Gottstein G., Shvindlerman L.S. Grain Boundary Migration in Metals: Thermodynamics, Kinetics, Applications
Подождите немного. Документ загружается.

224 3 Grain Boundary Motion
0
H
m
[eV]
0.2
0.4
0.6
0.8
ϕ [deg]
352515 45
H
m
[kJ/mol]
80
60
40
20
0
0
H
m
[eV]
0.2
0.4
0.6
0.8
ϕ [deg]
352515 45
H
m
[kJ/mol]
80
60
40
20
0
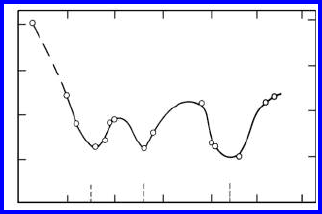
FIGURE 3.51
Measured activation enthalpies H
m
vs. misorientation angle ϕ for 100 tilt
boundaries in zone refined lead [182].
and its kinetic parameters (enthalpy of activation and pre-exponential factor)
was investigated for 100, 111 and 110 tilt grain boundaries with a total
amount of impurities of (2 − 5) · 10
−4
at% by the grain boundary half-loop
technique (N9, Table 3.3) (Fig. 3.52) [273]–[275].
The misorientation dependency of the activation enthalpy of migration for
10
¯
10 and 11
¯
20 tilt boundaries in zinc with a total impurity content of
5·10
−4
at% and 5 ·10
−3
at%, respectively, are shown in Fig. 3.53 [70, 239, 276].
The data were obtained by the reversed-capillary technique (technique N6,
Table 3.3). Qualitatively, the behavior of the migration activation enthalpy in
Zn is akin to fcc metals. Evidently, the dependency H
m
(ϕ) reflects structural
peculiarities of the grain boundaries. However, the situation is complicated
by the fact that exact CSLs for hexagonal close-packed structures exist only
for rotations around 0001 and for hypothetical structures with a rational
ratio c/a. This problem can be alleviated by including also superlattices close
to coincidence. A comparison of the misorientation dependencies of mobil-
ity (Fig. 3.53) with the computed misorientation dependency of Σ revealed
for hcp metals that there is a correlation between the extrema of the curves
A
b
(ϕ), H
m
(ϕ), A
0
(ϕ)(ϕ — angle of rotation) and the misorientation of low
Σ grain boundaries. We will refer to low Σ coincidence boundaries also as
special grain boundaries in the following.
In essence, all misorientation dependencies of grain boundary mobility and
its parameters considered are non-monotonous, and the corresponding ex-
trema correspond to low Σ misorientations of the CSL lattice. The magnitude
of the oscillations is large: for the grain boundary mobility about 2 orders of
magnitude, for the activation enthalpy ∼60-120kJ/mol. Therefore, one has to
address the question of what is the origin of this misorientation dependence. In
other words, is there a fundamental correlation between the structural prop-
erties of a grain boundary, e.g. the value of Σ, and grain boundary mobility?
© 2010 by Taylor and Francis Group, LLC
3.5 Experimental Results 225
13 14 15 16
