Goldfarb D. Biophysics DeMYSTiFied
Подождите немного. Документ загружается.

192 Biophysics DemystifieD
do not absorb wavelengths in the green area of the spectrum. Since the green light
is not absorbed, it is reflected; this accounts for the green color of most plants.
Cell Life Cycle
At the beginning of this chapter we noted that all cells come from preexisting
cells through the process of cell division. The phases of cell growth and cell
division make up what is known as the cell cycle. There are two main phases to
O
O
N
N
N
–
N
–
Mg
++
O
O
O
H
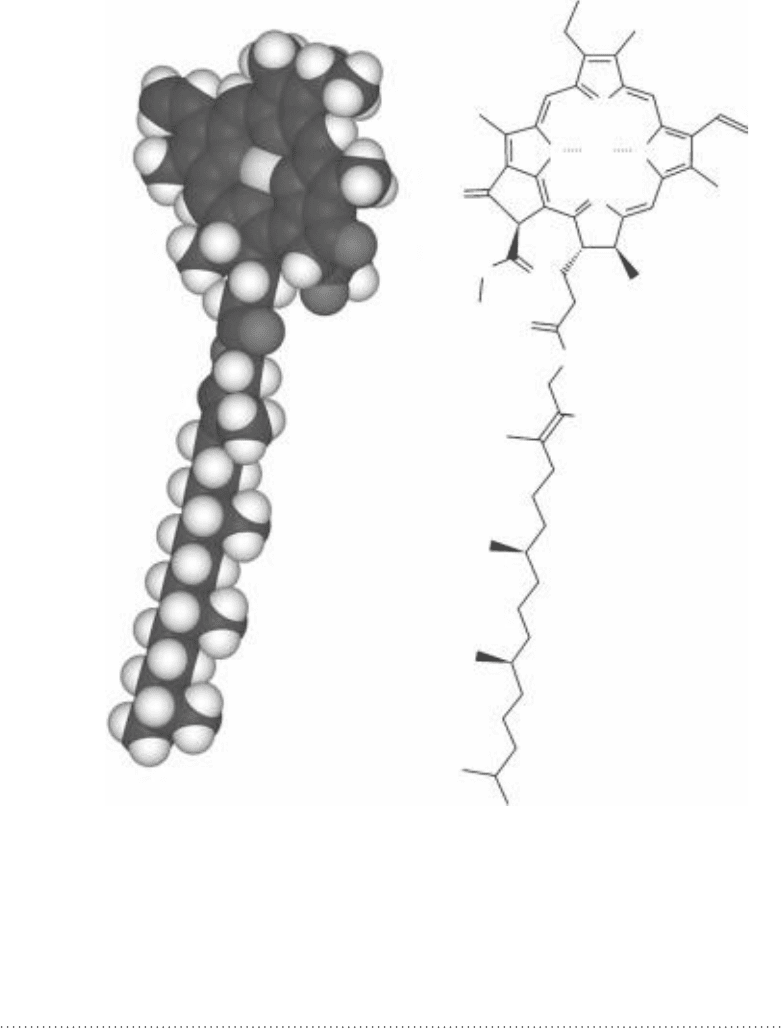
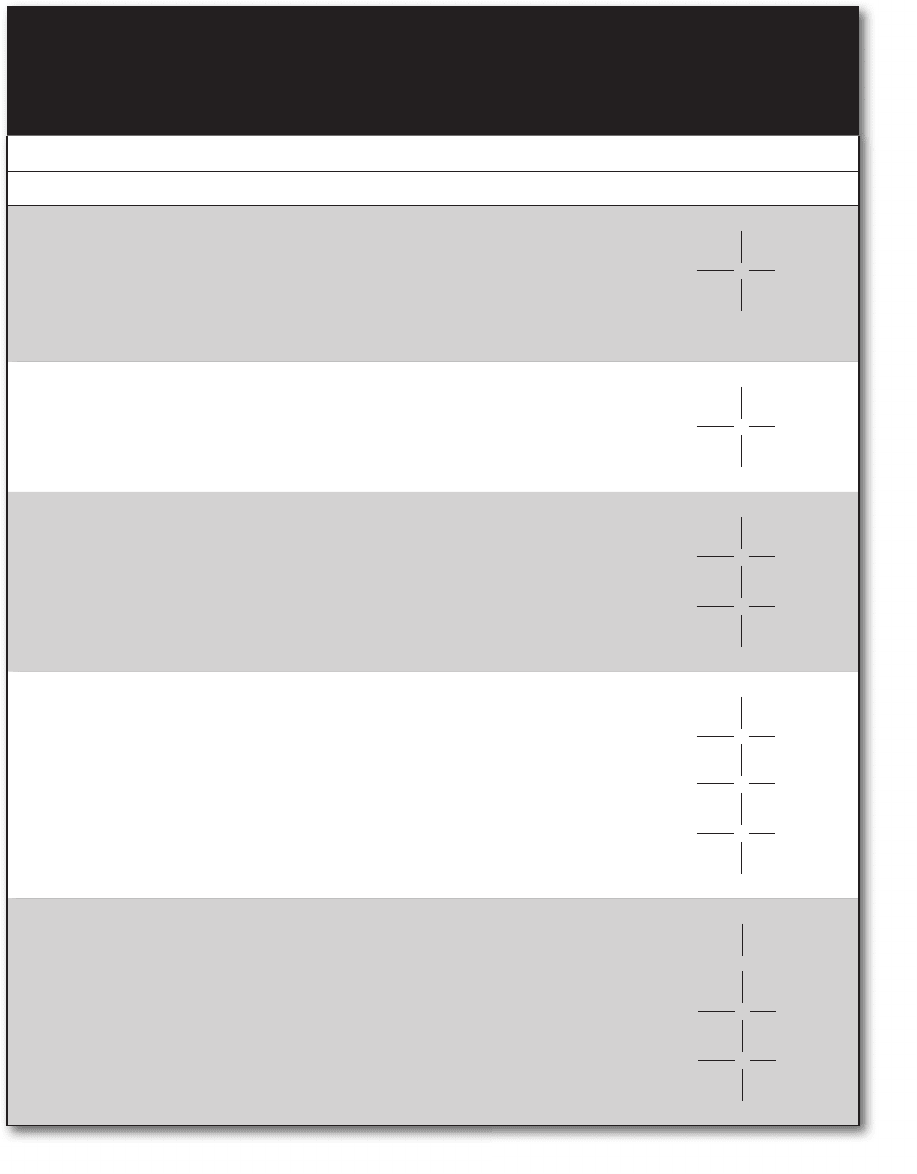
FIgure 8-14 • Chlorophyll a: space filling model (left) and structural formula with bound
magnesium ion (right).
chapter 8 The cell 193
the cell cycle, each of which is broken up into subphases. The two main phases
of the cell cycle are interphase and cell division. See Fig. 8-15.
There is a third phase, called quiescent, where the cell has essentially left
the cycle and entered into a resting phase where the cell continues to utilize
energy to do work, but has stopped dividing. The quiescent or resting phase
is abbreviated G
0
.
The interphase of the cell cycle is where the cell puts most of its energy into
