Gottstein G., Shvindlerman L.S. Grain Boundary Migration in Metals: Thermodynamics, Kinetics, Applications
Подождите немного. Документ загружается.


144 3 Grain Boundary Motion
planes that constitute the surfaces of the adjoining grains in a bicrystal have
a different surface tension Δγ
s
, the boundary feels a driving force
P =2Δγ
S
/λ (3.12)
where λ is the thickness of the crystals. Decreasing the thickness, e.g. by fab-
rication of a thin sheet, not only increases the driving force, but, as will be
shown below, a drag from the free surface. It is felt that the optimal value of
P in this case is about 10
−4
MPa.
The list of potential driving forces for grain boundary migration is very
long and cannot be comprehensively covered here. Other major driving forces
are: a temperature gradient (∇T (P =ΔSδ∇T/(Ω
a
)), where ΔS is the en-
tropy difference between the bulk of a grain and the grain boundary, δ is the
grain boundary thickness, and Ω
a
the molar volume. Under optimal condi-
tions ∇T
∼
=
10
5
K/m, ΔS ≈ ΔS
m
≈ R, P
∼
=
10
−5
MPa; an anisotropy of the
elastic constants (P = τ
2
/[2 · [1/(E
1
) − 1/(E
2
)]]), where τ is the stress, E
1
,
E
2
are Young’s moduli of the differently oriented crystals. For a stress on the
order of 10 MPa one obtains a driving force P
∼
=
10
−5
− 10
−4
MPa.
The origin of these driving forces and their approximate magnitudes are
listed in Table 3.1.
3.3 Drag Effects During Grain Boundary Motion
3.3.1 Origin of Drag Effects
Whenever there is an interaction between a grain boundary and other imper-
fections in the crystal, this interaction will affect grain boundary motion. Such
imperfections can be vacancies, dislocations, interface boundaries or external
crystal surfaces. Their interaction with the grain boundary is of very different
nature and thus will be treated in separate sections below.
3.3.2 Impurity Drag in Ideal Solute Solutions
It is a common experience that grain boundary motion progresses much faster
in pure metals than in alloys, even dilute alloys. This must be due to the effect
of solute atoms on grain boundary motion [193]–[196]. If there is an interaction
energy U (energy gain) between boundary and impurity atoms, these solute
atoms will tend to segregate to the boundary. In fact, if thermal equilibrium
could be established at all temperatures, and the boundary could adsorb an
unlimited number of impurities — like in the case of the Henry isotherm —
all solute atoms would end up in the boundary at T = 0 K. Owing to thermal
agitation (entropy effect), for T>0 the concentration in the boundary will
© 2010 by Taylor and Francis Group, LLC
3.3 Drag Effects During Grain Boundary Motion 145
be
c
b
= c
0
exp
U
kT
(3.13)
where c
0
is the volume impurity concentration
4
. When the grain boundary
moves, the segregated atoms will attempt to remain in the boundary, i.e. the
boundary has to drag its impurity load and can only migrate as fast as the
slowly moving impurities.
In the simplest approximation [193] one can assume that the segregated
impurities have to move along with the boundary and, therefore, exert a drag
force P
v
P
v
= n · f = n
0
c
b
· f = n
0
f · c
0
· e
U/kT
(3.14)
where n is the number of foreign atoms per unit area of the boundary, f is
the attraction force between boundary and a foreign atom (see, for instance,
[193]), n
0
is the number of lattice sites per unit area of the boundary.
If boundary and foreign atoms move together the net velocity of the segre-
gated solutes read
v = B · f =
D
kT
f (3.15)
where D = D
0
exp
−Q
D
kT
is the bulk diffusion coefficient of the solute atoms.
The boundary moves according to Eq (3.6) with the velocity
v = m
b
· (P − P
v
)=m
b
(P − nf )=m
b
P −
n · vkT
D
(3.16)
and, therefore
v =
Pm
b
1+
n·m
b
kT
D
≈
PD
0
c
0
kT
exp
−
H
D
+ U
kT
(3.17)
When the driving force increases it will finally reach a critical value where the
segregated impurities will no longer be able to keep up with the boundary.
