Gottstein G., Shvindlerman L.S. Grain Boundary Migration in Metals: Thermodynamics, Kinetics, Applications
Подождите немного. Документ загружается.

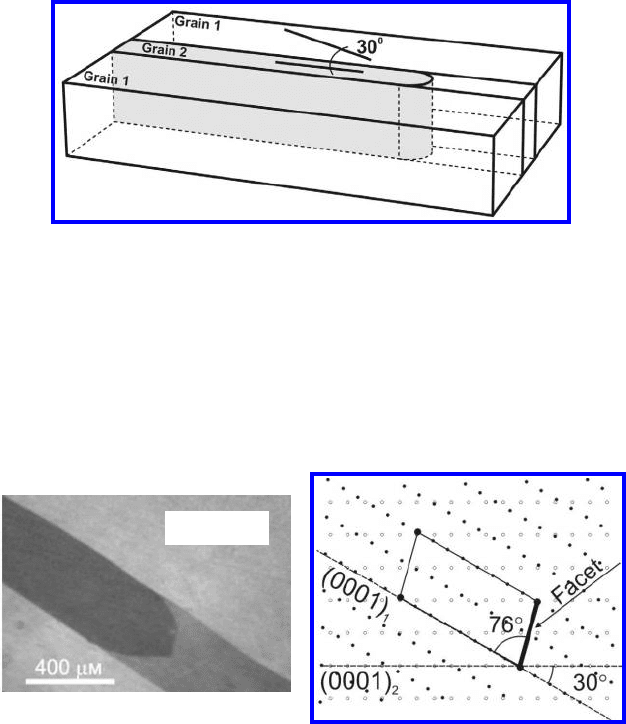
254 3 Grain Boundary Motion
FIGURE 3.80
Scheme of a Zn bicrystal containing [10
¯
10] tilt GBs with misorientation angles
of 30
◦
. The lines on the top faces of the crystals denote the orientation of basal
plane (0001) in each grain [320].
(a) (b)
T = 673 KT = 673 K
(a) (b)
T = 673 KT = 673 K
FIGURE 3.81
(a) Video frame of a moving grain boundary half-loop with facets [320]. (b)
Section of CCSL perpendicular to the [10
¯
10] tilt axis for GBs with misori-
entation angle θ of 30
◦
. Filled and empty circles mark the sites of the two
misoriented Zn lattices. Large circles mark CCSL sites. The reciprocal den-
sity of coincidence sites is Σ = 17. The unit cell of the respective CCSL, the
position of the basal plane (0001) for grain 2 and the CCSL plane parallel to
the facet on the moving GB are also shown [320].
© 2010 by Taylor and Francis Group, LLC
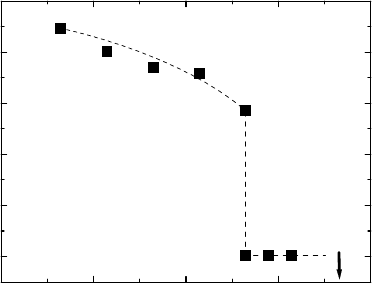
3.5 Experimental Results 255
620 640 660 680 700
0,0
0,1
0,2
0,3
0,4
0,5
T
m
Normalized facet length l
Temperature (K)
FIGURE 3.82
Observed temperature dependence of the facet length [320].
steady-state motion of the individual grain boundary — [10
¯
10] and [11
¯
20] tilt
grain boundary half-loop (Figs. 3.80, 3.81) — and in situ recording of grain
boundary motion. Above a certain temperature (673K for [10
¯
10] boundaries)
the migrating GB half-loop was continuously curved. Below this temperature
a facet appeared and coexisted with the curved GB. The length of the facet
increased with decreasing temperature (Fig. 3.82 [320]). The formation of a
grain boundary facet is usually due to a low grain boundary energy for the
specific facet inclination. From geometrical arguments, a low GB energy can
be associated with a high density of coincidence points of a coincidence site
lattice (CSL) [166, 316, 322]. The c/a ratio of the lattice spacing a in the basal
plane (0001) and c perpendicular to (0001) is irrational in Zn. Therefore, exact
coincidence site lattices exist in Zn only for GBs with [0001] rotation axis. In
all other cases, including [10
¯
10] tilt GBs, a so-called constrained coincidence
site lattice (CCSL) exists [323]. A section of the CCSLs perpendicular to the
[10
¯
10] tilt axis is shown in Fig. 3.81 for a GB with misorientation angle of 30
◦
.
