Gupta D. (Ed.). Diffusion Processes in Advanced Technological Materials
Подождите немного. Документ загружается.


42 DIFFUSION PROCESSES IN ADVANCED TECHNOLOGICAL MATERIALS
Figure 1.17(a) Enhancement of
195
Au diffusion in the Au-1.2 at.% Ta alloy com-
pared with that in pure Au. The activation energies are 143 and 170 kJ/mol,
respectively. See also Fig. 1.4 for the mechanism.
[68]
Ch_01.qxd 11/30/04 8:36 AM Page 42
DIFFUSION IN BULK SOLIDS AND THIN FILMS, GUPTA 43
Figure 1.17(b) Grain boundary and sub-boundary diffusion of
195
Au tracer in
Au-1.2 at.% Ta alloy compared with that in pure Au in the boundaries of both kinds.
The activation energies are 121 and 92 kJ/mol for grain boundaries in Au-Ta alloy
and pure Au, respectively. The activation energy for sub-boundaries in both the
cases is 121 kJ/mol. (After Gupta and Rosenberg
[68, 89]
)
Ch_01.qxd 11/30/04 8:36 AM Page 43

1.5 General Characteristics of Grain
Boundary Diffusion
This section discusses general characteristics of grain boundary diffu-
sion and relates these characteristics to the lattice diffusion. It also touches
on some important properties of grain boundaries, as well as their
anisotropy and energy. Because this subject is vast, it cannot be covered
here in depth. A recent special issue of Interface Science
[69]
discusses the
latest developments in the field. Sutton and Balluffi’s book
[45]
deals in
depth with the microstructure, defects, and kinetic properties in the vari-
ous interfaces in crystalline solids. The handbooks by Kaur et al.
[70]
and
Volume 26 in the Landholt-Börnstein series
[71]
contain discussions and
data on various materials.
1.5.1 Anisotropy of Diffusion in Grain Boundaries
A grain boundary is essentially a two-dimensional transition region
between two crystals that have different orientations but the same perio-
dicity. The transition region leads to a very complex crystallographic
description of the grain boundary since 9 degrees of freedom or inde-
pendent variables are involved, which may be specified as 3 for the mis-
orientations, 3 for the choice of the GB plane, and 3 for the rigid-body
translation.
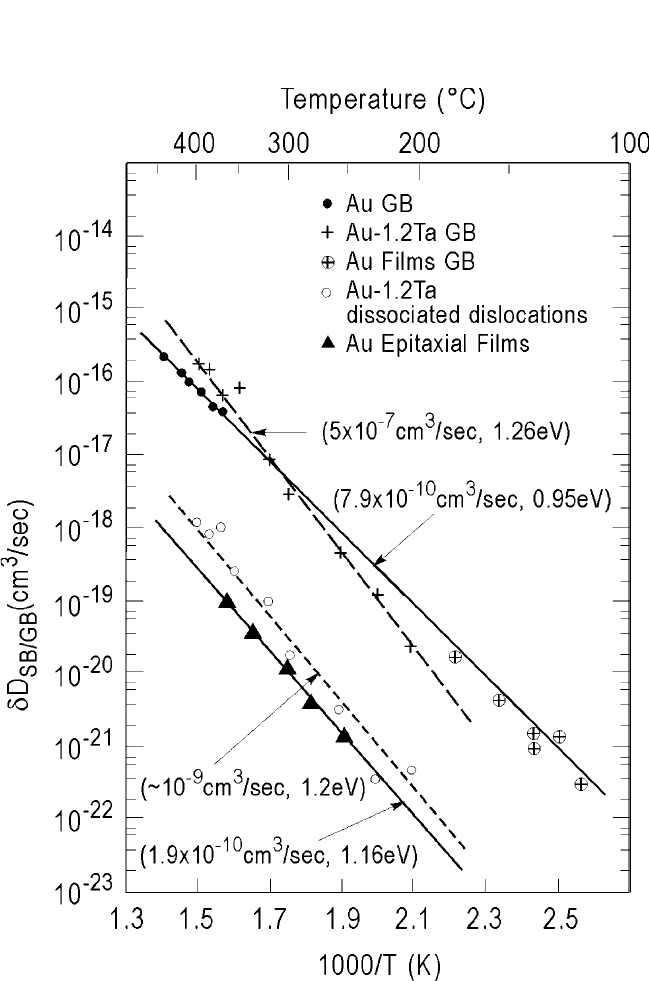
Figure 1.18(a) through (c) shows grain boundary diffusion of
119
Sn
radioactive tracer in polycrystalline Pb foils.
