Gupta D. (Ed.). Diffusion Processes in Advanced Technological Materials
Подождите немного. Документ загружается.


12 DIFFUSION PROCESSES IN ADVANCED TECHNOLOGICAL MATERIALS
where ∆H
f
and ∆H
m
are enthalpies for the formation and motion of the
defect, and S
f
and S
m
are the corresponding entropy terms, respectively.
The temperature dependence of the diffusion coefficient is generally of
the Arrhenius type and is written as:
D D
o
e
(QkT)
, (28)
with
D
o
1
6
a
2
Z f v
o
exp[(S
m
S
f
)k]. (29)
From the measurement of the diffusion coefficient, knowledge of the
crystal geometry (the coordination number and the lattice parameter), v
o
(related to Debye q
D
), and an independent measurement of the correla-
tion factor, f, it is possible to evaluate the total entropy factor. For the
separation of formation and motion components of the entropy and
enthalpy, separate experiments are needed, such as the resistance studies
of the quenched-in defects, the positron annihilation, and the simultane-
ous length and lattice parameters.
[10, 22]
Note that the diffusion coeffi-
cient and the various factors involved therein are basic properties of the
solids.
Table 1.1 lists self-diffusion data for several important pure elements.
While most metals diffuse by a vacancy mechanism, self-interstitial atoms
are important for diffusion in covalently bonded solids like Ge and Si, at
least at high temperatures.
[23]
When an interstitial atom is formed and a
vacancy is left behind, it is known as a Frenkel defect. Anotable example
for the formation of Frenkel defects is radiation damage in solids. The
energy involved in Frenkel defect formation equals the sum of the vacancy
and interstitial formation energies less the binding energy. The Frenkel
defect is usually very mobile and is accompanied by mass transport. The
interstitial atoms may also be of the impurity type, which fit into the inter-
stices in lattice easily by virtue of their small size. Consequently, their for-
mation energy is negligible, and in Eq. (27), the terms S
f
and ∆H
f
may be
omitted. Hence, diffusion of the foreign or impurity interstitials may be
very fast. Gas atoms such as H, C, O, and N commonly occur as intersti-
tials in BCC metals such as Fe, W, Nb, and Ta, and in HCPmetals such as
Ti, Zr, and Hf. In the Si lattice as well, most transition metal impurities
enter as interstitial atoms, although their solubilities rarely exceed one
part per million.
[14, 23, 24]
Ch_01.qxd 11/30/04 8:35 AM Page 12

13
Table 1.1. Self-Diffusion in the Lattice in Some Pure Elements
a
Activation Energy Frequency Factor Comments/
Element Q
l
(kJ/mol) D
l
o
(10
4
m
2
/sec) References
Aluminum 123 0.047
Antimony ‘ c 200.6 56.0
⊥ c 149.6 0.1
Beryllium ‘ c 164.7 0.62
⊥ c 157.2 0.52
Cadmium ‘ c 76.1 0.05
⊥ c 79.8 0.10
Chromium 435 970
Cobolt-Paramagnetic 283.0 0.83
Copper 199 0.16
Germanium 299 32
Gold 170 0.04
a-Hafnium 370 0.86
Indium ‘ c 78.2 2.7
⊥ c 78.2 3.7
a-Iron-Paramagnetic 284 0.49
g-Iron 284 0.49
283.7 0.18
d-Iron 238.3 1.9
Lead 109 1.37
Lithium 56.39 0.39
Magnesium ‘ c 134.6 1.0
⊥ c 135.8 1.5
Molybdenum 385.4 0.1
Nickel 278 0.92
Niobium 401.2 1.1
Palladium 265.9 0.21
Platinum 285.1 0.33
Potassium 40.8 0.31
Silicon 484 1460
Silver 170 0.04
Sodium 35 0.004
Tantalum 412.6 0.124
a-Thallium ‘ c 95.7 0.4
⊥ c 94.5 0.4
b-Thallium 83.4 0.7
b-Tin ‘ c 107.0 7.7
⊥ c 105.0 10.7
a-Thorium 320.2 1.2
a-Titanium 169 6.6 10
5
b-Titanium A 130.4; B 250.8 (1) 3.58 10
4
; (2) 1.09 2 exponentials fit
b
Tungsten 586 1.88
g-Uranium 110.8 1.12 10
3
Vanadium 307.9 0.036 880 to 1360°C
393.5 214.0 1360 to 1830°C
Zinc ‘ c 91.5 0.13
⊥ c 96.14 0.18
b-Zirconium A 116; B 273 (1) 8.5 10
5
; (2) 1.34 2 exponentials fit
b
a. Data are obtained from N. L. Peterson, Solid State Phys., 22:429–430 (1970), and from a more
recent compilation in Gupta and Ho.