Filled and open circles, respectively, mark the lattice points of the two mis-
oriented Zn lattices. Large gray circles mark the CCSL sites. Evidently, the
points of both lattices do not coincide exactly, and the difference may reach a
few percent of the lattice spacing. This situation is similar to near-coincidence
GBs of materials with cubic lattice, when the misorientation angle is close but
not equal to the misorientation of exact coincidence θ
Σ
, although still inside
the range for special GBs [320, 324]. The reciprocal density of coincidence
© 2010 by Taylor and Francis Group, LLC
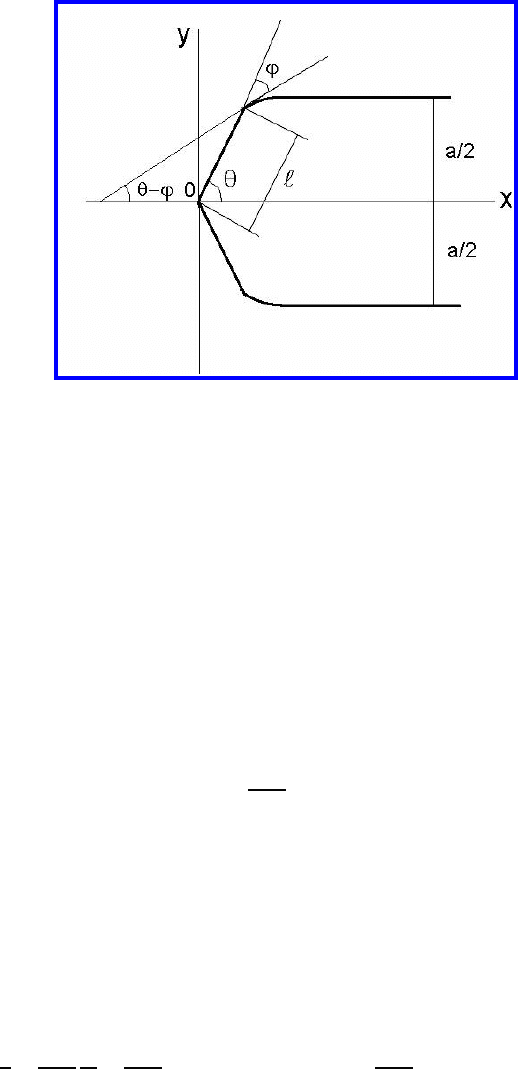
256 3 Grain Boundary Motion
FIGURE 3.83
Geometry of a grain boundary half-loop with a facet [320].
sites for the studied 30
◦
[10
¯
10] GB is Σ = 17, for the 85
◦
[11
¯
20] grain boundary
Σ = 11.
Let us consider the steady-state motion of a grain boundary half-loop with
a facet. A schematic sketch of the considered faceted boundary is given
in Fig. 3.83 [320]. It resembles closely the experimentally observed shape
(Figs. 3.80, 3.81).
The equation of motion and the boundary conditions which define the
steady-state shape and the velocity V of a moving half-loop are given in [320].