-6
-5
-4
-3
335375415455495
10 20 30 40 50 60
-19
-17
-15
-13
-11
-9
-7
-5
10 20 30 40 50 60
-19
-17
-15
-13
-11
-9
-7
-5
10 20 30 40 50 60
-19
-17
-15
-13
-11
-9
-7
-5
0 10 20 30 40 50 60
0
1
2
3
4
0
100
200
300
1000/T [1/K]
1.3 1.4 1.5 1.6
10
-7
10
-8
10
-9
10
-10
1
2
3
4
5
ϕ [deg]
10
-5
10
-9
10
-13
10
-17
10 20 30 40 20 30 40 20 30 4050 5060 60
<100> <111> <110>
500°C
400°C
300°C
ϕ [deg]
0
1
2
3
4
010 6050403020
0 10 20 30 40 50 60
0
4
8
12
16
20
A
0
[m
2
/s]
10
12
10
4
10
-4
<100>
<111>
<110>
ϕ [deg]
010
6050403020
H
m
[kJ/mol]
300
200
100
0
335370405450495
T [°C]
A
b
[m
2
/s]
A
b
[m
2
/s]
H
m
[eV]
(a)
(b)
(c)
(d)
<100>
<111>
<110>
13 14 15 16
-6
-5
-4
-3
335375415455495
10 20 30 40 50 60
-19
-17
-15
-13
-11
-9
-7
-5
10 20 30 40 50 60
-19
-17
-15
-13
-11
-9
-7
-5
10 20 30 40 50 60
-19
-17
-15
-13
-11
-9
-7
-5
0 10 20 30 40 50 60
0
1
2
3
4
0
100
200
300
1000/T [1/K]
1.3 1.4 1.5 1.6
10
-7
10
-8
10
-9
10
-10
1
2
3
4
1
2
3
4
5
ϕ [deg]
10
-5
10
-9
10
-13
10
-17
10 20 30 40 20 30 40 20 30 4050 5060 60
<100> <111> <110>
500°C
400°C
300°C
ϕ [deg]
0
1
2
3
4
010 6050403020
0 10 20 30 40 50 60
0
4
8
12
16
20
A
0
[m
2
/s]
10
12
10
4
10
-4
<100>
<111>
<110>
ϕ [deg]
010
6050403020
H
m
[kJ/mol]
300
200
100
0
335370405450495
T [°C]
A
b
[m
2
/s]
A
b
[m
2
/s]
H
m
[eV]
(a)
(b)
(c)
A
b
[m
2
/s]
A
b
[m
2
/s]
H
m
[eV]
(a)
(b)
(c)
(d)
<100>
<111>
<110>
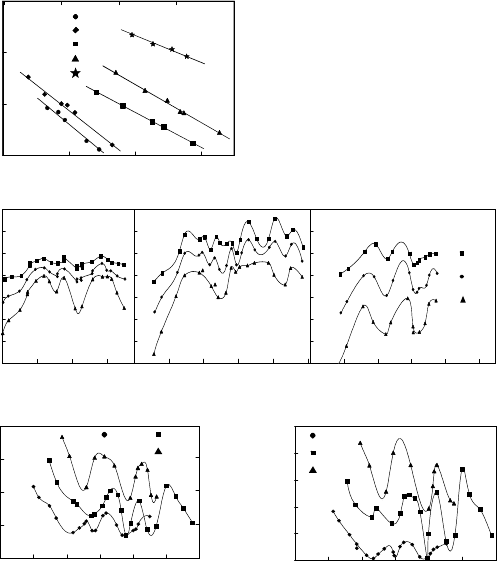
FIGURE 3.52
Temperature and misorientation dependencies of reduced grain boundary mo-
bility for 100, 111 and 110 tilt grain boundaries in aluminum. (a) Tem-
perature dependence, 111 tilt grain boundaries with various angles ϕ of
misorientation 1 − ϕ =33
◦
;2− 35.5
◦
;3− 28
◦
;4− 36.5
◦
;5− 45
◦
.(b)Mis-
orientation dependence at 500
◦
, 400
◦
and 300
◦
C, respectively. (c) Enthalpy
of activation vs. misorientation angle ϕ for motion of 100, 111 and 110
tilt boundaries in Al. (d) Pre-exponential factor of reduced grain boundary
mobility for tilt boundaries in Al.
© 2010 by Taylor and Francis Group, LLC
226 3 Grain Boundary Motion
H
m
[eV]
H
m
[eV]
H
m
[kJ/mol]
H
m
[kJ/mol]
1.5
0.5
1.0
10 30 50 70 90
ϕ
[deg]
(a)
100
50
150
1.50
1.0
0.5
50
100
150
15 30 45 75 9060
(b)
ϕ
[deg]
H
m
[eV]H
m
[eV]
H
m
[eV]H
m
[eV]
H
m
[kJ/mol]H
m
[kJ/mol]
H
m
[kJ/mol]H
m
[kJ/mol]
1.5
0.5
1.0
10 30 50 70 90
ϕ
[deg]
(a)
100
50
150
1.5
0.5
1.0
10 30 50 70 90
ϕ
[deg]
(a)
100
50
150
1.50
1.0
0.5
50
100
150
15 30 45 75 9060
(b)
ϕ
[deg]
1.50
1.0
0.5
50
100
150
15 30 45 75 9060
(b)
ϕ
[deg]
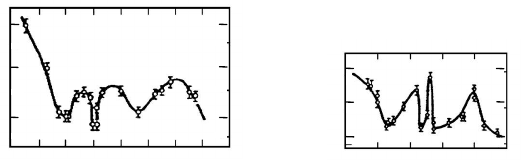
FIGURE 3.53
Misorientation dependence of activation enthalpy of grain boundary motion
for 10
¯
10 (a) and 11
¯
20 (b) tilt boundaries in Zn.