[72]
Contact auto-radiograms
were made on the entrance and exit sides of the foil after annealing at 90°C
for 100 hours, at which time the
119
Sn tracer emerged at the back surface.
To prevent cross talk between the two surfaces, the Pb foil thickness
(64 mm) was chosen to exceed 10 times the half-absorption thickness for
10 keV x-rays from
119
Sn with an absorption coefficient r 1000 cm
1
.
The auto-radiograms are compared with the grain boundary microstruc-
ture. Only a few grain boundaries, the high-angle ones, are seen in the
auto-radiograms through which the
119
Sn tracer diffused. The microstruc-
ture is in fact much finer, showing a variety of grain boundaries. Notably,
the twin boundaries are totally absent in the auto-radiograms. This is obvi-
ously due to variable diffusion kinetics in grain boundaries of differing
orientations. Grain boundary diffusion in polycrystalline Pb was subse-
quently measured by serial sectioning technique using
203
Pb radioactive
tracer.
[73]
In Fig. 1.19, GB self-diffusion coefficients (dD
b
cm
3
/sec) using the
203
Pb radioactive tracer from Gupta and Kim
[73]
are shown in an Arrhenius
plot and are compared with earlier data of Stark and Upthegrove
[74]
as a
44 DIFFUSION PROCESSES IN ADVANCED TECHNOLOGICAL MATERIALS
Ch_01.qxd 11/30/04 8:36 AM Page 44

DIFFUSION IN BULK SOLIDS AND THIN FILMS, GUPTA 45
function of orientation. The data in polycrystalline Pb are seen to go
through mostly the high-angle grain boundaries. These observations are
in accordance with the studies of Turnbull and Hoffman,
[75]
and Couling
and Smoluchowski,
[76]
on anisotropy of GB diffusion and its dependence
on the orientation angle q. In Ag tilt boundaries,
[100]
diffusion of
110
Ag
showed anisotropy of ∼15 for parallel and perpendicular directions at
Figure 1.19 Grain boundary diffusion in polycrystalline Pb and Pb bicrystals.
[73, 74]
Figure 1.18 Contact autoradiograms of the
119m
Sn tracer after diffusion in a Pb foil
at 90°C. (A) The plated or entrant surface, (B) the exit surface, and (C) the micro-
structure. The field of view is 1.25 cm in all cases. Note the similarity in the grain
structure. (After Gupta and Campbell
[72]
)
Ch_01.qxd 11/30/04 8:36 AM Page 45

450°C. The anisotropy progressively declined for orientations near
Σ 13 (q 23 degrees), Σ 17 (q 28 degrees), Σ 5 (q 36 degrees),
and so forth. The anisotropy of diffusion within the GB plane can be
understood on the basis of the distribution and nature of GB dislocations,
which predict channels of free volume parallel to the tilt axis in large-
angle boundaries. The diffusion coefficients and penetration distance
along GBs are also expected to show discontinuities and cusps as a func-
tion of misorientation angle similar to the GB energies because the con-
tributing factor in both cases should be the coincident lattice sites and the
GB dislocations. The activation energy for GB diffusion, however,
appears to remain independent of the orientation and Q
b
Q
l
≈ 0.4
(±20%), as in the case of GB diffusion in Pb, where Q
b
42 kJ/mol (0.44
eV) and Q
l
106 kJ/mol (1.1 eV). Thus, the anisotropy and orientation
dependence of GB diffusion may be largely due to changes in the pre-
exponential term D
b
o
.