In case of a partially faceted boundary the respective equations of motion and
the boundary conditions (a − c) for the curved boundary read (Fig. 3.83)
y
= −
V
γm
b
y
1+y
2
(a) y
( cos θ)=tan(θ − ϕ)
(b) y(∞)=a/2 (3.161)
(c) y( cos θ)= sin θ
Since the facet is flat with inclination θ (Fig. 3.83) the shape of the curved
part of the moving half-loop with a facet is given by the equation
y =
a
2
−
m
b
γ
V
π
V
+
m
b
γ
V
arc cos
sin(θ − ϕ)exp
V
m
b
γ
( cos θ − x)
(3.162)
© 2010 by Taylor and Francis Group, LLC

3.5 Experimental Results 257
The velocity of the moving half-loop can be extracted from the boundary
condition (c) which renders
V =
m
b
γ(θ − ϕ)
a
2
− sin θ
(3.163)
Since for steady-state motion the faceted and curved segments of the half-loop
have to move with the same velocity, the migration rate of the grain boundary
half-loop with a facet is also controlled by the facet motion. Formally the facet
velocity can be expressed as
V = m
f
γ
f
κ (3.164)
where m
f
,γ
f
are the mobility and surface tension of the facet, γ
f
= γ cos ϕ
(see Fig. 3.83). The equivalent curvature of the facet κ can be found from the
so-called weighted mean curvature approach [73, 318]. The weighted mean
curvature is defined by the reduction of the total interfacial free energy in
the course of an infinitesimal facet displacement divided by the volume swept
by the displaced facet. Then the velocity of the facet can be expressed as
(Fig. 3.83))
V =
m
f
γ sin ϕ sin θ
(3.165)
From Eqs. (3.162) and (3.165) we obtain the length of a moving facet
=
a
2
sin θ +
m
b
(θ−ϕ)
m
f
sin ϕ sin θ
(3.166)
Eq. (3.166) allows us to derive the mobility of the facet and its temperature
dependence from experimentally measured values of the facet length. For ex-
ample, the normalized facet mobility (m
f
/m
b
) for the facet in a [10
¯
10] grain
boundary system as extracted from experimental data is presented in Fig. 3.84
[320].
Apparently, a reduction of the facet length with rising temperature is mainly
caused by a decrease in the ratio m
f
/m
b
. On the other hand, the absolute
value of the facet mobility can be extracted if the grain boundary mobility is
known from an independent experiment (Fig. 3.85) [320, 321]. As can be seen
the facet has a high mobility with a very low activation enthalpy (∼ 0.1eV).
An increase in the facet length with increasing facet mobility seems coun-
terintuitive, since it is a frequent observation that faceted boundaries move
with low mobility [310, 324]. The high mobility of the facet is associated with
a low activation energy and attributed to a high step mobility on the facet
[247, 320]. Such interpretation tacitly assumes that the production rate of
steps on the facet is sufficiently high and will not affect the facet velocity.
For instance, steps may be injected into the facet from the shrinking part of
the boundary. This may not be true, though, for smooth low energy facets
like coherent twin grain boundaries. In such case the migration rate may be
© 2010 by Taylor and Francis Group, LLC

258 3 Grain Boundary Motion
640 645 650 655 660 665 670 675 680
0
5
10
15
20
m
f
/m
b
Temperature, K
370 375 380 385 390 395 400 405 410 415
0
2
4
6
8
10
12
m
f
/m
b
Temperature,K
(K)
m
f
/
m
b
370 375 380 385 390 395 400 405 410 415
0
2
4
6
8
10
12
m
f
/m
b
Temperature,K
(K)
m
f
/
m
b
(a) (b)
640 645 650 655 660 665 670 675 680
0
5
10
15
20
m
f
/m
b
Temperature, K
370 375 380 385 390 395 400 405 410 415
0
2
4
6
8
10
12
m
f
/m
b
Temperature,K
(K)
m
f
/
m
b
370 375 380 385 390 395 400 405 410 415
0
2
4
6
8
10
12
m
f
/m
b
Temperature,K
(K)
m
f
/
m
b
(a) (b)
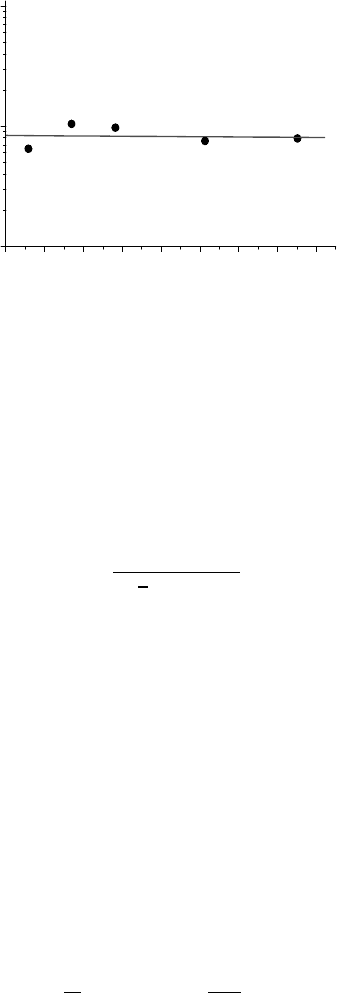
FIGURE 3.84
(a) Temperature dependence of the ratio of the normalized facet mobility
m
d
/m
b
for a [10
¯
10] tilt grain boundary [320]. (b) Temperature dependence of
the ratio of the facet mobility and boundary mobility m
f
/m
b
for a [11
¯
20] tilt
grain boundary [321].