growth and DNA replication. Interphase is broken up into three subphases: G
1
,
S, and G
2
. During G
1
and G
2
the cell grows. Protein synthesis is very active dur-
ing G
1
and G
2
, and the enzymes built from this protein synthesis go on to cata-
lyze other reactions that digest carbohydrates for energy and manufacture lipids
and other structures needed for cell growth. In between G
1
and G
2
is the
S-phase when DNA replication takes place. During the S-phase, protein syn-
thesis is for the most part limited to those proteins that are needed for DNA
replication. For example, the enzymes involved in replication and, in eukary-
otes, the structural proteins that make up histones for the packaging of chro-
matin. See Fig. 8-16.
After the G
2
phase, the cell enters the cell division phase, also called the mitosis
or M-phase. Mitosis itself is broken into several subphases. In the first stage of
mitosis, called prophase, the chromatin condenses into visible chromosomes.
(Remember from earlier in this chapter that sometimes DNA is spread out in a
Cell cycle
Interphase: Cell growth
and DNA replication
Cell division: mitosis
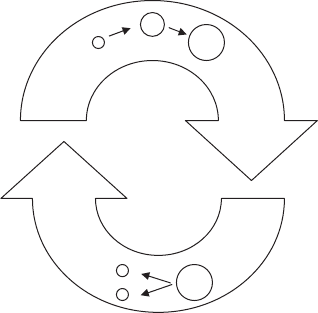
FIgure 8-15 • The two parts of the cell
cycle, interphase and cell division.
