Geckeler K.E., Nishide H. (Eds.) Advanced Nanomaterials
Подождите немного. Документ загружается.


16.2 Tissue Engineering and Regeneration 529
mimic those of natural bone, is a goal to be pursued. It is well known that natural
bone consists of nanosized, plate - like crystals of HAp grown in intimate contact
with an organic matrix which is rich in collagen fi bers. One novel approach to
fabricating nanocomposite bone grafts, using strategies found in Nature, has
recently received much attention and is perceived to be benefi cial over conven-
tional methods. A variety of production methods have been employed for the
formation of collagen – HAp composite gels, fi lms, collagen - coated ceramics,
ceramic - coated collagen matrices and composite scaffolds for spine and hard
tissue repair [17] .
Stem cells are cells from an embryo, fetus, or adult that have the ability to repro-
duce for long periods, and can also give rise to specialized cells that comprise the
tissues and organs of the body. When implanted onto immunodefi cient mice,
stem cells were shown to combine with mineralized 3 - D scaffolds to form a highly
vascularized bone tissue. Cultured cell – bioceramic composites can be used to treat
defects across the bone diaphysis, with excellent integration of the ceramic scaffold
with bone, and a good functional recovery [18] . Excellent innovative studies with
nanobioceramics are currently in progress, and clinical applications are becoming
relatively common.
Vago and coworkers [19] have introduced a novel 3 - D biomatrix obtained from
the marine hydrocoral Millepora dichotoma as a scaffold for hard - tissue engineer-
ing. M. dichotoma was biofabricated under both fi eld and laboratory conditions,
and 3 - D biomatrices prepared in order to convert mesenchymal stem cell s ( MSC s)
to exemplify an osteoblastic phenotype. The effect of the biomatrices on the pro-
liferation and differentiation of MSCs was then examined at 2, 3, 4, 7, 10, 14, 21,
28, and 42 days. The investigations included light microscopy, scanning electron
microscopy ( SEM ) and energy dispersive spectroscopy ( EDS ), in addition to moni-
toring calcium incorporation into newly formed tissue (with Alizarin red staining),
bone nodule formation (von Kossa staining), fat aggregate formation (oil red O
staining), collagen type I immunofl uorescence, DNA concentrations, alkaline
phosphatase ( ALP ) activity, and osteocalcin concentrations. The MSCs seeded onto
M. dichotoma biomatrices showed higher levels of calcium and phosphate incor-
poration, and higher type I collagen levels, than did control Porites lutea biomatri-
ces. In addition, the ALP activity revealed that those MSCs seeded on M. dichotoma
biomatrices were highly osteogenic compared to those on control biomatrices. The
osteocalcin content of MSCs seeded on M. dichotoma remained constant for up to
two weeks, before surpassing that of seeded P. lutea biomatrices after 28 days. The
investigators reported that M. dichotoma biomatrices enhanced the differentiation
of MSCs into osteoblasts, and hence showed excellent potential as bioscaffolding
for hard - tissue engineering.
As emerging areas, both tissue and implant engineering are evolving to address
the shortage of human tissue and organs. Feasible and productive strategies have
been aimed at combining a relatively traditional approach, such as bioceramic
implants, with the acquired knowledge applied to the fi eld of cell growth and dif-
ferentiation of osteogenic cells. The core of the tissue engineering and regenerative
medicine is the fabrication of scaffolds, in which a given cell population is seeded,

530 16 Nanoceramics for Medical Applications
proliferated, and differentiated with the introduction of functional cell types from
many different sources [20] .
Nanostructured materials and their modifi ed forms offer some attractive pos-
sibilities in the fi elds of tissue and implant engineering, taking advantage of the
combined use of living cells and 3 - D ceramic scaffolds (Figure 16.2 ) to deliver vital
cells to the damaged site of the patient. Recently, bone - like nanostructure scaffolds
have been developed using the technology of composites to imitate natural bone
in bone - tissue engineering [21] .