Then the boundary will detach from the impurity cloud and move as a free
boundary with the speed
v = m
b
· P (3.18)
In this very simple model all atoms will detach from the boundary at the
same time. For a more realistic approach one has to consider the diffusion
of the solute atoms with the moving boundaries as proposed by Cahn and
L¨ucke [194, 195].
Due to the motion of the grain boundary the concentration distribution of
solute atoms will be altered. We are interested in the steady-state concentra-
tion (c(x, y, z)) of solute atoms in the presence of a boundary moving with
4
This is only true if there is no interaction between the solutes in the boundary. If this
constraint is relaxed a different isotherm has to be used, as will be addressed in Sec. 3.3.3.
© 2010 by Taylor and Francis Group, LLC
146 3 Grain Boundary Motion
constant velocity v. If we assume a planar boundary moving in x-direction,
the problem can be reduced to a one-dimensional diffusion equation
∂c
∂t
= D
∂
2
c
∂x
2
+
∂
∂x
Dc
kT
dU
dx
(3.19)
with D the volume diffusion coefficient and c = c
0
for x →±∞. This diffusion
equation is time dependent, since the grain boundary changes position with
time, as does the concentration distribution attached to it. In a coordinate
system affixed to a grain boundary moving with constant speed the concen-
tration distribution remains stationary, i.e. ∂c/∂t = 0. For sake of simplicity,
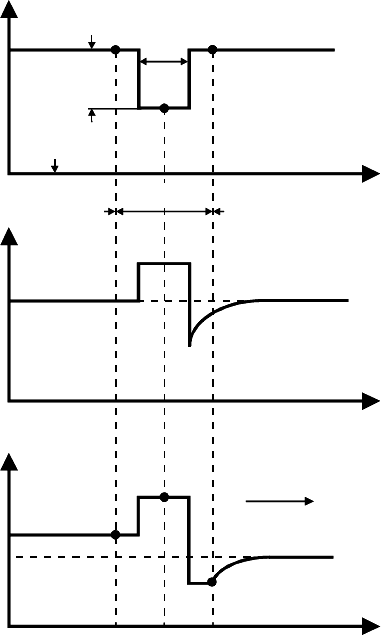
we shall assume (Fig. 3.5a) that
U(x)=
0 |x|≥a
−H
0
|x|≤a
(3.20)
Thus, dU/dx = 0 except for x = ± a. Using the Galilei transformation
x = x
− vt (3.21)
we obtain the diffusion equation in the coordinate system moving along with
the grain boundary at its origin
∂c
∂t
= D
∂
2
c
∂x
2
+ v
∂c
∂x
= 0 (3.22)
(x = ±a; c = c
0
,x= ±∞) which yields the solution
c(x)=c
a
+ c
b
e
−
vx
D
(3.23)
with c
a
, c
b
being integration constants. For range I (behind the boundary)
c
I
(x)=c
0
(3.24a)
because of c(x)=c
0
for x = −∞.
For range II (in front of the boundary)
c
II
(x)=c
0
+(c
2
− c
0
) e
−
vx
D
(3.24b)
with c
2
= c(+a).
Owing to the discontinuity of the potential U for x = ± a the constant c
2
and the concentration distribution inside the boundary cannot be calculated
in closed form
5
. However, during steady-state migration the diffusive flux
5
A closed form solution is possible, when another shape of the potential U(x)isused,e.g.a
triangular potential, where |dU/dx| < ∞. This renders the solution of the diffusion equation
more difficult for the range dU/dx = 0, but the analysis yields a result very similar to the
solution given here.
© 2010 by Taylor and Francis Group, LLC
3.3 Drag Effects During Grain Boundary Motion 147
must be constant everywhere, i.e. equal to the convective flux j
c
= vc
0
.For
a boundary comprising a single atomic layer B (Fig. 3.5c) we obtain for the
flux through the interface: range II/ boundary (B) (x = a)
c
b
D
b
e
−
H
0
kT
− c
2
D
b
− vc
2
= −vc
0
(3.25a)
and the interface: boundary (B) / range I (x = − a)
c
1
D
b
− c
b
D
b
e
−
H
0
kT
− vc
b
= −vc
0
(3.25b)
The first two terms of Eq. (3.20a) represent the diffusion flux through the
interface B/II in terms of the difference of the number of atomic jumps from
plane B to plane 2 and from plane 2 to plane B:
c
b
ν exp
−
H
0
+ H
D
kT
− c
2
ν exp
−
H
D
kT
(3.26)
where H
D
is the activation enthalpy of volume diffusion. The same holds for
Eq. (3.25b). Eqs. (3.24b, 3.25a,b) can be solved with regard to c
2
and c
b
.