[11]
b. D
l
D
l
01
e
AkT
D
l
02
e
BkT
.
Ch_01.qxd 11/30/04 8:35 AM Page 13

1.2.2.2 Effect of Substitutional Impurity Atoms
The presence of substitutional impurity atoms in single crystals mod-
ifies diffusion of the host atoms in addition to their own diffusion, largely
because of their interaction with the vacancies by virtue of their differing
electronic structure. The former is known as solvent diffusion enhancement.
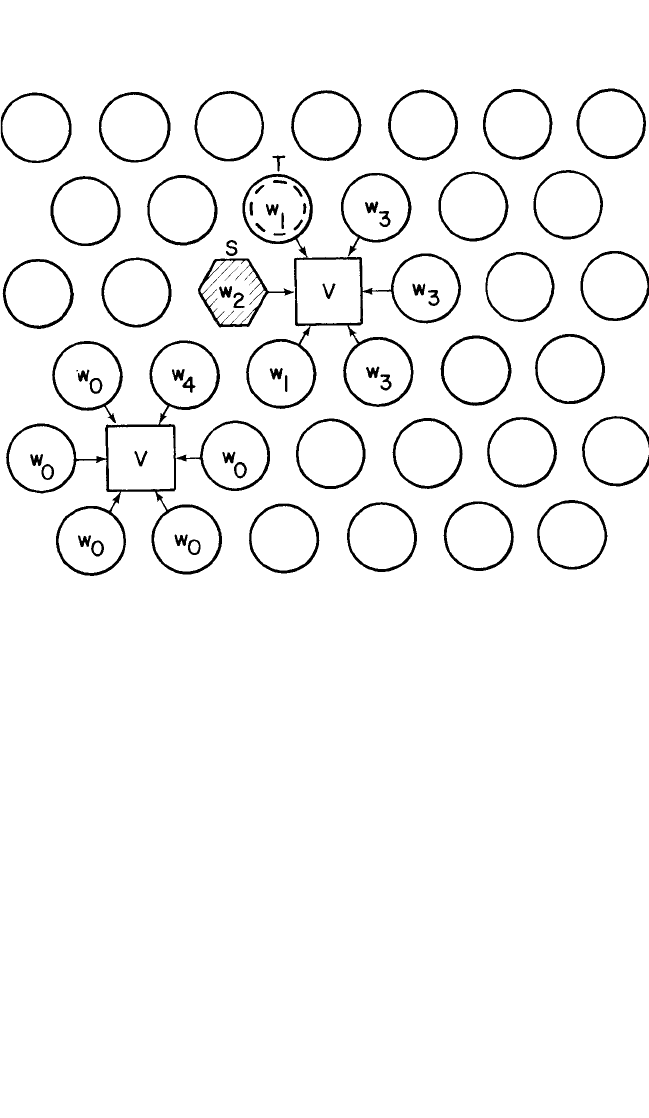
In Fig. 1.4, a five-frequency model for vacancy–atom jumps in FCC
crystal is shown in a dilute alloy after Manning
[20, 21]
and LeClaire.