One is tempted to attribute the observed orientation dependence to the mis-
orientation dependence of grain boundary structure (Chapter 2), specifically
to changes in the apparently structure-dependent transfer of atoms across the
boundary. However, while the observed misorientation dependence certainly
reflects the variance of grain boundary structure with changing misorientation,
it is not an effect of migration mechanism but rather an effect of misorienta-
tion dependence of grain boundary segregation. If grain boundaries segregate
less, they are less hindered by impurity drag and thus more mobile. Special
boundaries are considered to be more perfectly structured and, therefore, less
liable to segregation. This would explain the observation that special bound-
aries are more mobile than non-special boundaries.
The classical work in this field is Aust and Rutter, who studied the in-
fluence of tin additions on the velocity of grain boundary migration and its
activation energy in lead [180]–[182]. They observed that the migration rates
of boundaries with misorientation close to a coincidence relationship — i.e.
special grain boundaries — and others — the random boundaries — were
substantially different: lead dopped with tin the special boundaries moved
faster than the random ones, and the migration rate of random boundaries
depended much more strongly on tin content than the migration rate of special
boundaries (Fig. 3.54). The situation for the energy of activation was similar:
for special grain boundaries the activation energy of migration was practically
unaffected by the tin content, whereas for random grain boundaries the acti-
vation energy rose dramatically with increasing tin concentration (Fig. 3.55).
The structure dependence of segregation is most convincingly demonstrated
by mobility measurements on Al and Zn of different purity [239, 255, 273, 276,
278, 279]. Fig. 3.56 shows the dependence of the activation enthalpy for the
mobility of 100 tilt boundaries vs. misorientation in Al with different con-
tents of impurities [274]. For low-angle boundaries the activation enthalpy
is seen to decrease with increasing misorientation. For high-angle boundaries
(ϕ ≥ 20
◦
) the activation energy depends strongly on impurity level. For both
© 2010 by Taylor and Francis Group, LLC
3.5 Experimental Results 227
264
“special”
grain boundaries
“random”
grain boundaries
10.0
1.0
0.1
0.01
0.001
0.0001
0
v
[
m
m
/
m
i
n
]
c [10
-3
wt.%]
264
“special”
grain boundaries
“random”
grain boundaries
10.0
1.0
0.1
0.01
0.001
0.0001
0
v
[
m
m
/
m
i
n
]
c [10
-3
wt.%]
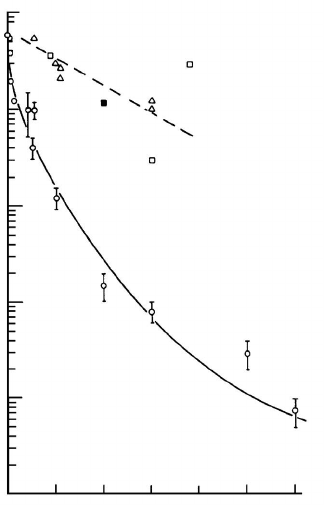
FIGURE 3.54
Migration rate vs. Sn concentration in Pb bicrystals according to Aust and
Rutter [180, 277]. Special and random boundaries behave differently.
ultrapure (99.99995%) and low purity (99.98%) materials the activation en-
ergy does not depend on the misorientation angle, but the absolute value of
the activation energy is higher for the impure material by a factor of three. For
a less-than-ultrapure material (99.9992%), referred to as high purity material,
the activation energy oscillates and attains minima for special misorientations,
which correspond to low Σ CSL rotations. For these special misorientations
the activation energy is practically identical for high purity and ultrapure
material. Thus, experimental results of single grain boundaries confirm the
finding that in high purity materials special boundaries have a smaller migra-
tion activation enthalpy [180]–[182] and as a consequence, in some cases (to
be considered below) a higher mobility than random boundaries.