We obtain, using Φ = bv/D and Ψ = e
−H
0
/kT
c
1
= c
0
(3.27a)
c
b
= c
0
1+Φ
Ψ+Φ
≥ c
0
(3.27b)
c
0
=
1 −
Φ(1− Ψ)
(1+Φ)(Ψ+Φ)
(3.27c)
The corresponding concentration distribution is given in Fig. 3.5c.
The concentration c
1
behind the boundary is here always equal to the initial
concentration c
0
.SinceΨ< 1, the boundary concentration c
b
>c
1
.Further,
c
2
<c
0
, i.e. there is a concentration dip in front of the boundary.
As discussed above, the elementary step of grain boundary motion is as-
sumed to consist of the jump of single atoms across the boundary from one
crystal to the other. We consider boundary motion from left to right in Fig.
3.5 which corresponds to a net atom jump to the left. By such a jump the
total free energy of the system may be altered for two reasons:
(i) If there is a driving force P (free energy per cm
3
) causing the boundary
to move to the right, the energy is decreased by the amount P Ω
a
≈ Pb
3
per jump, where Ω
a
is the atomic volume. Conversely, for backward
jumps the energy is increased by this amount.
(ii) If the boundary moves by one atomic step to the right, the impurities
located before in plane 2 are now situated in plane B and the ones of
plane B are now in plane 1. This corresponds to an energy change per
atom jump ΔH = c
2
H
0
− c
b
H
0
. A backward jump leads to an increase
ΔH = c
b
H
0
− c
1
H
0
.
© 2010 by Taylor and Francis Group, LLC
148 3 Grain Boundary Motion
U
c
c
(b)
(c)
Plane
H
0
1B2
Range II
v
c
1
c
2
c
B
2a
x
x
x
(a)
B
Range I
U
c
c
(b)
(c)
Plane
H
0
1B21B2
Range II
v
c
1
c
2
c
B
2a
x
x
x
(a)
BB
Range I
FIGURE 3.5
One-atomic boundary (a) energy level of an impurity in bulk and boundary;
(b) impurity concentration dependence across the boundary; (c) concentration
dependence in atomistic model; 1,2 denote atomic positions next to boundary
in the crystal, and B position in the boundary.
© 2010 by Taylor and Francis Group, LLC
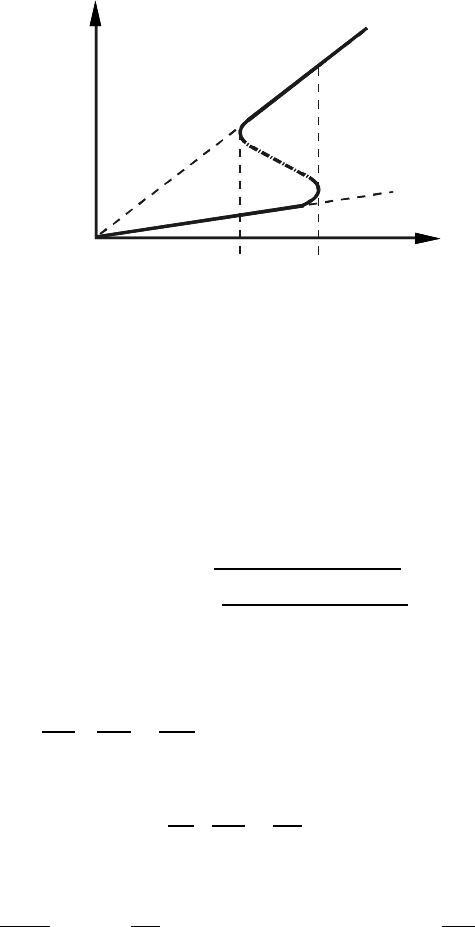
3.3 Drag Effects During Grain Boundary Motion 149
Free
Loaded
TP
v
Free
Loaded
TP
v
FIGURE 3.6
Dependence of grain boundary migration rate on driving force in the presence
of impurity drag. In the interval denoted by T the transition from the loaded
to the free boundary and vice versa occurs discontinuously.
