irra-
diation. By far the most important process caused by the interaction between the cosmic rays
and matter is ionization. The ejected electron may have enough energy to further ionize and
excite the ambient molecules resulting in second-generation ions, radicals, electrons, photons,
and rovibronically excited species. The primary ions may participate in various ion–molecule
reactions and eventually be neutralized by secondary electrons to form reactive neutral species
or stable molecules. The formed radicals may react with other radicals or molecules to form the
terminal products.
The laboratory studies of energetic-particle irradiation on ices relevant to astrochemical inter-
est have been extensively carried out over the last decade (Kaiser and Roessler, 1998; Moore and
Hudson, 1998; Hudson and Moore, 1999; Roser et al., 2002, 2003). However, studies on the electron
irradiation of solid lms is only very limited. In order to investigate the role of secondary electrons
formed by the cosmic rays in dust grains that may play major roles in the chemical evolution in cold
interstellar medium (ISM), we studied the low-energy (10–300eV) electron irradiation on the water
ice
containing 10% methane at 10
K
(Wada et al., 2006).
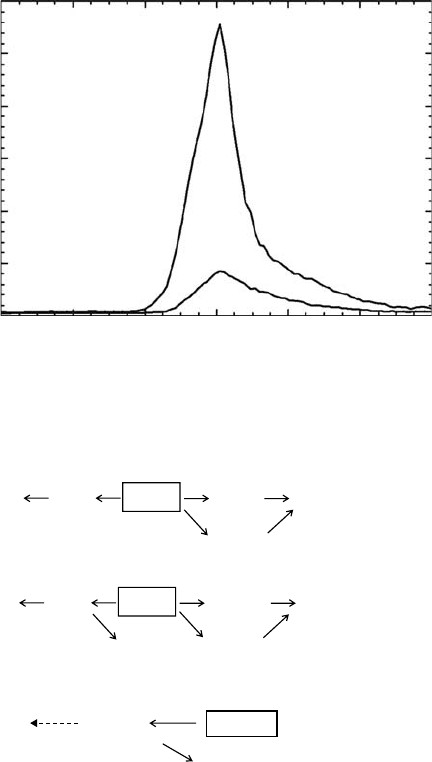
Figure
29.13 shows the conceptual idea of the apparatus (ARIOS, Inc. Akishima, Tokyo). The
H
2
O and CH
4
gases were pre-mixed in the stainless steel gas reservoir (300cm
3
) in a predeter-
mined mixing ratio (H
2
O/CH
4
= 10/1). The sample gases were deposited on the cold substrate. The
hot-lament electron gun was installed on the vacuum manifold. The electron beam was raster-
scanned in order to irradiate the sample surface homogeneously. Electron ux was calibrated by

828 Charged Particle and Photon Interactions with Matter
using aFaraday cup. The angle of the electron beam to the substrate was 45° to the surface normal.
The
quantitative analysis of reaction products was performed by TDS.
The
real-time and in situ observation of electron-induced reactions was made by FT-IR (Nicolet
Nexus 670) in the region of interest, 900–4000cm
−1
. Figure 29.14 shows the IR spectrum, obtained
by simultaneous sample deposition/e
−
-irradiation experiments for 11h. As seen in the gure, the
dominant peak at ∼3400 cm
−1
, and small peaks at 3030cm
−1
and 1303cm
−1
are due to the reactants,
water and methane, respectively. The formation of methanol as a major product is easily recognized
by
the absorption peaks appearing at 1014 and 1122
cm
−1
(see inset in Figure 29.14).