The results of recent studies have suggested that bone marrow stromal cells might
be a potential source of osteoblasts and chondrocytes, and can be used to regener-
ate damaged tissues using a tissue - engineering approach. However, these strate-
gies require the use of an appropriate scaffold architecture that can support the
formation de novo of bone and/or cartilage tissue, as in the case of osteochondral
defects. Oliveira et al . [14, 22] developed a novel hydroxyapatite/chitosan ( HAp/
CS ) bilayered scaffold by combining a sintering and a freeze - drying technique,
aiming to show the potential of such scaffolds to be used in tissue engineering
for osteochondral defects. Subsequently, in vitro (Phase 1) cell culture studies were
carried out to evaluate the capacity of the HAp and CS layers to separately support
the growth and differentiation of goat bone marrow stromal cell s ( GBMC s) into
osteoblasts and chondrocytes, respectively. The data showed not only that the
GBMCs were able to adhere and proliferate but also that the constructs exhibited
a great potential for use in tissue - engineering strategies, leading to the formation
of adequate tissue substitutes for the regeneration of osteochondral defects.
The effects of surface chemistry modifi cations of titanium alloy (Ti - 6Al - 4V) with
zinc, magnesium, or alkoxide - derived nanocrystalline carbonate hydroxyapatite
( CHAp ) on the regulation of key intracellular signaling proteins in human bone -
derived cell s ( HBDC s) cultured on these modifi ed Ti - 6Al - 4V surfaces, have been
investigated in Australia by Zreiqat et al . [23] . The surface modifi cation with
nanocrystalline CHAp was shown to contribute to successful osteoblast function
and differentiation at the skeletal tissue – device interface.
The role of gene therapy in aiding wound healing and treating various diseases
or defects has become increasingly important in the fi eld of tissue engineering.
The use of 3 - D scaffolds in gene delivery has emerged as a popular and necessary
delivery vehicle for obtaining controlled gene delivery. Ko et al . [24] described the
techniques to synthesize composite scaffolds by combining natural polymers such
as agarose and alginate with calcium phosphate ( CaP ). Alginate has been used
extensively in various applications such as cell encapsulation seeding, gene deliv-
ery, and antibody or growth factor entrapment and release, while agarose has been
used as a scaffold involved in cartilage repair. The incorporation of CaP into the
agarose or alginate hydrogels was performed in situ . Ko et al . concluded that, by
incorporating CaP into the agarose or alginate hydrogel, they were able to synthe-
size a scaffold that was mechanically strong and chemically suitable for use as a
gene - delivery vehicle in tissue engineering.
For many implants, a sustained and controlled release of antibacterial agents
into the wound site is desirable for combating infection. A further advantage of
nanostructured sol – gel - derived glasses is that silver, which is known to have anti-

16.2 Tissue Engineering and Regeneration 531
bacterial properties, can be incorporated into the glass composition. The addition
of silver ions to bioactive glasses has also been investigated by Jones et al . [25] , for
the production of glasses with bactericidal properties. A bioactive glass scaffold
containing 2 mol% silver was shown to release silver ions at a rate shown previ-
ously to be bactericidal in, but not cytotoxic to, bone cells.
16.2.2
Liposomes
Liposomes are the most clinically established nanometer - scale systems currently
used to deliver nontoxic and antifungal drugs, genes, and vaccines; they are also
being used as imaging agents. Liposomes consist of a single layer, or multiple
concentric lipid bilayers, that encapsulate an aqueous compartment. The outstand-
ing clinical profi le of liposomes, compared to other delivery systems, is based on
their biocompatibility, biodegradability, reduced toxicity, and capacity for size and
surface manipulations [26] .
Nanometer - sized particles, such as superparamagnetic iron oxides, semicon-
ducting nanocrystals, silica nanoparticles, and calcium phosphate, each possess
novel functions that include unique magnetic, optical, therapeutic, and medical
properties. The encapsulation of these nanoparticles within liposomes may lead
to an enhanced nanoparticle hydrophilicity, stability in plasma, and an overall
improvement in their biocompatibility [26 – 28] . Furthermore, by utilizing the
ability of liposomes to carry hydrophilic and hydrophobic moieties, combinatory
therapy/imaging modalities can be achieved by incorporating therapeutics and
diagnostic agents into a single liposome - delivery system [26] .
Semiconductor nanocrystals, known as quantum dot s ( QD s) are fl uorescent
nanoparticles with a diameter in the range of 1 to 10 nm. QDs offer distinct spec-
trofl uorometric advantages over traditional fl uorescent organic molecules, with
typical fl uorescence characteristics 10 to 20 - fold brighter than conventional dyes.