If one supposes that the basic activation energy H
m
for such a jump is de-
creased in the case of a forward jump and increased in the case of a backward
jump by half of these energy changes, one obtains for the net velocity
v = bν
⎧
⎨
⎩
exp
−
H
m
−Pb
3
/2−H
0
(c
2
−c
b
)/2
kT
−exp
−
H
m
−Pb
3
/2+H
0
(c
b
−c
1
)/2
kT
⎫
⎬
⎭
(3.28)
Assuming that Pb
3
kT and H
0
(c
2
− c
b
) kT the exponential function
can be expanded to give
v =
D
m
b
Pb
3
kT
−
H
0
2kT
(c
1
− c
2
)
= m (P − P
v
(v)) (3.29a)
or
P =
kT
b
3
·
vb
D
m
+
H
0
2b
3
(c
1
− c
2
) (3.29b)
with
m =
D
m
b
2
kT
,P
v
=
H
0
2b
3
· (c
1
− c
2
) ,D
m
= νb
2
exp
−
H
m
kT
(3.29c)
m is the mobility of the grain boundary without impurity atmosphere, and
P
v
is the retarding force due to the impurities. The important point is that c
1
and c
2
and thus also P
v
depend upon the velocity v of the boundary so that
the dependence v = v(P ) is contained only implicitly in Eq. (3.29a). Only the
© 2010 by Taylor and Francis Group, LLC

150 3 Grain Boundary Motion
inverse function P = P (v) can explicitly be written (3.29b).
The introduction of the expressions for c
1
(Eq. (3.27a)) into Eq. (3.29b)
leads to an equation f(v, P) = 0 which is of third order in v.Thepositive
roots of this equation represent the function v = v(P ). In general, the solution
v(P ) cannot be given in closed form; only for very high (v
H
)andverylow(v
L
)
velocities are approximate expressions possible. In zero order approximation
one obtains
v
F
=
b
2
D
m
kT
P ; (3.30a)
v
L
=
2b
3
D
H
0
c
0
[exp (H
0
/kT ) − 1]
· P (3.30b)
The velocity v
F
describes the velocity of a free boundary, i.e. a boundary
broken away from the impurity atmosphere. The velocity v
L
is determined
by the diffusion of the impurities together with the moving boundary. At
intermediate values of v a transition from the boundary loaded with impurities
to the broken away boundary occurs. This transition range (T in Fig. 3.6) is
characterized by a very rapid increase in the velocity with increasing driving
force P . However, it is stressed that in this transition regime the boundary
also will move either as a loaded or as a free boundary or at some point
can change from one state to the other, as confirmed experimentally (Fig.
3.7). This implies that the boundary cannot remain in the transition state
over an extended period of time as claimed previously to explain a nonlinear
relationship between grain boundary velocity and driving force.
3.3.3 Impurity Drag in Regular Solutions
Eq. (3.30) predicts that the mobility of the loaded grain boundary ought to
decrease with increasing impurity concentration m ∼ 1/c
0
and that the activa-
tion enthalpy H
m
of the mobility is different for the free and loaded boundary,
but in both cases H
m
is independent of impurity concentration. This, how-
ever, is at variance with experimental results (Fig. 3.8). The concentration
dependence of the activation energy indicates that segregated atoms in the
boundary cannot be treated as a dilute solid solution, i.e. without interaction
of the segregated atoms. If there is solute-solute interaction in the bound-
ary, the impurity concentration in the boundary cannot be represented by
Eq. (3.13) (the Henry isotherm) anymore. Instead, the solutes in the bound-
aries have to be treated as a regular solution rather than an ideal solution
[197]. To simplify the mathematical treatment, we shall assume in the follow-
ing that the concentration profile of solute is always given by the equilibrium
concentrations in the bulk c
0
and in the boundary c
b
.