[25]
The
solute atoms by themselves diffuse using vacancies as first-nearest neigh-
bors with the frequency w
2
, so that their diffusion coefficient D
2
can be
expressed as:
D
2
a
2
w
2
f
2
exp[(G
v
f
G
sv
)kT], (30)
where G
sv
is the solute-vacancy-association free energy. The correlation
factor f
2
is no longer constant and depends on all the jump frequencies in
a complex way. Furthermore, the activation energy for D
2
can also be per-
turbed to the extent of 25% of the value for the solvent self-diffusion.
The general expression for solvent enhancement of diffusion, [(D
1
(c)
D
1
(0))D
1
], usually measured by the solvent tracer, may be written as:
D
1
(c) D
1
(0){1 b
1
c b
2
c
2
.....}, (31)
where c is the concentration of the solute and the subscript 1 is used to
denote the solvent species. For a dilute alloy with 1% solute, the higher
order terms are negligible and the diffusion enhancement is generally lin-
ear. The strength of enhancement, measured by the term b
1
, is related to
the perturbation caused by the solute-vacancy binding to the first-nearest
neighbor jump frequencies in an FCC lattice. Figure 1.4 shows that w
o
is
the general atom frequency in the presence of an unbound vacancy. w
1
and
w
2
are jumps in which vacancy remains bound to the solute atom. In the w
3
jump, the vacancy dissociates from the solute atom. Finally, the w
4
jump
again brings the vacancy adjacent to the solute atom. Manning
[21]
and
LeClaire
[25]
provided the following kinetic relationship among these jumps:
b
1
18 4
w
w
4
o
w
w
1
3
7
2
, (32)
where b
1
(1/c)[(D
1
(c) D
1
(0))D
1
(0)] in the linear regime. The vacancy-
solute binding energy is defined by the temperature dependence of
w
4
w
3
e
E
VS
b
kT
. Furthermore, the frequencies w
1
and w
o
are also temperature-
14 DIFFUSION PROCESSES IN ADVANCED TECHNOLOGICAL MATERIALS
Ch_01.qxd 11/30/04 8:35 AM Page 14
DIFFUSION IN BULK SOLIDS AND THIN FILMS, GUPTA 15
dependent, so that w
1
w
o
e
BkT
. The solute-vacancy binding energy
E
b
VS
≈ 10 kJ/mol (0.1 eV); perhaps B also has the same magnitude. The
individual measurements E
VS
b
and B are usually very complex and involve
knowledge of correlation factors as well. In any event, while the enhance-
ment factor is determined by exponential terms, it may not always be pos-
itive; at certain temperatures and compositions, it may even be negative.
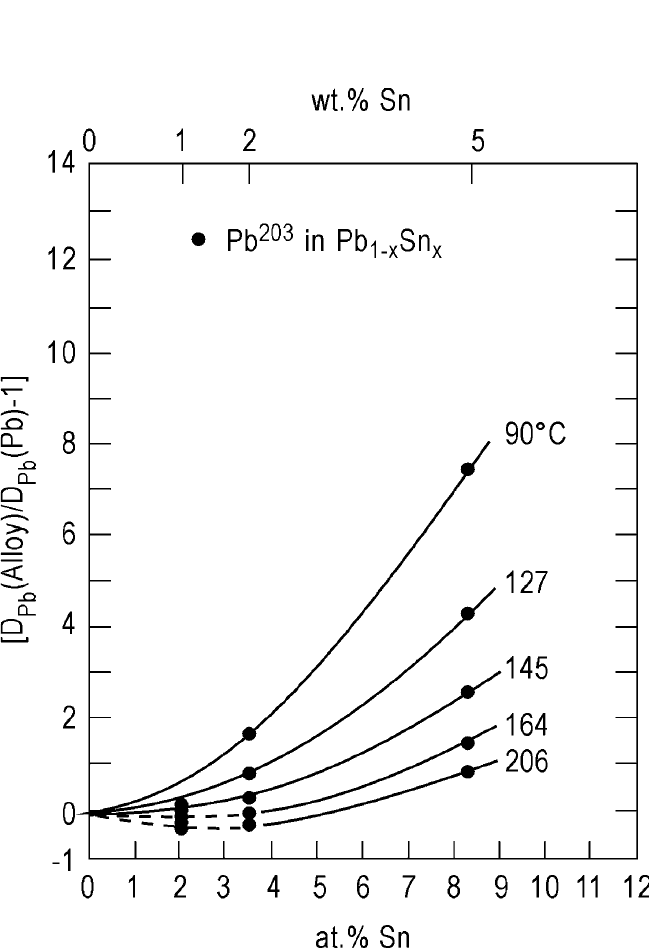
Figure 1.5 provides an example in the self-diffusion measurements
in Pb and Pb-Sn alloys.