From the bicrystal experiments on differently pure materials, it can be con-
cluded that the orientation dependence of grain boundary mobility is due to
segregation effects [279]. In fact, high-angle tilt grain boundaries in ultrapure
metals do not show any misorientation dependence of boundary mobility at
© 2010 by Taylor and Francis Group, LLC
228 3 Grain Boundary Motion
0
0.5
H
m
[eV]
1.0
1.5
2.0
2.5
1
510152025
c
Sn
[10
-4
wt.%]
“random” boundaries
“special” boundaries
50
100
150
200
H
m
[kJ/mol]
0
0.5
H
m
[eV]
1.0
1.5
2.0
2.5
1
510152025
c
Sn
[10
-4
wt.%]
“random” boundaries
“special” boundaries
50
100
150
200
H
m
[kJ/mol]
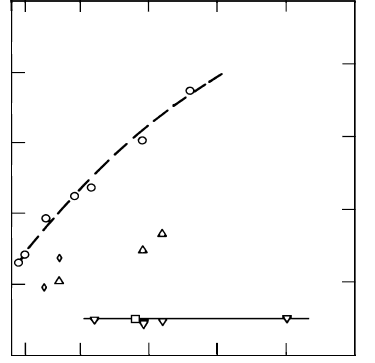
FIGURE 3.55
Activation enthalpy of migration for experiments shown in Fig. 3.53.
all. Correspondingly, the orientation dependence of grain boundary mobility
for high purity material merely reflects the orientation dependence of segre-
gation such that special grain boundaries are less liable to segregation than
random grain boundaries and, therefore, less affected by impurity drag. It
should be remembered that the segregation capability is an intrinsic grain
boundary property which is strongly connected with the boundary structure.
In this regard the orientation dependence of grain boundary mobility and its
parameters reflect the structural properties of a grain boundary. But the influ-
ence of impurity drag, which in turn is determined by boundary segregation, is
much greater than the intrinsic orientation dependence of boundary mobility.
Therefore, we can state with reasonable confidence that the misorientation
dependence of boundary mobility on concentration is mainly determined by
boundary segregation.
This effect and, therefore, the distinction between the mobility of special
and random boundaries is, however, limited to a relatively small range of im-
purity content. At a purity level of 99.98%, all tilt grain boundaries in Al
were found saturated with impurities, irrespective whether special or random
boundaries. The ability of special boundaries to resist segregation appears to
be very sensitive to the atomic arrangement even for low Σ boundaries. For
the same impurity level special 10
¯
10 tilt boundaries in zinc demonstrate
selective segregation while 11
¯
20 tilt boundaries remain unaffected by segre-
gation (Figs. 3.57a,b).
© 2010 by Taylor and Francis Group, LLC
3.5 Experimental Results 229
10 20 30 40 50
H
m
[
e
V
]
ϕ [deg]
0
0.5
1.0
1.5
2.0
2.5
50
200
150
100
H
m
[
k
J
/
mo
l
]
10 20 30 40 50
H
m
[
e
V
]
ϕ [deg]
0
0.5
1.0
1.5
2.0
2.5
0
0.5
1.0
1.5
2.0
2.5
50
200
150
100
H
m
[
k
J
/
mo
l
]
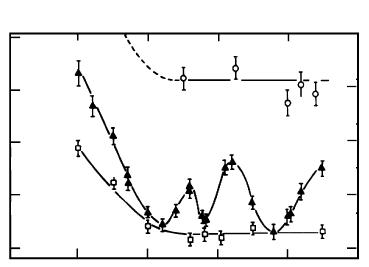
FIGURE 3.56
The dependence of the activation enthalpy of migration for 100 tilt grain
boundaries in Al of different purity: — 99.99995at%; — 99.9992at%;
◦ — 99.98at%.
Thus, we can conclude that the orientation dependence of grain bound-
ary mobility is a consequence of segregation and can only be observed in a
relatively small window of impurity content. The extent of this regime also
depends on misorientation. Outside of this concentration range the mobility
of high-angle grain boundaries apparently does not depend on misorientation.
This conclusion has serious consequences for our understanding of the bound-
ary mobility and the mechanism of impurity influence on it. The results on
the purest material seem to indicate that grain boundary mobility does not
depend on misorientation. However, owing to the lack of extremely pure ma-
terials and with our current level of information on the behavior of ultrapure
metals, it cannot be ruled out that the apparent invariance of mobility with
misorientation represents only an intermediate case and with increasing pu-
rity of the material the mobility again becomes dependent on orientation.