QDs also exhibit a greater photostability, a broad excitation wavelength range, a
size - tunable spectrum, and a narrow and symmetric emission spectrum. On the
basis of these photophysical characteristics QDs are currently being investigated
as potential imaging agents, primarily in fl uorescence - based diagnostic applica-
tions [26] .
The self - assembly of organized nanoscopic structures has been the subject of
much interest in both colloidal and nanomaterials science. Indeed, some recent
studies have shown that nanoscale liposomes can be used as a nanoscale template
for the deposition of silica, so as to create a hollow silica nanoshell. These silicate
materials have been used to encapsulate fl uorescent dyes, enzymes, polymer par-
ticles, and liquids [27] . Moreover, the liposome – silica nanoparticle hybrid systems
thus formed can be used in the design of biosensors, whereby the physical char-
acteristics of silica can be matched with the biocompatibility and pharmaceutical
and pharmacodynamic properties of liposomes [28] .
Calcium phosphate - based hybrid nanoparticles have shown great promise as
candidates for drug delivery and bone regeneration systems, based on the excellent
biocompatibility of calcium phosphate [29] . Recently, hydroxyapatite - coated

532 16 Nanoceramics for Medical Applications
liposome s ( HACL s) were successfully manufactured and fi lled with a model
hydrophobic (lipophilic) drug, namely indomethacin ( IMC ) [29] . In this process,
the HAp layer was precipitated onto the liposomes, the aim being to provide the
HACL with two functions: (i) that the inner core liposome provides a sustained
drug release; and (ii) that the outer HAp layer provides the osteoconductivity for
bone cells. The liposomes were formed from 1,2 - dimyristoyl - sn - glycero - 3 - phos-
phate ( DMPA ) and 1,2 - dimyristoyl - sn - glycero - 3 - phosphocholine ( DMPC ). The
results reported by Xu et al . [29] indicated that precipitating HAp onto the liposome
reduced the release rate of IMC compared to uncoated liposomes. In fact, under
the conditions used, the 5 h period required to release 70% of the IMC from the
liposomes was extended to 20 h when the liposomes were coated with HAp.
Perhaps, more importantly, IMC release from the uncoated liposomes occurred
more rapidly at pH 7.4 than at pH 4, whereas the HAp coating reduced the release
rate at pH 7.4 compared to that at pH 4. Based on these fi ndings, Xu et al .
suggested that this effect might open up the possibility of creating “ smart ”
(pH - controlled) targeted drug delivery devices.
When Hang et al . [30] used liposome - coated HAp and tricalcium phosphate as
bone implants in the mandibular bony defect of miniature swine, they found the
liposome - coated materials to be biocompatible. Moreover, the clinical endpoint
was enhanced compared to that in the absence of liposomes. It was hypothesized
that coating hydroxyapatite and tricalcium phosphate with negatively charged
liposomes might improve the nucleation process for new bone formation. In
experiments conducted in miniature swine, artifi cial bony defects on one side were
implanted with either HAp - coated or tricalcium phosphate - coated liposomes,
while defects on the other side served as controls. Histology and radiography
performed at three and six weeks after surgery showed the coated liposome materi-
als to be biocompatible. At three weeks, the implant material was surrounded by
dense connective tissues, whilst by six weeks, new bone formation was visible near
the implanted material. Liposomes immobilized in agarose gel and implanted in
the defects also showed new bony bridge formation.
16.3
Nanohydroxyapatite Powders for Medical Applications
Bone mineral is composed of nanocrystals or, more accurately, nanoplatelets
which originally were described as HAp, and similar to the mineral dahllite . Today,
it is agreed that bone apatite may be better described as CHAp, and approximated
by the formula (Ca,Mg,Na)
10
(PO
4
CO
3
)
6
(OH)
2
. The composition of commercial
CHAp is similar to that of bone mineral apatite. Bone pore sizes range from 1 to
100 nm in normal cortical bone, and from 200 to 400 nm in trabecular bone tissue,
with the pores being interconnected.