[26]
Initially, addition of the Sn solute results in
de-enhancement; only at sufficiently higher concentrations and lower
temperatures is real enhancement of the solvent species observed.
1.2.3 Pressure and Mass Dependence of Diffusion
Pressure and mass dependence of diffusion are discussed briefly here.
They are addressed in detail in Chapter 2.
Figure 1.4 Five-frequency exchange model for host atoms in a dilute alloy: The
position of the solute atom is shown at S by a hexagon and of vacancy at V by a
square. (After Manning
[20]
and LeClaire
[25]
)
Ch_01.qxd 11/30/04 8:35 AM Page 15
16 DIFFUSION PROCESSES IN ADVANCED TECHNOLOGICAL MATERIALS
Figure 1.5 Relative enhancement of solvent (Pb) lattice diffusion in Pb-Sn alloys.
(Gupta and Oberschmidt
[26]
)
Ch_01.qxd 11/30/04 8:36 AM Page 16

DIFFUSION IN BULK SOLIDS AND THIN FILMS, GUPTA 17
1.2.3.1 Pressure Dependence
The diffusion coefficient may be expected to be pressure-dependent in
view of Maxwell’s thermodynamic relationship:
∂
∂
G
P
T
V, (33)
where volume V is to be associated with the diffusion process. From
Eqs. (27) and (28), the dependence of D on the hydrostatic pressure may
be written as:
∂
∂
ln
P
D
T
∆
kT
V
∂ ln
∂
a
P
2
g
G
f
T
, (34)
where g
G
is the Grüneisen compressibility coefficient. The activation
volume may be further separated into a sum of the formation and
motion terms, V
f
V
m
, for processes involving point defects in solids,
similar to Eq. (27). Furthermore, the activation volume, entropy, and
enthalpy are interrelated by the volume thermal expansion coefficient
a and the isothermal compressibility b given by Lawson
[27]
and
Keyes,
[28]
respectively, as:
S aVb (35)
and
V 4b Q. (36)
The values of ∆V are typically of the order of an atomic volume (V
o
),
even in closed packed lattices, which imply only a small relaxation around
a vacancy. Table 1.2 lists activation volumes for some pure metals and ele-
mental semiconductors. Rice and Nachtrieb
[29]
have shown that ∆V and Q
can also be empirically related to the heat of melting L
melt
and the volume
of melting V
melt
as:
Q L
melt
VV
melt
. (37)
Experimentally, measurable changes in the diffusivity (10%) occur
at hydrostatic pressures of the order of a few kilobars in metals and at
temperature 0.5T
melt
.