1.5.2 Diffusion Mechanisms in Grain Boundaries
This section briefly examines the structure of grain boundaries and
the associated defects to explain possible diffusion mechanisms in
grain boundaries. This subject is exhaustive and complex, and only a
qualitative description of the gross features of grain boundaries is pos-
sible here. Sutton and Balluffi
[45]
and Balluffi
[77]
discuss this subject in
depth. Grain boundaries have been shown to be a world in themselves,
with a potpourri of defects such as the vacancies, interstitials, and dis-
locations. Because a grain boundary is a transition structure between
two crystals, perfectly two-dimensional periodic arrays would only
develop at some discrete choices of GB variables. To account for any
arbitrary choice of orientations and translations, incorporation of line
defects is required.
Extensive grain boundary structure studies have been conducted
over the past several decades
[78, 79]
using techniques such as high-reso-
lution transmission microscopy and computer stimulation, from which
the following broad features of grain boundaries have emerged (see
Chapter 3). First, the grain boundary is found to be relatively narrow,
on the order of a few atomic distances only. Second, the atomic pack-
ing in the GB core is only slightly less dense than in the perfect crys-
tal. Although the core structure has finite excess volume, much of it
may be considered to be relaxed by the development of a variety of
atomic groupings and localized configurations. Some boundaries have
short wavelength periodicity running parallel to the coincident site
46 DIFFUSION PROCESSES IN ADVANCED TECHNOLOGICAL MATERIALS
Ch_01.qxd 11/30/04 8:36 AM Page 46

DIFFUSION IN BULK SOLIDS AND THIN FILMS, GUPTA 47
lattice (CSL) and are generally of low energy compared to the bound-
aries with long periodicity; the former may be termed special bound-
aries. In the case of arbitrary misorientation between two crystals, the
grain boundary minimizes its energy by preserving patches of the spe-
cial low-energy-ordered boundary and introducing therein arrays of
intrinsic grain boundary dislocations. It is also possible to have partial
grain boundary dislocations and extrinsic grain boundary dislocations
in the grain boundaries.
There is indirect experimental evidence from which a localized
structure of vacancies may be inferred with little relaxation of the
neighboring atoms. First, there was the measurement of pressure
dependence of the GB self-diffusivities in Ag by Martin et al.,
[80]
which
resulted in a value of ∆V
b
V
o
1.1, where V
o
is the activation volume
for GB self-diffusion defined analogous to Eq. (34) and V
o
is the atomic
volume. The value of ∆V
b
is of the order of atomic volume in grain
boundaries and compares very favorably to its counterpart in the lattice
(Table 1.2).
Second, the isotope effect measurement for self-diffusion in Ag grain
boundaries by Robinson and Peterson
[81]
has also resulted in a relatively
large value of ∆E
b
∼ 0.46, which is temperature-insensitive. The corre-
sponding value of the isotope effect for self-diffusion in lattice is 0.65
∆E
l
0.71; the variation is due to some temperature dependence (Table
1.2). If the mobile defects responsible for GB diffusion were partially dis-
sociated or highly relaxed vacancies, a correlated movement of a number
of atoms would be required for diffusion to occur. Therefore, only a small
isotope effect value would have been observed. The experimental data,
however, neither support nor rule out contribution of interstitial atoms to
grain boundary diffusion. Atomistic computer modeling of point defects
in grain boundaries, using various techniques such as kinetic Monte Carlo
and molecular dynamics, have shown that vacancies as well as interstitial
atoms are stable, depending on the misorientation of the boundary and the
host crystal lattice.