determined by the production rate of steps on the facet rather than by step
migration and consequently, a steady-state motion with a facet of finite length
may not be possible according to Eq. (3.166). Yoon [325] proposed that be-
sides the faceting transition there may also be a roughening transition where
smooth facets become rough owing to entropy-driven defect generation (steps,
vacancies).
It is noted that the dynamic facet length given by Eq. (3.166) is equiva-
lent to a maximum rate of free energy decrease [321]. As shown in Sec. 3.4.3
the velocity V of a half-loop as a whole corresponds to an extremum of the
function Ψ(V ) (3.146). Only maxima of this function correspond to stable
steady-state motion. For a linear dependence of the velocity on the driving
force (M (V ) = const.) the extremum of the function Ψ(V )isequivalentto
the maximum rate of reduction of free energy of the system (dissipation rate),
in other words, the system tries to reduce the free energy as fast as possible,
i.e. with the maximum possible rate.
For the steady-state motion of a grain boundary half-loop with a facet the
average mobility can be defined in two ways, either by the facet motion or by
the motion of the curved boundary. In the first case — the half-loop motion
is controlled by the facet motion — the average mobility can be expressed as
[321]
M
f
(V )=
V
2γ/a
=
m
f
a sin ϕ sin θ
2
(3.167)
© 2010 by Taylor and Francis Group, LLC
3.5 Experimental Results 259
1,48 1,49 1,50 1,51 1,52 1,53 1,54 1,55 1,56
10
-8
10
-7
10
-6
γ
b
m
f
, m
2
/s
1000/T, K
-1
FIGURE 3.85
Temperature dependence of the facet mobility for a [10
¯
10] tilt grain boundary
[321].
When the half-loop migration rate is determined by the motion of the curved
grain boundary the average mobility is
M
b
(V )=
m
b
(θ − ϕ)a
2
a
2
− sin θ
(3.168)
A maximum rate of free energy reduction of the system is obtained for a
constant average mobility
M
f
(V )=M
b
(V ) (3.169)
Obviously condition (3.169) is satisfied by relation (3.166). In essence, the
facet changes its length in the course of motion in order to permit the system
to reduce its free energy with the highest rate, which in turn is related to the
Onsager principle of irreversible thermodynamics.
3.5.8 Effect of Pressure on Grain Boundary Migration:
Activation Volume
As derived in Sec. 3.5.2, the grain boundary mobility is given by Eqs. (3.157)
and (3.158)
m
b
=
v
P
= m
0
exp
−
H
m
kT
H
m
= E
m
+ pV
∗
© 2010 by Taylor and Francis Group, LLC

260 3 Grain Boundary Motion
If an experiment is carried out at constant pressure the pre-exponential factor
m
0
and the enthalpy of activation H
m
can be derived from the temperature de-
pendency of grain boundary mobility. However, the enthalpy of activation and
the pre-exponential factor are not independent of each other (compensation
effect, Sec. 3.7). Therefore, from mobility measurements at different temper-
atures only information on a single activation quantity can be obtained.