194 Biophysics DemystifieD
spaghetti-like structure called chromatin. At other times the cell organizes indi-
vidual DNA molecules into tightly packed chromosomes.) In the metaphase of
mitosis, the chromosomes line up in pairs. A portion of the cytoskeleton known
as the mitotic spindle begins pulling the paired chromosomes apart, so that each
daughter cell will have its own set of chromosomes. After the chromosomes have
separated, they begin decondensing back into chromatin. In the final phase of
mitosis, called cytokinesis, the cell membrane pinches in toward the center of the
cell and divides the cell in two. See Fig. 8-16.
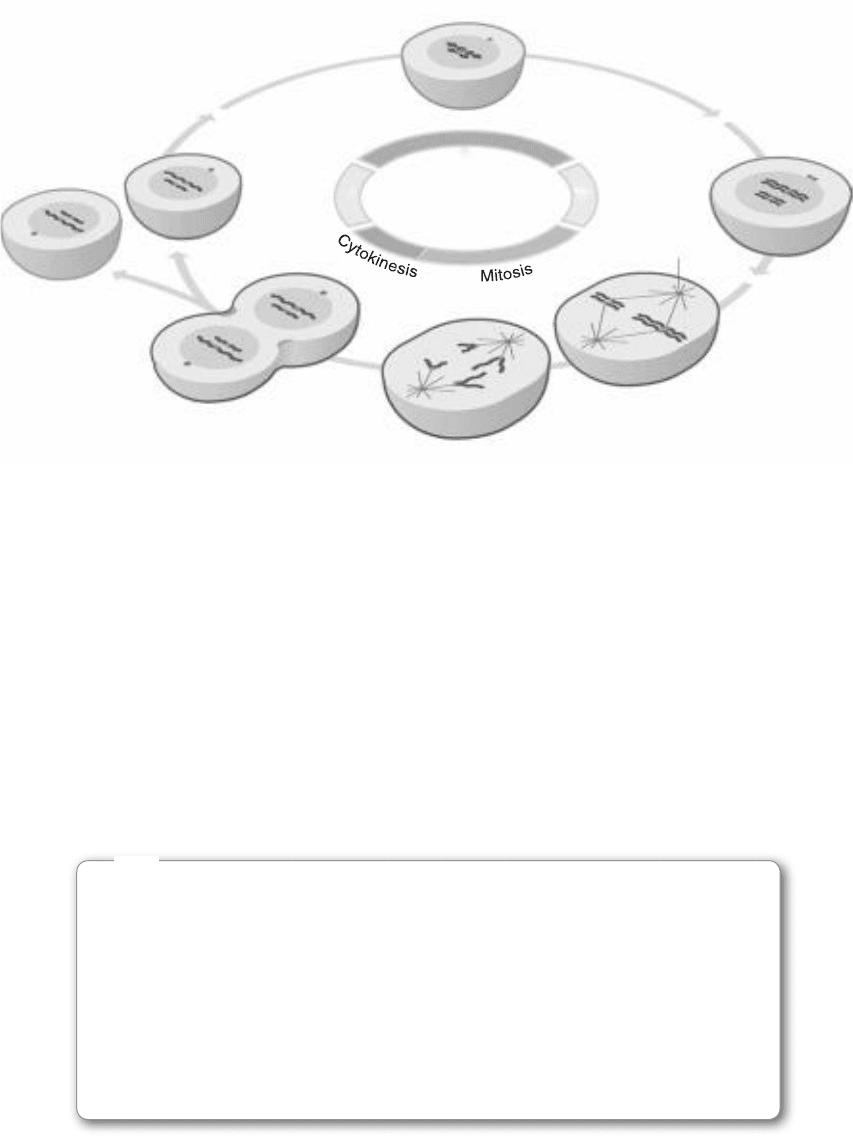
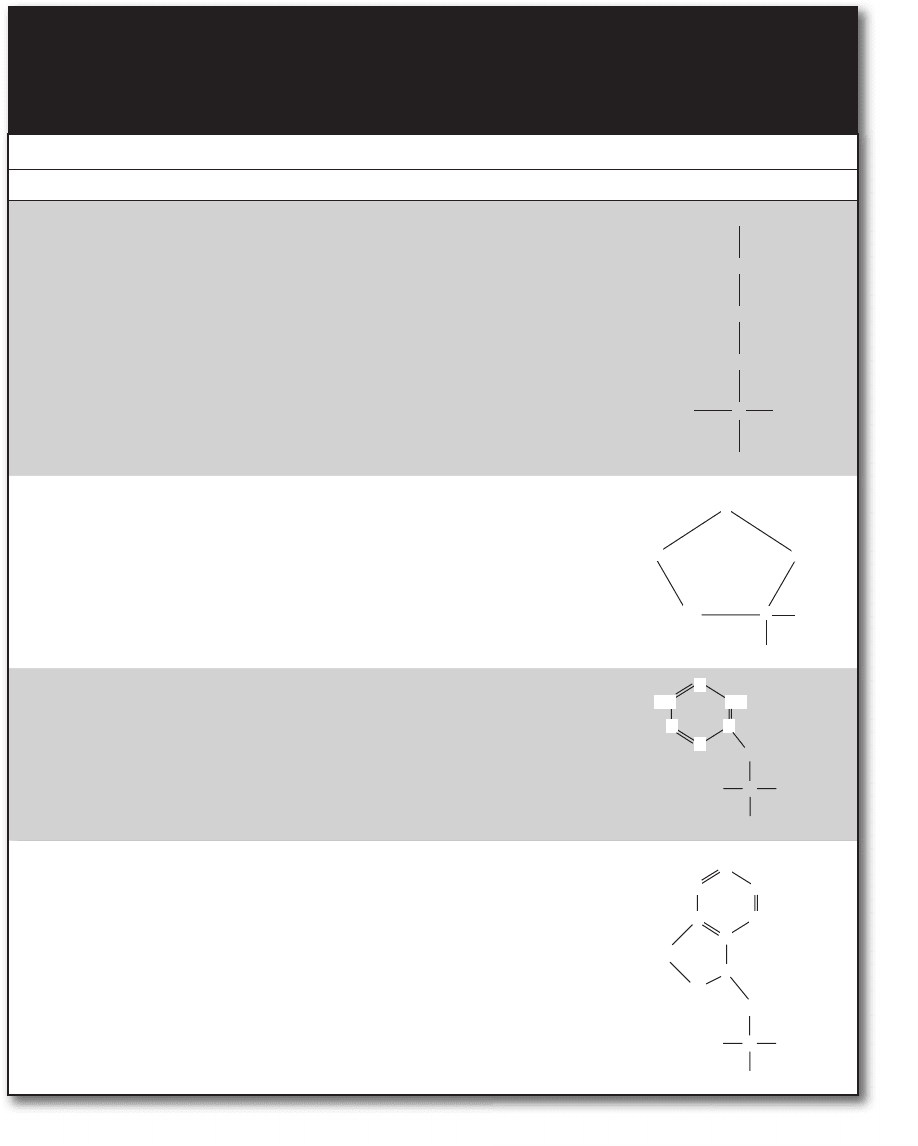
Anaphase
M
G
2
G
1
S
Metaphase
Mitotic spindle
FIgure 8-16 • Cell cycle. (Courtesy of David O. Morgan, The Cell Cycle: Principles of Control, New Science Press.)
still struggling
This chapter is largely descriptive. The main point is to learn some vocabulary
and to gain some understanding of the context for the biophysical studies that
we will learn about in the rest of the book. Do not feel that you have to have all
of the terms in this chapter memorized. As you encounter some of these terms
later in the book, use this chapter as a reference as needed, or refer to the glos-
sary in the back of the book.
?

chapter 8 The cell 195
Quiz
Refer to the text in this chapter if necessary. Answers are in the back of the
book.
1. Which of the following is not true about cells?
A.
The cell is the basic unit of life.
B.
cells are surrounded by a phospholipid bilayer membrane.
C. Prokaryotes always have nucleic acids.
D. Eukaryotes are always multicellular organisms.
2. Which of the following statements are true?
S1. Eukaryotes are typically larger that prokaryotes.
S2. Eukaryotes divide more rapidly than prokaryotes.
S3. Eukaryotes compartmentalize various functions into membrane-bound
organelles.
S4. Eukaryotes adapt more quickly to drastic changes in their environment.
A. S1 and S3
B. s2 and s4
C. S1 and S4
D.
s2 and s3
E. S1, S3, and S4
3. What are receptors?
A.
Antenna-like projections from the cell that receive radio signals.
B.
Membrane proteins that receive other cells.
C. Molecules on the surface of the cell membrane that signal specific outside molecules to
come and bind to the membrane.