[82]
Both defects have similar attractive binding ener-
gies in the grain boundaries, are mobile, and account for diffusion under
certain conditions. Suzuki and Mishin
[82]
have studied the role of intersti-
tial atoms and vacancies in Cu grain boundaries in detail. They noted
that diffusion in the Σ 5(310) [001], commonly considered a typical
grain boundary, was dominated by the interstitial atoms, whereas other
boundaries, the Σ 5(210)[001], Σ 9(12
–
2)[011], Σ 11(31
–
1)[011],
Σ 7(23
–
1)[111], and Σ 13(34
–
1)[111], were dominated by vacancies. In
any event, grain boundary diffusion is many orders of magnitude faster
than in the lattice. In Sec. 1.5.3, a thermodynamic model is discussed to
explain this difference.
Ch_01.qxd 11/30/04 8:36 AM Page 47

1.5.3 Interrelationship Among Grain Boundary,
Lattice Diffusion, and Energy
As mentioned in Sec. 1.5.2,
[45]
grain boundaries have lower density of
the interfacial material, which implies additional energy associated with
the microstructure and consequently an easier atomic diffusion. In the
past four decades, several investigators, notably Borisov et al.,
[83]
have
attempted to correlate the energy of the interfaces with diffusion kinetics.
The terms grain boundary and interface are used interchangeably
because this discussion will also include interfaces in several multiphase
alloys. The basic postulate in obtaining interface energy (g
i
) from self-
diffusion data is that it is the difference between the Gibbs free energies
(∆G
l
and ∆G
i
) for vacancy diffusion in the lattice and the interface, as
shown schematically in Fig. 1.20. The difference is positive because dif-
fusion in interfaces is many orders of magnitude faster than that in the
adjoining lattice. Hence the interface energy can be written as:
g
i
1
2
(∆G
l
∆G
i
) (66)
The factor of 1/2 enters because the interface energy is shared
between the two adjoining crystals. The Arrhenius nature of the coeffi-
cient, D, is given by Eqs. (27) through (29). The subscripts l and i denote
the lattice and grain boundary diffusion processes, respectively. The dif-
ference between the free energies, ∆G
l
and ∆G
i
, is then expressed as:
g
i
(Jmol)
1
2
(∆G
∆G
i
)
1
2
RT {n (D
i
D
)} (67)
or
g
i
(Jm
2
) r{(Q
Q
i
) RT n(D
o
i
D
o
)}, (68)
where D
o
l
and D
o
i
(m
2
/sec) are the pre-exponential terms for lattice and
interface diffusion, respectively; the conversion parameter r 12a
2
N
o
,
with a the mean distance between atoms at the interface, to the first
approximation, equal to one lattice parameter; and N
o
is Avogadro’s num-
ber. Q
l
and Q
i
, and R, the gas constant within the brackets, are to be taken
in J/mol.
To test that grain boundary energies computed from diffusion data are
indeed meaningful, diffusion parameters and the resulting interface ener-
gies obtained from Eq. (68) for several pure metals are compared in Table 1.4
with the direct measurements of interface energies using nondiffusion
48 DIFFUSION PROCESSES IN ADVANCED TECHNOLOGICAL MATERIALS
Ch_01.qxd 11/30/04 8:36 AM Page 48

DIFFUSION IN BULK SOLIDS AND THIN FILMS, GUPTA 49
techniques such as the zero creep and transmission electron microscopy
(TEM). Figure 1.21 shows a plot of the two kinds of interfacial energies.
Despite the semi-empirical nature of the basic postulate in Eqs. (66)
through (68), the two kinds of interfacial energies agree well, within a few
percent.
Because the diffusion processes, in general, are related to the absolute
melting temperature of the host,
[84]
the grain boundary energies should also
be thus related. Physically, such a dependence stems from the cohesive
energy of the solid that controls the atomic motions as well as the energy
of the grain boundary itself. Chapter 2 discusses this topic in detail. The
Figure 1.20 Schematic representation of grain boundary energy (g
i
) and free ener-
gies for diffusion in lattice and grain boundaries. Note the decrease in g
i
by ∆G
upon addition of a solute. (After Borisov et al.