Ch_01.qxd 11/30/04 8:36 AM Page 17

18 DIFFUSION PROCESSES IN ADVANCED TECHNOLOGICAL MATERIALS
Table 1.2. Isotope Effect for Various Diffusion Mechanisms in Some
Elements
Mechanism/ Correlation Kinetic Activation
Host Factor Factor (K) Isotope Effect Volume
(Isotopes) 0 f 1 [Eq. (38)] (E)(VV
o
)
A. Vacancy
FCC Metals
Ag/Ag*(110,105): 0.782 0.92 0.718(1000K)
a
0.9
Lattice 0.639(1150K)
Ag/Ag*(110,105): ... ... 0.46
b
1.1
c
Grain boundary
Au/Au*(199,195) 0.782 0.9 0.704(1127K)
d
0.9
Lattice 0.653(1327K)
d
Cu/Cu*(67,64) 0.782 0.87 0.669(1200K)
a
Lattice
BCC Metals
Na 0.727 0.5
e
0.348(300–350K)
f
0.33
Diamond Lattice
Ge/Ge*(77,71) 0.5 0.86 0.26(1173K)
g
0.33
B. Interstitial 1.0 0.68 – –
C. Divacancy 0.475 1.45 – –
D. Interstitialcy 0.667 1.99 – –
E. Interchange 0.969 2.66 – –
a. N. L. Peterson, “Self diffusion in metals,” J. Nucl. Mater., 69–70:3–37 (1978)
b. J. T. Robinson and N. L. Peterson, “Correlation effects in grain boundary diffusion,” Surf. Sci.,
31:586 (1972)
c. G. Martin, D. A. Blackburn, and Y. Adda, “Autodiffusion au joint de grains de bicristaux
d’argen soumis a une press hydrstatique,” Phys. Status Solidi, 23:223 (1967)
d. C. Herzig, H. Eckseller, W. Bussmann, and D. Cardis, “The temperature dependence of the
isotope effect for self-diffusion and Co impurity-diffusion in Au,” J. Nucl. Mater., 69–70:61
(1978)
e. M. D. Feit, “Dynamical theory of diffusion, II. Comparison with rate theory and impurity iso-
tope effect,” Phys. Rev., B5:2145 (1972)
f. J. N. Mundy, “Effect of pressure on the isotope effect in Na self-diffusion,” Phys. Rev., B3:2431
(1971)
g. D. R. Campbell, “Isotope effect for self diffusion in Ge,” Phys. Rev., B12:2318 (1975)
As mentioned earlier, the total activation volume is actually com-
posed of formation and motion components for the mobile defect and is
analogous to Eq. (27). The individual components can be measured by
monitoring resistance with pressure on and off. This technique is similar
Ch_01.qxd 11/30/04 8:36 AM Page 18

DIFFUSION IN BULK SOLIDS AND THIN FILMS, GUPTA 19
to quenching experiments.
[22]
The measurements of the activation volumes
have been very useful in determining the nature of the diffusing defect
and, in fact, supplement the measurement of the isotope effect and the
underlying correlation factor, which are discussed in the following section.
Thin metallic films held on substrate usually have a bi-axial tensile stress
of the order 10
9
dynes/cm
2
(10
8
Pa), as discussed in Chapter 8, which is of
the magnitude mentioned above. Consequently, we may expect ∼10%
enhancement of the diffusivity, which is minuscule and insignificant.
1.2.3.2 Mass Dependence
In Eqs. (26) and (27), a correlation factor f was introduced to account
for the nonrandom character of the atom-vacancy jumps in tracer diffu-
sion measurements. Therein, a reverse jump has a greater than random
probability since the vacancy is still located adjacent to the tracer atom.
It measures the fraction of jumps that is effective in causing net dis-
placement of the tracer atom. For self-diffusion in cubic lattices, f is a
geometrical factor and depends on the crystal type and the mobile defect
responsible for diffusion. It is also generally temperature-independent.
The values of f for various diffusion mechanisms can be readily com-
puted from the direction of jumps. Table 1.2 lists the values of the corre-
lation factors for various diffusion mechanisms possible in FCC lattices.
Since correlation factors differ markedly for different mechanisms, their
measurement provides clues to the mobile defect responsible for diffu-
sion. The correlation factor is measured by diffusing two radioisotopes
simultaneously in a single crystalline sample. The resulting isotope effect
∆E is related to their diffusivities (D
a
and D
b
), masses (m
a
and m
b
), and
a kinetic factor ∆K as:
E [D
a
D
b
1]
m
m
a
b
12
1
f K. (38)
The kinetic factor ∆K is defined as the fraction of kinetic energy at the
saddle point associated with atomic motion in the jump direction. To
obtain reasonable precision in ∆E, a mass difference of at least 5% is
sought between the two isotopes. The isotopes are generally co-diffused
so that the errors in temperature, time, diffusion distance measurements,
and so forth are the same and do not figure in the relative diffusivities.