Orthopedic implants used mainly for joint replacement and fracture fi xation
include metallic (cobalt chromium or titanium alloys) implants, screws, plates
and nails, and their various permutations and combinations. The most important

16.3 Nanohydroxyapatite Powders for Medical Applications 533
parameters for these implants are that they have the necessary wear resistance,
allow for an adequate attachment to bone, and display the required strength,
ductility, and elasticity. At a bone - implant – load - carrying interface, the greater the
implant material stiffness, the greater load it can carry, compared to the sur-
rounding tissues. This imbalance in load, which is known as stress shielding , can
cause the bone tissue to be resorbed. An implant that is too rigid may also
increase the likelihood of bone fracture, as the bone becomes osteoporotic
(thinned) due to the excessive protection generated by the stress - shielding effect
of the implant.
Macro - textured implants nanocoated with calcium phosphate and possessing the
appropriate bioactivity characteristics, bonding ability, and design, may be the
answer to this serious problem. A range of new nanomaterial production compa-
nies are in the process of applying this new technology in orthopedic, cardiovas-
cular, and dental implants.
Nanotechnology has opened up novel techniques for the production of bone - like
synthetic nanopowders and coatings of HAp. Indeed, the availability of HAp nano-
particles has opened up new opportunities for the design of superior biocompat-
ible coatings for implants, and the development of high - strength orthopedic and
dental nanocomposites.
Although, bone - like HAp nanopowders and nanoplatelets (Figure 16.3 ) can be
synthesized by a range of production methods, one very promising approach is
to synthesize these materials via a sol – gel solution. The results of earlier studies
have shown that, while biphasic sol – gel HAp products are easily synthesized,
monophasic HAp powders and coatings are more diffi cult to produce. Many
companies have successfully synthesized HAp nanoparticles with diameters in
the range of 15 to 20 nm, and with HAp coatings 70 nm thick (Nanocoatings Pty.
Ltd., Australia). The nanoparticles and nanoplatelets of HAp provide excellent
bioactivity for integration into bone, which arises from their very high surface
areas [31, 32] .
Figure 16.3 Nanocrystalline carbonate apatite platelets formed by a sol – gel process.

534 16 Nanoceramics for Medical Applications
Several new production and surface - modifi cation techniques, including some
sol – gel techniques, chemical vapor deposition ( CVD ), and plasma spray, have
resulted in bonds with an excellent adhesive strength between the HAp and the
substrate material, while others may be poor (e.g., electrophoretic deposition,
various solution dip - coating systems, thermal spray). Currently, several companies
and research groups are producing nanocomposites (e.g., NanoCoatings Pty Ltd,
Australia; Mitsubishi Materials; ApaTech Ltd; Dentsply International; BioMet Int.;
Wyeth BioPhamia; and Medtronic Sofamor Danek) that incorporate the macropar-
ticles and nanoparticles of HAp and organic and biogenic materials (e.g., polyeth-
ylene, synthetic peptides and collagen, growth factors). Such a combination
provides mechanical strength that is not achievable by using nanoparticles alone.
In addition, for some of these materials an enhanced bioactivity and mechanical
properties have been reported in orthopedic and dental applications, such as bone
cements and dental fi llings [33] .
Saiz and coworkers [34] have focused on the sintering of porous HAp scaffolds
fabricated using two techniques based on manipulation of the HAp slurries,
namely infi ltration of the polymer foams and robocasting :
• The fi rst method involves the infi ltration of a polymer sponge with a ceramic
slurry until the inner polymer walls are completely coated by the ceramic
powders. Subsequently, the sample is fi red to remove the polymer and form a
ceramic skeleton that is strengthened by sintering at high temperature.
• The second technique involves the use of computer - driven rapid prototyping
techniques to produce porous ceramic with anisotropic microstructures. This
so - called “ robocasting ” is a simple technique used to produce porous ceramic
parts with complex shapes. In robocasting, a ceramic ink is extruded through a
thin nozzle to build a part layer - by - layer, following a computer design. Sintering
in air at temperatures ranging between 1100 ° C and 1200 ° C yields dense
materials with narrow, grain - sized distributions.
It has been stated that both techniques can be used to fabricate scaffolds with an
adequate pore size capable of promoting bone ingrowth.