When a boundary moves together with its segregated impurities, the ve-
locity of the impurity atoms v
im
should be equal to the boundary velocity
© 2010 by Taylor and Francis Group, LLC
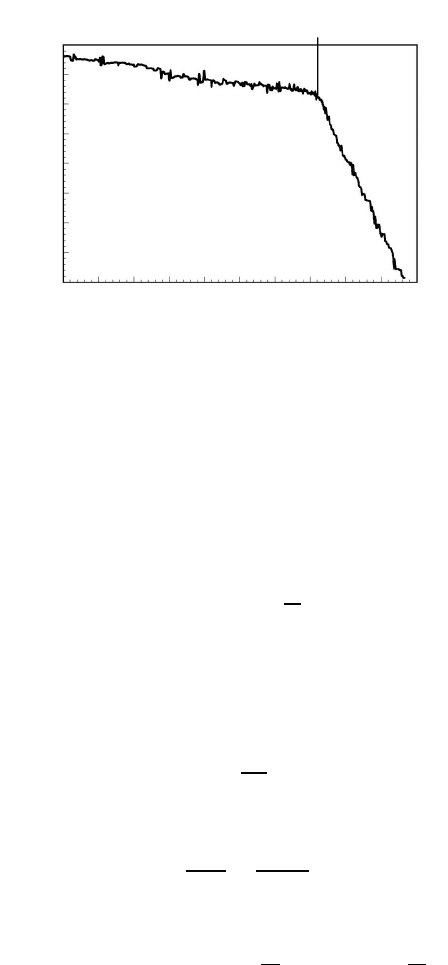
3.3 Drag Effects During Grain Boundary Motion 151
position [µm]
time [s]
t
d
v = 1.8µm/s
v = 25.2µm/s
0 20 60 80 10040 200180160140120
0
400
800
1200
1600
position [µm]
time [s]
t
d
v = 1.8µm/s
v = 25.2µm/s
0 20 60 80 10040 200180160140120
0
400
800
1200
1600
FIGURE 3.7
Recording of grain boundary position with annealing time in an Al bicrystal.
At t
d
the grain boundary unzips from its impurity cloud and moves freely.
Note discontinuous transition without intermediate stage.
v
b
. If the boundary moves under the action of an external driving force P ,it
imparts the same driving force to the impurities carried along
v
b
= m
b
P = v
im
P
Γ
(3.31)
where m
b
and m
im
are the mobilities of boundary and impurity atoms, re-
spectively. Γ = c
b
−c
0
is the difference between the concentration of adsorbed
impurities in the boundary c
b
and the bulk concentration c
0
.WiththeNernst-
Einstein relation
m
im
=
D
kT
(3.32)
where D is the respective diffusion coefficient, Eq. (3.31) yields
m
b
=
m
im
Γ
=
D
Γ · kT
(3.33)
For dilute (volume) solutions one may use the Henry isotherm
Γ=z · B · c
0
− c
0
= c
0
zB
0
e
H
i
kT
− 1
∼
=
zB
0
e
H
i
kT
c
0
(3.34)
for c
0
c
b
.(B = e
G
i
/kT
,B
0
= e
−S
i
/k
,G
i
— Gibbs free energy of adsorp-
tion, S
i
— adsorption entropy, H
i
— interaction enthalpy of impurity atoms
with the boundary, z — number of adsorption sites in the boundary.) Eqs.
© 2010 by Taylor and Francis Group, LLC
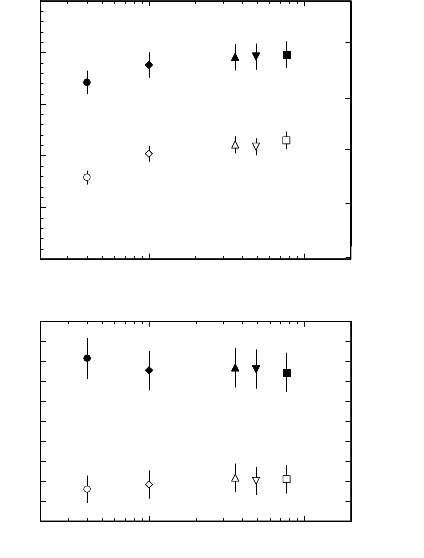
152 3 Grain Boundary Motion
10
-4
10
-3
c [% at.]
0.5
1.0
1.5
2.0
2.5
3.0
H
[
e
V
]
10
-4
10
-3
H
[
k
J
/
m
o
l
]
100
150
200
250
50
c [at.%]
10
-4
10
-3
0.5
1.0
1.5
2.0
2.5
3.0
50
100
150
200
250
10
-04
10
-03
c [% at.]