D. Molecules on the surface of the cell membrane that bind specific outside molecules,
thereby signaling the cell to do something.
4. What are the Golgi apparatus and the ER?
A. The Golgi apparatus is a device used to study cellular function, and the ER is a reticulating
structure found within the cell.
B.
They are both membranous organelles.
C. The Golgi apparatus stores vesicles, and the ER stores ribosomes.
D. The Golgi apparatus is a species of eukaryote, and the ER is a species of prokaryote.
5. What do ribosomes do for the cell?
A.
Process DNA
B.
Generate polypeptides
C. Distinguish between the smooth and rough ER
D. synthesize lipids
196 Biophysics DemystifieD
6. What are the two main parts of the cell cycle?
A. The handle bars and pedals
B. interphase and outerphase
C. Growth and division
D.
expansion and contraction
E. Separation and division
7. Microtubules are part of what structure?
A. Tubulin
B. Mitochondria
c. Bones
D. Cytoskeleton
e. capillaries
8. What does the nucleus of the cell contain?
A.
Protons and neutrons
B.
The nuclear envelope and DNA lamina
C. DNA and histones
D. DNA and ribosomes
9. What are chloroplasts?
A. Mitochondria
B. photosynthetic proteins
C. Plant organelles responsible for photosynthesis
D.
Green leafy plants
10. What do mitochondria contain?
A. DNA
B. Folded membranes
C. ATP
D.
A and B
e. B and c
F. A and C
G.
A, B, and C

197
In Chap. 7 you learned some basics of protein structure and function. In this
chapter we will delve a little deeper into the physics and biophysical chemistry
involved. Proteins, in order to function properly, need to fold up into a specific
shape. Therefore, much of protein biophysics is concerned with gaining a deep
understanding for the physics of protein folding and protein conformational
changes.
CHAPTer OBJeCTIVeS
In this chapter, you will
Learn about protein structure and its relationship to protein function.
•
study the forces and factors that stabilize protein structures.
•
Learn how membrane protein folding differs from cytoplasmic protein folding.
•
Learn ways to analyze peptide bond angles.
•
Learn the two most common protein secondary structures: the alpha helix and
•
the beta sheet.
Quar
k
w
B
E
De Broglie’s photon
sin
sin
S
D
ec
ec
2
B
B
Electr yclosity
E
lec
ty closit
y
Relativist
Ong
in
ca
a
a
e
a
e
ev
m
m
k
e
chapter
9
Protein Biophysics
198 Biophysics DemystifieD
Protein Folding
When a protein is folded into its correct shape, it is said to be in its native state.
The native state can include one or a small number of conformations that are
involved in the natural functioning of the protein. When a protein is unfolded,
so that it is no longer in its native state, we say that it is denatured.
Experimentally, we can expose proteins to denaturing agents, such as heat,
changes in pH, or exposure to certain chemicals. Denaturing agents destabilize the
protein’s higher-order structure (i.e., quaternary, tertiary, and/or secondary struc-
ture) causing the polypeptide chain to unfold. Frying an egg makes the clear part
of the egg become hard and white, because the protein in the egg white, albumen,
becomes denatured. Frying an egg is irreversible, because the peptide chains from
neighboring albumen molecules become entangled in one another. However,
under carefully controlled experimental conditions, it is possible to avoid entangl-
ing of the denatured chains (e.g., by keeping the protein concentration low and
using a gentle chemical denaturing agent). In such a case denaturing is reversible.
We can remove the denaturing agent and allow the protein to fold back into its
native state. In this way we can experimentally reproduce the folding and unfold-
ing of a protein in order to study the physics of protein folding.
Protein folding is, for the most part, an example of self-assembly. Self assembly
means that the forces driving the molecules to assemble in the appropriate con-
figuration or quaternary structure are inherent in the molecules themselves, and in
their interactions with solvent molecules (such as water and small ions in solution).
Only molecules that are part of the final structure are involved. Solvent molecules
such as water and small ions in solution are part of the final structure in that they
remain associated with the folded protein (or other self-assembled structure) form-
ing hydrogen bonds and other interactions that contribute to stabilizing the
structure.
Not all protein folding is self-assembly. Many proteins do require assistance
from other molecules, called folding moderators or chaperones, in order to achieve
their native state. In some cases the folding moderators (often proteins them-
selves) catalyze a step in the folding process that would otherwise occur very
slowly. In other cases a folding moderator may temporarily bind to the peptide
chain and bring certain parts of the molecule closer together or may provide a
sequestered environment that fosters the protein’s native state.