Khalyfa and coworkers [35] have developed a powder mixture comprising tetra-
calcium phosphate ( TTCP ) as the reactive component and β - tricalcium phosphate
( β - TCP ) or calcium sulfate as a biodegradable fi ller, which can be printed with an
aqueous citric acid solution. Two post - processing procedures – a sintering and a
polymer infi ltration process – were established to substantially improve the
mechanical properties of the printed devices. Specimens of different shapes and
sizes have been printed to study the usability of the developed powder - binder
systems in the 3 - D printing process; in this way a 3 - D scaffold with a thoroughly
open channel system was produced. The printing of a human cranial segment,
together with all its fi ligree structures, was successfully achieved using both pow-
der - binder systems, based on computed tomography scanning data of a human
cranium. In all cases, the printed objects were strong enough to be handled manu-
ally, without damaging the integrity of the devices. Preliminary examinations on
relevant application properties, including in vitro cytocompatibility testing, indi-

16.4 Nanocoatings and Surface Modifi cations 535
cated that the new powder - binder system represented an effi cient approach to the
creation of patient - specifi c ceramic bone substitutes and scaffolds for bone - tissue
engineering.
Ordered tubular structures with open porosity were created by de Sousa and
Evans [36] by using the microextrusion freeforming of a tubular latticework. The
extrudate was a suspension of fi ne HAp powder in isopropyl alcohol with a
polyvinyl butyral binder. The extruder consisted of a stepper - driven syringe fi tted
with a miniature tube extrusion die. In this way, tubular lattice scaffolds and
microsprings were successfully prepared from crowded ceramic suspensions of
HAp. The lattices were then sintered at 1250 ° C to produce a ceramic that had
potential as a bone scaffold and could accommodate growth promoters in a slow
release form.
16.4
Nanocoatings and Surface Modifi cations
16.4.1
Calcium Phosphate Coatings
When considering an ideal material to replace and mimic bone, synthetic calcium
phosphates are an obvious choice, as they can replicate the structure and composi-
tion of HAp, a bone mineral. However, despite having a similar composition and
chemistry to that of human bone, the mechanical properties of calcium phosphate
are far from being close to those of human bone, which limits their use for load -
bearing applications.
Today ’ s solutions of materials for bone replacement are still far from ideal, with
metallic implants remaining the fi rst choice for load - bearing applications. As all
metallic orthopedic and dental implants are bioinert and do not bond chemically
to bone, the only means of fi xation is by mechanical interlocks, whereby the
implant must be manufactured in such a way that it possesses suitable surface
roughness by micro - and macro - texturing. By increasing the surface roughness,
the surface area is increased and this in turn increases the area of fi xation. Other
current methods for fi xing implants fi rmly in place are the use of screws or bone
cement, which are both used in dental and orthopedic implants.
Most published information on HAp is classifi ed under calcium phosphate, to
which HAp belongs. Therefore, the chemical properties will be viewed from the
standpoint that HAp is calcium phosphate, although it will have different solubility
and reactivities from other phosphates within the physiological environments.
Calcium phosphates are characterized by particular solubilities, such as when
bonding to the surrounding tissues, and their ability to degrade and be replaced
by advancing bone growth. As the calcium phosphate or HAp comes into contact
with body fl uid, its surface ions can be exchanged with those of the aqueous solu-
tion; alternatively, various ions and molecules, such as collagen and proteins, can
be adsorbed onto the surface [31, 32] .

536 16 Nanoceramics for Medical Applications
The goal of calcium phosphate as a bioactive coating is to achieve a rapid biologi-
cal attachment to bone. Biological fi xation is defi ned as the process by which
prosthetic components become fi rmly bonded to the host bone by bone in - growth,
without the use of adhesive or mechanical fi xation.
Coatings offer the possibility of modifying the surface properties of surgical -
grade materials to achieve improvements in performance, reliability, and biocom-
patibility. More recently, techniques such as physical vapor deposition, thermal
and electron beam evaporation, plasma metalorganic chemical vapor deposition
( MOCVD ), electrochemical vapor deposition, thermal or diffusion conversion and
sol – gel processing have been used to produce both macro - and nanocoatings.
HAp - coated implants have demonstrated extensive bone apposition in animal
models. The development of good implant – bone interfacial strength is thought to
be a result of the biological interactions of released calcium and phosphate ions.
Quality HAp - coated implants heal faster and attach more completely to the bone.