10
00
10
02
10
04
10
06
10
08
10
10
A
0
[
m
2
/
s
]
0.0001 0.001
1
10
100
1000
10000
100000
1E+006
1E+007
1E+008
1E+009
1E+010
A
0
[m
2
/s]
c [at.%]
10
-4
10
-3
10
10
10
8
10
6
10
4
10
2
10
0
(a)
(b)
H
m
[kJ/mol]
H
m
[eV]
10
-4
10
-3
c [% at.]
0.5
1.0
1.5
2.0
2.5
3.0
H
[
e
V
]
10
-4
10
-3
H
[
k
J
/
m
o
l
]
100
150
200
250
50
c [at.%]
10
-4
10
-3
0.5
1.0
1.5
2.0
2.5
3.0
50
100
150
200
250
10
-04
10
-03
c [% at.]
10
00
10
02
10
04
10
06
10
08
10
10
A
0
[
m
2
/
s
]
0.0001 0.001
1
10
100
1000
10000
100000
1E+006
1E+007
1E+008
1E+009
1E+010
A
0
[m
2
/s]
c [at.%]
10
-4
10
-3
10
10
10
8
10
6
10
4
10
2
10
0
(a)
(b)
H
m
[kJ/mol]
H
m
[eV]
FIGURE 3.8
Dependence of activation enthalpy H and preexponential factor A
0
of the
reduced grain boundary mobility for 38.2
◦
(open symbols) and 40.5
◦
(filled
symbols) 111 tilt grain boundaries on impurity content in pure Al: ◦, • —
AlI; ♦, —AlII;, — AlIII; , —AlIV;, —AlV.
© 2010 by Taylor and Francis Group, LLC

3.3 Drag Effects During Grain Boundary Motion 153
(3.31)–(3.34) render the known expression for the mobility of a boundary with
impurities:
m
b
=
D
0
e
−
(
H
D
+H
i
)
kT
zB
0
kT · c
0
(3.35)
where D
0
is the diffusion pre-exponential factor, H
D
is the activation enthalpy
for (volume) diffusion of the impurity atoms. According to Eq.(3.35) the ac-
tivation enthalpy for grain boundary migration is the sum of two activation
enthalpies, impurity diffusion and impurity adsorption. The pre-exponential
mobility factor changes inversely proportionally to the impurity concentration
contrary to experimental results. As evident from Fig. 3.8, even at the low-
est impurity content, the activation enthalpy rises with increasing impurity
concentration, whereas the pre-exponential factor remains essentially at the
same level.
If there is a strong tendency toward segregation, the boundary impurity
concentration may be high although the volume impurity concentration is
small. For high impurity concentrations in the boundary it is necessary to
take into account the mutual interaction of adsorbed atoms in the bound-
ary. Also, grain boundaries are inhomogeneous, i.e. not every site in the grain
boundary is equally favorable for impurity segregation. The essential point
here is that this inhomogeneity plays an important role in the adsorption dur-
ing the process of migration and thus has to be taken into account.
In the following we shall consider the interaction of impurities with grain
boundaries in terms of adsorption. This allows us to express an influence of
adsorption on both the activation enthalpy and the pre-exponential mobility
factor. We consider a true binary system with bulk concentrations c
1
and c
2
and assume that the grain boundary chemistry is in equilibrium with the bulk,
in spite of grain boundary motion
μ
b
1
γ
1
,T,c
b
1
= μ
v
1
(p, T, c
1
) (3.36)
μ
b
2
γ
2
,T,c
b
2
= μ
v
2
(p, T, c
2
) (3.37)
where μ
b
1
, μ
b
2
,andμ
v
1
, μ
v
2
are the chemical potentials of the first and second
component in the bulk and in the boundary, respectively, and c
b
1,2
denote the
respective boundary concentrations. The activities of atoms in both the bulk
(a
1
,a
2
) and the boundary
a
b
1
,a
b
2
are related by
a
b
1
a
1
=
a
b
2
a
2
ω
1
ω
2
· e
ω
1
(γ
2
−γ
1
)
kT
(3.38)
where γ
1
and γ
2
are the grain boundary surface tensions of the pure first and
second component, respectively, and
ω
1
= −
∂μ
b
1
∂γ
1
p,T,γ
2
and ω
2
= −
∂μ
b
2
∂γ
2
p,T,γ
1
(3.39)
© 2010 by Taylor and Francis Group, LLC