Under ideal conditions, the relative difference between the two diffusivi-
ties can be measured with a precision of ∼5%. This has been possible
because a surprisingly large number of isotope combinations are found to
Ch_01.qxd 11/30/04 8:36 AM Page 19

fulfill the seemingly stringent mass criterion. In addition, imaginative
schemes for nuclear counting have been used based on discrimination of
decay time and energy of radioactive emissions, which are discussed in
detail by Rothman.
[30]
The kinetic factor ∆K has the lower and upper bounds of zero and
unity, respectively, for point defects. For FCC and HCPlattices, the meas-
ured values of ∆K are on the order of 0.9 for metals such as Cu, Ag, and
Zn. For BCC lattices, smaller values of ∆K 0.68 are observed for met-
als like Na and Cr and are attributed to a relatively open saddle-point
structure rather than a change of diffusion mechanism. Finally, note that
the factor ∆K from the isotope effect and the activation volume measure-
ments [Eq. (34)] show numerically similar values for the same crystal lat-
tice and the diffusing defect. Thus the values of ∆K and ∆VV
o
are con-
sidered complementary in deciding the diffusion mechanism. For a
vacancy mechanism in close-packed metals, both ∆K and ∆VV
o
0.9. In
open lattices like BCC, the corresponding values are of the order of 0.68
and 0.5, respectively, for the vacancy mechanism. For the interstitial dif-
fusion mechanism, ∆K ∼ 1 and ∆VV
o
0 may be expected. For extended
defects, such as the divacancies, interstitialcy, and grain boundaries, as
listed in Table 1.2, ∆K and ∆VV
o
1 may be expected.
1.2.4 Linear Chemical Diffusion Regime: Finite
Driving Force F on Individual Atoms
In Fig. 1.6, the nonzero driving force F on individual atoms results in
a net flux in the right-hand direction. Consequently, the mass velocity
term v in Eq. (1) cannot be neglected. The driving force F makes the
20 DIFFUSION PROCESSES IN ADVANCED TECHNOLOGICAL MATERIALS
Figure 1.6 Schematic diagram for diffusion showing displacement of an atom in
the lattice when the driving force is finite, defined by the chemical potential gradi-
ent ∂m∂x. (After Manning
[21]
)
Ch_01.qxd 11/30/04 8:36 AM Page 20
DIFFUSION IN BULK SOLIDS AND THIN FILMS, GUPTA 21
energy at successive lattice sites differ by:
∆G
m
aF. (39)
Assuming that the energy barrier undergoes an equal change on either
side, the net number of jumps can be written as:
Γ
Γ
Γ(e
e
e
e
), (40)
where Γ has been defined in Eq. (25) and e is given by:
e
1
2
∆G
m
kT aF2kT. (41)
Combining Eqs. (40) and (41), the external force term may be written as:
2
sinh aFkT. (42)
Some atomic driving forces are listed in Table 1.3. These forces, with
the exception of perhaps centrifugal force, are commonly encountered in
Table 1.3. Driving Forces in Diffusion
Atomic Governing
Driving Force Force (F) Parameter Example
Electro-Migration ZeE Ze (effective Thin-film stripe failures
charge) under high currents
Thermo-Migration
Q
T
*
∂
∂
T
x
Q* (heat of Soret effect in ULSI
transport) solder failures
Chemical Inhomogeneity
[non-ideal chemical
potential gradient kT
∂
∂
x
ng
g (activity Kirkendall void formation
(∂m∂x)] coefficient)
Centrifugal Force mw
2
rm(effective Creep in aero-engines,
molecular mass) isotope enrichment
Stress Field
∂
∂
U
x
U (interaction Hydrostatic pressure,
energy) stresses in thin-film,
diffusion creep
Ch_01.qxd 11/30/04 8:36 AM Page 21