Proteins are polymers of amino acids. There are 20 major amino acids com-
monly found in proteins. Other amino acids are also found in proteins (many of
which are slight variations of the major 20), but these 20 are by far the most
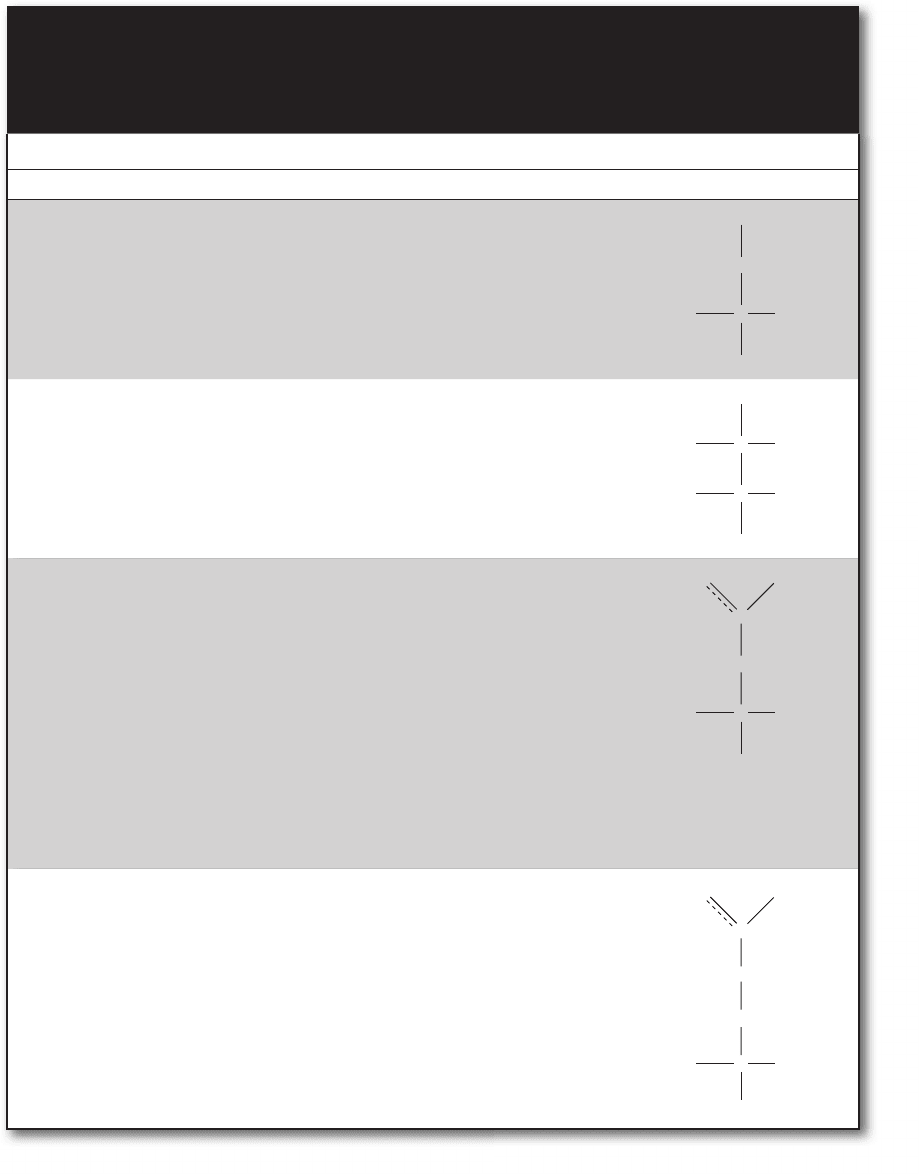
common. Table 9-1 lists the 20 major amino acids and some of their properties.
chapter 9 protein Biophysics 199
TABLE 9-1 the 20 major amino acids most commonly found in proteins are typically classified into the fol-
lowing three groups: nonpolar (hydrophobic), uncharged polar (hydrophilic), and charged (some-
times called charged polar) (hydrophilic). Among the charged amino acids there are those that are
acidic (release protons and carry a negative charge) and those that are basic (absorb protons and
carry a positive charge). (courtesy of Biochemistry Demystified.)