It is conceivable that in the absence of impurities, random grain boundaries
move more easily than special boundaries, since the lower energy and well-
ordered structure of special boundaries would provide an enhanced resistance
to a temporary modification of the structure, as necessary for grain boundary
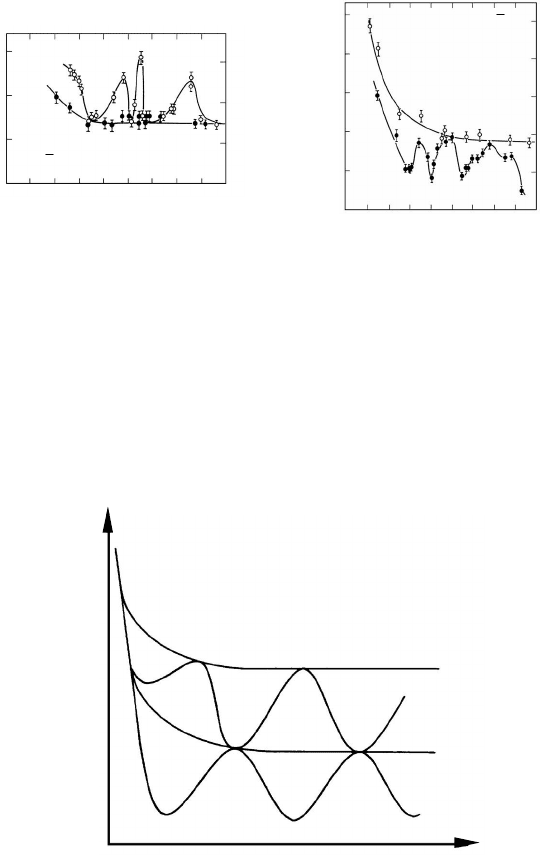
motion. Correspondingly, four regimes of purity level may be distinguished,
which reflect a different orientation dependence of grain boundary mobility
(Fig. 3.58) [279]. Three branches (1–3) in Fig. 3.58 have been experimentally
confirmed, while the fourth still remains at the level of speculation.
© 2010 by Taylor and Francis Group, LLC
230 3 Grain Boundary Motion
<>
101 0
20 40 60 80
ϕ [deg]
2.5
2.0
1.5
0.5
1.0
0
H
m
[eV]
(b)
0
H
m
[kJ/mol]
20 40 60 80
ϕ [deg]
<>
1120
1.5
1.0
0.5
0
H
m
[eV]
(a)
0
H
m
[kJ/mol]
50
150
100
0
50
150
100
200
250
0
<>
101 0
20 40 60 80
ϕ [deg]
2.5
2.0
1.5
0.5
1.0
0
H
m
[eV]
(b)
0
2.5
2.0
1.5
0.5
1.0
0
H
m
[eV]
(b)
0
H
m
[kJ/mol]
20 40 60 80
ϕ [deg]
<>
1120
1.5
1.0
0.5
0
H
m
[eV]
(a)
1.5
1.0
0.5
0
H
m
[eV]
(a)
0
H
m
[kJ/mol]
50
150
100
0
50
150
100
200
250
0
FIGURE 3.57
The dependence of the activation enthalpy of migration on angle of rotation
for (a) 11
¯
20 and (b) 10
¯
10 tilt grain boundaries in Zn of different impurity:
◦ — 99.995at%; • — 99.9995at%.
1
4
2
3
ϕ
H
m
1
4
2
3
ϕ
H
m
FIGURE 3.58
Schematic dependence of activation enthalpy of grain boundary mobility for
differently pure materials. 1 — low purity; 2 — high purity; 3 — ultra high
purity; 4 — completely pure material.