The measured activation enthalpies for grain boundary motion are difficult
to interpret. The problem of impurity influence on boundary mobility and,
in particular, on migration activation enthalpy was discussed above. It was
shown that despite a decrease in the activation enthalpy of boundary migra-
tion with rising impurity content, the activation enthalpy of migration for
most grain boundaries is significantly greater than the activation enthalpy of
grain boundary diffusion and, in many instances, can even exceed the activa-
tion enthalpy of bulk diffusion (Figs. 3.51, 3.55, 3.56). Even if the interaction
between the adsorbed atoms is taken into account, at least in a very pure
material, there is a drastic difference between the rate theory of single atom
hopping and experimental results.
A more direct measure of the migration mechanism is the activation vol-
ume. The activation volume reflects the difference between the volume of the
system in the activated and in the ground state. The activation volume V
∗
can be obtained from measurements of the pressure dependence of Gibbs free
energy of activation
G = H −TS = E + pV
∗
− TS (3.170)
∂ ln v
∂p
-
-
-
-
T
= −
V
∗
RT
(3.171)
∂ ln v
∂1/T
-
-
-
-
p
= −
H
R
(3.172)
For instance, the activation volume for bulk self-diffusion ought to be in the
order of one atomic volume. In this case the activated state consists of the va-
cancy production and the local lattice expansion caused by the diffusing atom
in the saddlepoint configuration. The relaxed volume of a vacancy roughly
corresponds to a little less than an atomic volume, and the lattice expansion
during the diffusive jump will be small compared to an atomic volume. The
activation volume for grain boundary diffusion is only a little less than that for
bulk diffusion (Table 3.7). This can be easily understood, because the density
of grain boundaries is not very different from the bulk density of the crystal
(even during melting the density of a metal drops only a few percent) (see
Chapter 1). With regard to grain boundary motion the value of the activation
volume is expected to provide information on essentials of the grain boundary
migration mechanism.
Despite the relative ease of interpretation of the activation volume com-
pared to the activation enthalpy, there have been only very few studies on
© 2010 by Taylor and Francis Group, LLC
3.5 Experimental Results 261
the pressure dependence of grain boundary migration [302]–[304]. The main
reason for this deficiency is the serious experimental problems that have to be
overcome in order to successfully conduct experiments on grain boundary mi-
gration at high hydrostatic pressure. Beginning with the pressurizing medium,
either a gas or a liquid should be stable up to high temperatures and must be
inert to the material of the sample to avoid contamination of the specimen.
Moreover, the high pressure device must be designed to provide a stable and
homogeneous temperature field. The displacement of a grain boundary during
annealing at high pressure is given by
= vt = v
0
t exp
−
E
m
RT
exp
−
pV
∗
RT
(3.173)
where v is the grain boundary velocity, t is the annealing time, v
0
is the pre-
exponential velocity factor. For a fluctuation of temperature δT and pressure
δp and an inaccuracy of time measurement δt, the relative error of the grain
boundary displacement reads [304]
Δ
=
E
m
RT
2
δT
+
V
∗
RT
δp
+
δt
t
(3.174)
Obviously, the uncertainty of grain boundary displacement will be essentially
determined by the stability of pressure and temperature in addition to the
magnitude of the activation energy and activation volume. For E
m
∼
=
1.3eV,
T
∼
=
10
3
K, V
∗
∼
=
10 cm
3
/mol, at a pressure of the order of 100 MPa, a minor
fluctuation of temperature by δT
∼
=
1 K is equivalent to a change of pressure
by 15 MPa. Therefore, a high stability of pressure and temperature is required
for an accurate measurement.