Name Abbreviation(s) Unique Features Structure
Nonpolar, hydrophobic side chains—found in protein interiors
Glycine Gly, G • Smallest
• R is a single proton
• Flexible
• Neuroinhibitor
• Role in biosynthesis of many
compounds, such as purines
H
C
H
COO
−
+
H
3
N
Alanine Ala, A • Methane
•
α
-Keto homologue is
pyruvate
• Role in nitrogen transport
from tissues to the liver
CH
3
C
H
COO
−
+
H
3
N
Valine Val, V • Butyl group
• Branched chain amino acid
found in high concentration
in muscles
CH
2
CH
3
C
H
C
H
COO
−
+
H
3
N
Leucine Leu, L • Branched chain amino acid
found in high concentration
in muscles
H
C
H
CH
3
CH
3
C
H
C
H
COO
−
+
H
3
N
Isoleucine Ile, I • Isomer of leucine
• Branched chain amino acid
found in high concentration
in muscles
H
C
H
3
C
CH
3
CH
2
C
H
COO
−
+H
3
N
200 Biophysics D emystifieD
TABLE 9-1 the 20 major amino acids most commonly found in proteins are typically classified into the fol-
lowing three groups: nonpolar (hydrophobic), uncharged polar (hydrophilic), and charged (some-
times called charged polar) (hydrophilic). Among the charged amino acids there are those that are
acidic (release protons and carry a negative charge) and those that are basic (absorb protons and
carry a positive charge). (courtesy of Biochemistry Demystified.) (Continued)
Name Abbreviation(s) Unique Features Structure
Nonpolar, hydrophobic side chains—found in protein interiors
Methionine Met, M • One of two amino acids
containing sulfur
• Contains a sulfur as an
ester
• Sulfur easily oxidized
• As
S-adenosyl-L-
methionine (SAM),
a methyl donor in many
bioreactions
CH
2
CH
2
CH
2
S
C
H
COO
−
+
H
3
N
Proline Pro, P •
α
-Carbon forms a ring
containing primary amine
• Inflexible
• Forms kinks in secondary
structures
H
2
H
H
2
COO
−
C
C
C
N
+
H
2
C
Phenylalanine Phe, F • Alanine plus a phenyl
• Converted to tyrosine,
which is, in turn,
converted to
L-dopa
• Interferes with the
production of serotonin
C
C
H
H
C
C
CH
C
C
2
H
+
H
3
N
HC
COO
−
Tryptophan Trp, W • Bulky, aromatic side
chains
• Indole group
• Precursor for serotonin
and niacin
C
C
H
H
H
HN
C
C
C
C
CH
C
CH
2
+
H
3
N
HC
COO
−
chapter 9 protein Biophysics 201
TABLE 9-1 the 20 major amino acids most commonly found in proteins are typically classified into the fol-
lowing three groups: nonpolar (hydrophobic), uncharged polar (hydrophilic), and charged (some-
times called charged polar) (hydrophilic). Among the charged amino acids there are those that are
acidic (release protons and carry a negative charge) and those that are basic (absorb protons and
carry a positive charge). (courtesy of Biochemistry Demystified.) (Continued)
Name Abbreviation(s) Unique Features Structure
Uncharged polar side chains—metabolically active and located on the exterior of proteins
Serine Ser, S • Hydroxyl group
• Found at the active site
of enzymes
• Aids in glycoprotein
formation
CH
2
OH
C
H
COO
−
+
H
3
N
Threonine Thr, T • Methyl and hydroxyl
group
• Found at the active site of
enzymes
• Aids in glycoprotein
formation
H
C
H
3
C
OH
C
H
COO
−
+
H
3
N
Asparagine Asn, N • Methyl group and
carboxyl group with a
highly polar uncharged
amine that readily forms
hydrogen bonds
• Found at the ends of
alpha helices and beta
sheets
• Aids in formation of
glycoproteins
• Input to urea cycle
•
α
-Keto homologue is
oxaloacetate
CH
2
C
C
NH
2
O
H
COO
−
+
H
3
N
Glutamine Gln, Q • Two methyl groups and
carboxyl group with a
highly polar uncharged
amine
• Forms isopeptide linkages
• Central role as nitrogen
donor in synthesis of
nonessential amino acids
• Provides nitrogen
transport to the liver
CH
2
CH
2
C
C
NH
2
O
H
COO
−
+
H
3
N