© 2010 by Taylor and Francis Group, LLC
3.5 Experimental Results 231
3.5.4 Correlation of Grain Boundary Migration and
Diffusion
While we pointed out in Sec. 3.1 that grain boundary migration is not to be
confused with diffusion across the boundary, it is interesting to compare the
misorientation dependence of grain boundary diffusion with the misorientation
dependence of grain boundary mobility. For this purpose the data for grain
boundary diffusion of zinc in 100 and 111 tilt boundaries in aluminum can
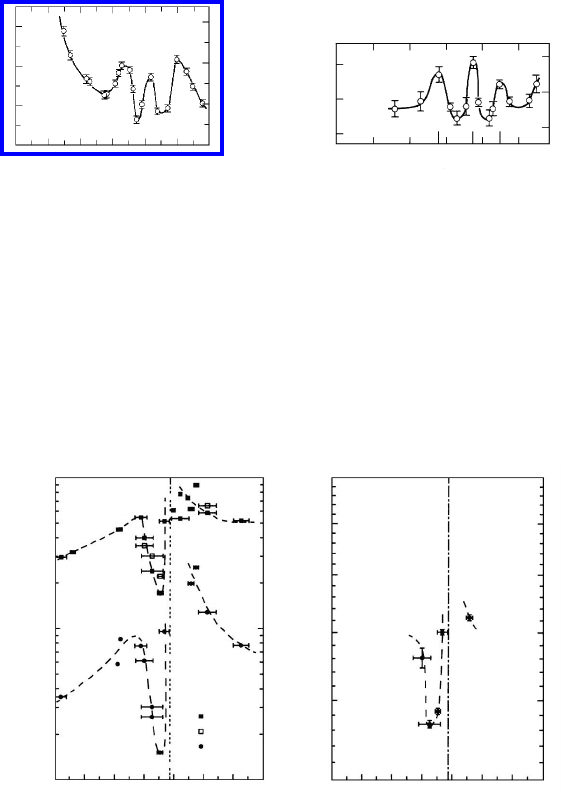
be utilized [280]–[282]. Fig. 3.59 shows the misorientation dependence of the
migration activation enthalpy for 111 tilt boundaries in Al and the activation
enthalpy for zinc diffusion along 111 tilt grain boundaries in aluminum. The
migration activation enthalpy of both migration (H
m
) and boundary diffusion
(H
DG
) depend upon the misorientation in a non-monotonous manner; maxima
on the diffusion activation enthalpy curve strictly correspond to minima on
the migration activation enthalpy curve, and vice versa. In both cases extrema
are observed at angles which correspond to special (CSL) boundaries. Appar-
ently, the misorientation dependence of boundary diffusion is complementary
to the misorientation dependence of boundary migration. Of course, it has to
be kept in mind that the measured diffusion data [280] reflect the diffusion
along grain boundaries, while migration is at most akin to diffusion across the
boundary. Also, boundary migration is more related to self-diffusion, whereas
impurity (zinc) diffusion was measured experimentally [280, 282]. Unfortu-
nately, there are no methods to measure the diffusion across grain boundaries.
In a high-accuracy study [283] the grain boundary diffusion of
195
Au and grain
boundary self-diffusion of
64
Cu along symmetrical 001 tilt boundaries with
misorientation close to Σ5 (36.9
◦
) were measured under identical experimental
conditions as a function of temperature and tilt angle (Fig. 3.60). The param-
eter π = SδD
b
(segregation factor S = 1 for grain boundary self-diffusion,
2 ≤ S ≤ 6 for Au in Cu; grain boundary width δ=0.5nm; D
b
grain boundary
diffusion coefficient) manifests the same characteristic orientation dependence
for both grain boundary self-diffusion and grain boundary impurity diffusion
of Au in Cu. A qualitatively similar orientation dependence was observed in
both cases. Furthermore, this orientation dependence was independent of the
purity of the Cu material used (Fig. 3.60a). High purity Cu bicrystals of two
different original Cu materials (Cu1 and Cu2) were grown. The total impurity
amount in both types of Cu was small and comparable, although different.
Clearly segregation effects cannot account for the observed variations of the
parameter π.
The activation enthalpy and pre-exponential factor of grain boundary mi-
gration do not correspond to the relevant parameters for diffusion, however.