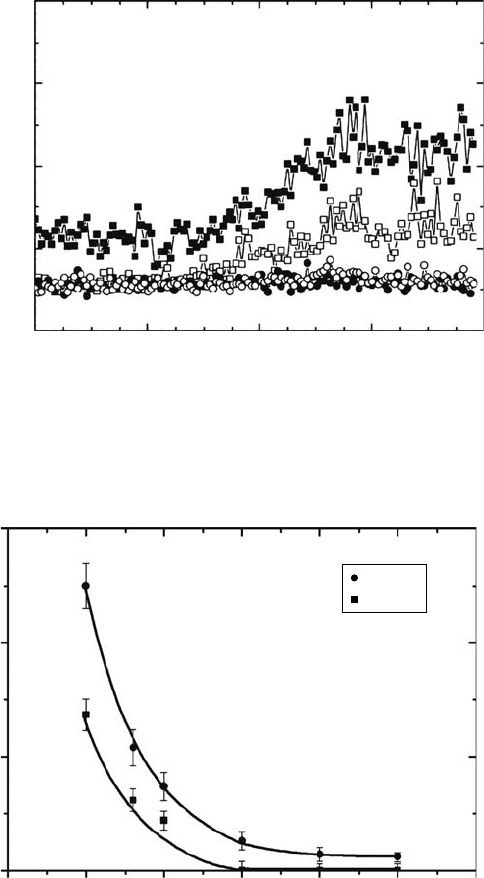
Figure 29.15 shows the dependence of yields of reaction products on the electron ux for the
sample H
2
O/CH
4
(10/1), using electron energy of 100eV (electron irradiation time of 22min). At the
Sample
reservoir
Capillary
(ID 0.1 mm)
IR beam
Sample inlet pipes
e–
Electron gun
Rotating feedthrough
Capillary plate
QMS
FT-IR
H
2
O
CH
4
/CD
4
Closed-cycle
helium refrigerator
Gold-coated
copper substrate
Figure 29.13 Schematic diagram of the experimental apparatus. QMS: quadrupole mass spectrometer.
(From
Wada, A. et al., Astrophys. J., 644, 300, 2006. Reproduced
with permission of the AAS.)
0.15
0.10
0.05
0.00
4000 3500 3000 2500
H
2
O/CH
4
(10/1), 300 ML, 100 eV, 300 nA cm
–2
, 10 K, 11 h
Wavenumbers (cm
–1
)
H
2
O
H
2
O
CH
4
3010 cm
–1
CO
2
2343 cm
–1
Absorbance
2000 1500 1000
100011001200
1122 cm
–1
CH
4
1303 cm
–1
CH
4
OH
1014 cm
–1
1300
0.01
0.02
0.03
0.04
0.05
Figure 29.14 Infrared absorption spectrum of H
2
O/CH
4
(10/1) ice irradiated continuously by 100eV electrons
with ux of 300nA cm
−2
during the sample deposition for 11h at 10K. Total thickness of the sample is 300ML.
The uence is 8eV molecule
−1
. (From Wada, A. et al., Astrophys. J., 644, 300, 2006. Reproduced with permission
of the AAS.)

Chemical Evolution onInterstellar Grains atLow Temperatures 829
lowest electron ux, that is, 30nA cm
−2
(1.5 eV molecule
−1
), H
2
CO is the most abundant product, fol-
lowed by C
2
H
4
, C
2
H
6
, CH
3
OH, and C
2
H
2
. The predominance of the formation of C2 hydrocarbons
with low-electron ux may be due to the enriched CH
4
on the solid surface due to its segregation
from the water ice. Methanol shows a steady increase with electron ux. The delayed appearance
of
C
2
H
2
suggests the occurrence of successive dehydrogenation reactions, C
2
H
6
→ C
2
H
4
→ C
2
H
2
.
Figure 29.16 shows the yield of methanol as a function of the incident electron energy obtained by
the simultaneous sample deposition/e
−
-irradiation experiments. Methanol, which could not be detected
with electron energy of 10eV, started to be detected at 30eV and its yield increases monotonically up
0 1000 2000 3000
Electron flux (nA cm
–2
)
0
1×10
15
2×10
15
3×10
15
4×10
15
H
2
O/CH
4
(10/1), 10 ML, 10 K
100 eV, 22 min
H
2
CO
CH
3
OH
C
2
H
4
C
2
H
6
C
2
H
2
Abundance (molecule)
Figure 29.15 Yields of CH
3
OH, H
2
CO, C
2
H
4
, C
2
H
6
, and C
2
H
2
as a function of electron ux. Electron
energy, 100eV. Simultaneous electron irradiation during deposition of the sample at 10 K for 22min. The total
sample thickness: 10ML. (From Wada, A. et al., Astrophys. J., 644, 300, 2006. Reproduced with permission
of the AAS.)
0 100
Electron energy (eV)
200 300
0
2×10
14
4×10
14
6×10
14
8×10
14
1×10
15
H
2
O/CH
4
(10/1), 10 ML, 10 K
100 nA cm
–2
, 22 min
Methanol abundance (molecule)
Figure 29.16 Yield of CH
3
OH as a function of electron energy. Simultaneous electron irradiation with ux
of 100nA cm
−2
during deposition of H
2
O/CH
4
(10/1) at 10K for 22min. The total sample deposited, 10 ML.
(From
Wada, A. et al., Astrophys. J., 644, 300, 2006. Reproduced
with permission of the AAS.)