[83]
)
Ch_01.qxd 11/30/04 8:36 AM Page 49
50 DIFFUSION PROCESSES IN ADVANCED TECHNOLOGICAL MATERIALS
Table 1.4. Diffusion in Lattice (Q
l
, D
o
l
), GB (Q
i
, dD
o
i
), and GB Energies (g
i
)
in Pure Metals
Lattice Grain Boundary
Host Q
l
D°
l
Q
i
DD°
i
Energy(g
i
) Reference
(Tracer) (kJ/mol) (10
4
m
2
/s) (kJ/mol) (10
16
m
3
/s) (mJ/m
2
)
Au(
195
Au) 169.6 0.04 84.8 3.1 396-0.016T a
Au* ... ... ... ... 400(1173K) b(*)
Ag(
110
Ag) 169.6 0.04 77.1 0.13 382(1173K) c
Ag(
110
Ag) 169.6 0.04 74.4 0.31 392(1173K) d
Ag* ... ... ... ... 400(1173K) e(*)
Cu(
110
Ag) 199.4 0.16 75.2 23 776-0.123T f
Cu(
64
Cu) 196.8 0.1 84.75 11.6 783-0.134T g
Cu(
67
Cu) 199.4 0.16 91.5 29 692-0.0054T h
Cu* ... ... ... ... 590(1123K) b
Ni(
63
Ni) 277.5 0.92 170.5 222 1126-0.214T i(*)
Ni* 277.5 0.92 ... ... 1373-0.4T j(*)
Pb(
203
Pb) 100 0.16 44.3 61 193-0.004T k
Pb(
119
Sn) 99.4 0.41 39.5 73 206-0.03T l
Pb* ... ... ... ... 200(588) m(*)
* Actual measurements
a. D. Gupta, Metall. Trans., 8A:1432 (1977)
b. J. E. Hilliard, M. Cohen, and B. L. Averbach, Acta Metall., 8:26 (1959)
c. D. Turnbull and R. E. Hoffman, Acta Metall., 2:419 (1954)
d. J. T. Robinson and N. L. Peterson, Acta Metall., 21:1181 (1973)
e. A. P. Greenough and R. King, J. Inst. Metall., 79:415 (1951
f. G. Barreau, G. Brunel, G. Cizeron, and P. Lacombe, Mem. Sci. Rev. Metall., 68:357 (1971)
g. T. Surholt and C. Herzig, Acta Mater., 45:3817 (1997)
h. D. Gupta, Defect Diffusion Forum, 156:43 (1998)
i. W. Lange, A. Hassner, and G. Mischer, Phys. Status Solidi, 5:63 (1964)
j. L. E. Murr, R. J. Horilev, and W. N. Lin, Philos. Mag., 22:52 (1970)
k. D. Gupta and K. K. Kim, J. Appl. Phys., 51:2066 (1980)
l. K. K. Kim, D. Gupta, and P. S. Ho, J. Appl. Phys., 53:3620 (1982)
m. K. T. Aust and B. Chalmers, Proc. Royal Soc., A204:359 (1950)
activation energies for diffusion in the lattice and grain boundaries, respec-
tively, are related to the absolute melting temperature (T
m
) of the host
[51]
as:
Q
l
142 T
m
Jmol (±20%) and Q
b
71 T
m
Jmole (±20). (69)
Consequently, Eq. (68) can be rewritten as:
g
b
2
7
a
1
2
T
N
m
o
(mJm
2
), (70)
Ch_01.qxd 11/30/04 8:36 AM Page 50

DIFFUSION IN BULK SOLIDS AND THIN FILMS, GUPTA 51
neglecting contributions of the log of pre-exponential terms in the dif-
fusion coefficients. As seen in Table 1.4, the temperature coefficient of
g
i
in pure metals is small and negative, implying a positive entropy
≈ 0.25 mJ/m
2
°C for grain boundaries. The value of g
b
≈ 0.24T
m
Figure 1.21 Comparison of diffusion-related grain boundary energies in pure met-
als with those measured directly.
Ch_01.qxd 11/30/04 8:36 AM Page 51