As evident from Figs. 3.52, 3.59, and 3.60, the activation enthalpy of grain
boundary migration for the majority of grain boundaries investigated is much
larger than that of grain boundary diffusion, frequently even larger than that
of bulk diffusion. The calculated values of the pre-exponential factor of grain
boundary mobility, based on the grain boundary diffusion data, are presented
© 2010 by Taylor and Francis Group, LLC
232 3 Grain Boundary Motion
0.4
0.6
0.8
01020304050
ϕ [deg]
H
Db
[eV]
13
7
19
(b)
0
1
2
3
010203040 6050
ϕ [deg]
H
m
[eV]
(a)
100
200
300
H
m
[kJ/mol]
H
Db
[kJ/mol]
40
60
80
0.4
0.6
0.8
01020304050
ϕ [deg]
H
Db
[eV]
13
7
19
(b)
0
1
2
3
010203040 6050
ϕ [deg]
H
m
[eV]
(a)
100
200
300
H
m
[kJ/mol]
H
Db
[kJ/mol]
40
60
80
FIGURE 3.59
(a) Migration activation enthalpy of 111 tilt grain boundaries in Al. (b)
Activation enthalpy of Zn diffusion along 111 tilt grain boundaries in Al.
10
-19
10
-20
10
-21
Σ
= 5
Σ
= 5
3534
Cu1
33 33 34 3536 36 3737 3838 3939 4040
Cu1
Cu2
6
7
8
9
10
8
7
5
5
6
10
9
T=661K, t=65.66d
64
Cu in Cu1
T=919K, t=9.6h
π
[m
3
/s]
π
[10
-20
m
3
/s]
ϕ
[deg]
(b)(a)
ϕ
[deg]
T=780K, t=68.8h
10
-19
10
-20
10
-21
Σ
= 5
Σ
= 5
3534
Cu1
33 33 34 3536 36 3737 3838 3939 4040
Cu1
Cu2
6
7
8
9
10
8
7
5
5
6
10
9
T=661K, t=65.66d
64
Cu in Cu1
T=919K, t=9.6h
π
[m
3
/s]
π
[10
-20
m
3
/s]
ϕ
[deg]
(b)(a)
ϕ
[deg]
T=780K, t=68.8h
FIGURE 3.60
Misorientation dependence of the quantity π(ϕ) of grain boundary diffusion
along 100 tilt boundaries in Cu for different temperatures.
© 2010 by Taylor and Francis Group, LLC
3.5 Experimental Results 233
in Fig. 3.52. For 100 tilt grain boundaries only a few of the calculated val-
ues correlate with experiments. As a rule, the experimental pre-exponential
factor is 2–15(!!) orders of magnitude larger than the factor calculated from
the theory of hopping motion of individual atoms across the grain bound-
ary. A difference between the mechanisms and activation parameters of grain
boundary migration and grain boundary diffusion was also found in atomistic
simulations (see Chapter 5).
3.5.5 Segregation Effects Other Than Misorientation
We have shown in Sec. 3.5.3 that the misorientation dependence of grain
boundary mobility is mostly due to segregation effects, and it is not clear
to date whether even in ultrapure material the intrinsic mobility of grain
boundaries was measured. In this sense even high purity material has to be
considered as a very dilute solution affected by segregation. In addition to the
influence on misorientation dependence of grain boundary mobility, there are
two important issues for a more quantitative treatment of segregation effects
on grain boundary migration.
1. The total impurity content is always composed of different elements,
but it is common experience that different elements have varying de-
grees of effect on grain boundary motion. Fe, Ti and Sc impurities are
known to very effectively hinder boundary migration in Al. P in Cu or
Nb in steels are comparably effective. Conversely, some other elements
may have only minor influence on boundary mobility, like Ag in Al. In
essence, the question has to be addressed which elemental concentration
is to be considered most relevant for the overall migration behavior of
the boundary.
2. Even if the impurity content in the volume is small, the concentration in
the boundary may be high, if the interaction energy with the boundary
is large. Then the impurity drag theory may fail, since it also assumes
a dilute solution of impurities in the boundary, i.e. it neglects impurity
interactions in the boundary.
In this context, the motion of pure 111 tilt boundaries with misorientation
angles in the vicinity of the special misorientation Σ7 (38.2
◦
) was studied un-
der the action of a constant driving force (technique N12, Table 3.3) [197, 255].
Here we will confine our consideration to the motion of 111 tilt boundaries
with misorientation ϕ =38.2
◦
(special misorientation Σ7) and 40.5
◦
.These
boundaries are of special interest because of their dominant role in recrys-
tallization of Al [284]. The velocity of grain boundary motion was measured
© 2010 by Taylor and Francis Group, LLC