© 2010 by Taylor and Francis Group, LLC
262 3 Grain Boundary Motion
TABLE 3.7
Parameters of Bulk Diffusion
Metal Specimen H
bulk
D
D
0
[m
2
/s] V
∗
bulk
/Ω
a
[eV]
Ag self- polycrystals 2.0 8.9 · 10
−3
diffusion
[I]
Ag self- 001 tilt 1.97 7.2 · 10
−5
diffusion grain boundary
[II] ϕ =28
◦
Ag self- polycrystals 1.87 2.78 · 10
−5
diffusion
[III]
Ag self- single crystal 0.66 [IV ]
diffusion “pure” silver 0.88 [V ]
polycrystals 1.28 2.45 · 10
−5
1.1 [VII]
Al-Zn ϕ =32
◦
[VI][VI]
ϕ =37
◦
1.35 1.4 · 10
−4
111 tilt [X][X]
grain boundary
Al self- 1.23 − 1.35
diffusion [XII]
Al-Cu single crystal 1.16
Al-Ag “pure” Al single 1.19
Al-Au crystal of Al 1.18
[V ]
Au self- polycrystals 1.8 9.1 · 10
−6
0.67 − 0.81
diffusion [XIII][XII]
Cd self- polycrystals Q
||
=0.79 = 1.3·⊥C
diffusion 99.9995 at% Q
⊥
=0.82
`
D
||
+2D
⊥
´
0.53 − 0.59
[XI]
D
⊥
0
=5· 10
−6
||C
D
0
=1· 10
−5
0.53 − 0.59
[XV ][XV II]
Pd self- Polycrystals 1.1 1.17 · 10
−4
single crystal
diffusion 99.95% [XV III][XV III]0.71 − 0.84 [XIX]
0.57 − 0.715 [XX]
Pd self- 001 tilt 1.07 6.26 · 10
−5
diffusion and twist grain [XXIV ][XXIV ]
boundary
99.999 at%
Sn self- polycrystals 0.99 7.8 · 10
−5
single crystal
diffusion 99.99% [XXI] ⊥ C
0.304 − 0.362
||C
0.321 − 0.329
© 2010 by Taylor and Francis Group, LLC
3.5 Experimental Results 263
TABLE 3.8
Parameters of Grain Boundary Diffusion
Metal H
b
D
[eV] (δD
b
)
0
[m
3
/s] V
∗
/Ω
a
Ag self- 0.92 5.76 · 10
−15
diffusion
[I]
Ag self- 1.05 2 · 10
−16
diffusion
[II]
Ag self- 0.67 1.16 · 10
−16
1.09
diffusion
[III]
Ag self-
diffusion
Al-Zn 0.54 [V III]3.1 · 10
−15
[V III]0.8 − 1.08 [IX]
0.52 ϕ =32
◦
0.87 ϕ =37
◦
111 tilt grain boundary [XI]
Au self- 0.88 [XIV ]3.1 · 10
−16
[XIV ]
diffusion
Cd self- 0.48 [XV I]3.35 · 10
−14
diffusion
Pd self- 0.68 [XV III]8.17 · 10
−14
diffusion
Pd self- Twist grain boundary [XXV ]
diffusion 0.35
tilt grain boundary
ϕ =30
◦
0.2
Sn self- 0.43 [XXIII]3.22 · 10
−15
diffusion
I — Hoffmann RE, Turnbull D. J. Appl. P hys., 22:634, 1951.
II — Turnbull D, Hoffmann RE. Acta Metall., 2:419, 1954.
III — Kaigorodov VN, Klotsman SM, Timofeev AN, Trakhtenberg IS.
P hys. Met. Metall., 25:910, 1968.
IV —Rein G, Mehrer H. Phil. Mag., A45:767, 1982.
V—BeyelerM,These Universit´eParis, 1968.
VI — Beke DL, Godeny I. Phil. Mag., A47:281, 1983.
VII—ErdelyiG,LojkowskiW,BekeDK,G¨odeny I, Kedves JL. in
Diffusion in Metals and Alloys, Kedves FJ, Beke DL,
editors. Aldermannsdorf, p. 398, 1983.
VIII — G¨odeny I, Beke DK, Kedves FJ. T rans. Japan. Inst. Metals
(suppl.), 27:525, 1986.
IX — Erdelyi G, Lojkowski W, Beke DK, G¨odeny I, Kedves JL. Phil.
Mag.,A56 : 673, 1987.
X—OvsienkoDY,ZasimcukIK.Phys.Met.Metall., 10:743, 1960.
© 2010 by Taylor and Francis Group, LLC