The long - term performance of a calcium - phosphate - coated implant depends on
coating properties such as thickness, porosity, phases and crystallinity, implant
surface roughness, and overall design.
In addition to the effects of surface topography and chemistry, thin depositions
of HAp or calcium phosphate crystals on implants were found to accelerate early
bone formation and increase the strength of the bond between implant and bone.
A histologic and histomorphometric evaluation of the implant – bone interface was
carried out by Orsini and coworkers [37] to determine the effects of a novel surface
treatment created by discrete crystalline deposition of nanometer - sized calcium
phosphate particles added to the dual acid - etched surface of dental implants placed
in the human posterior maxilla. The bone – implant contact evaluations indicated
that an increase in osteoconduction along the calcium phosphate - treated surface
occurs during the fi rst two months after implant placement. These authors also
stated that their results suggested that the nanometric deposition of calcium phos-
phate crystals could be clinically advantageous for shortening the implant healing
period, providing earlier fi xation, and minimizing micromotion, thus allowing
earlier loading and restoration of function for implants placed in areas with low -
density bone.
During the past 20 years, four general industrial coating methods have been
adapted for the production of bioactive coatings:
• Spray coating was developed by Ducheyne and colleagues, who used relatively
thick (100 μ m to 2 mm) calcium phosphate coatings for bone in - growth [38] .
• Thick bioglass coating was initiated and developed by Hench and colleagues for
surface bioactivity [39] .
• The “ self - assembly ” of biomimetics by precipitation in a simulated body fl uid
( SBF ) solution [40] .
• Nanocoatings , using a range of methods including dipping in sol – gel HAp
solutions to produce strong and bioactive coatings [31] .
The sol – gel method in particular represents an attractive and versatile method, as
it can be used to produce ceramic coatings from solutions by chemical means. It
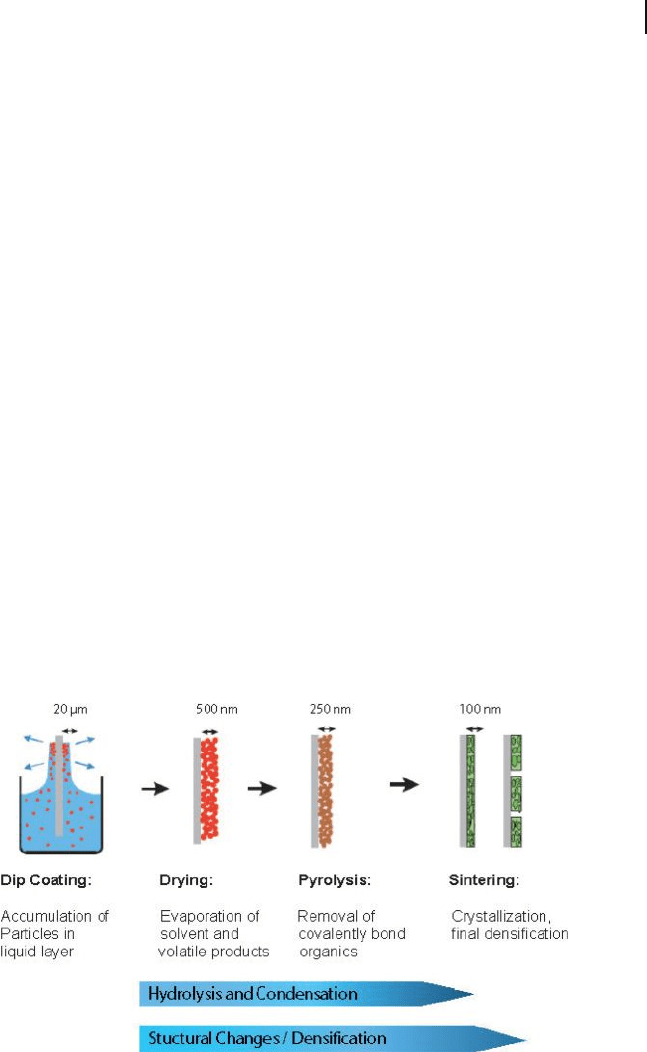
16.4 Nanocoatings and Surface Modifi cations 537
is relatively easy to perform, and complex shapes can be coated. It has also been
shown that the nanocrystalline grain structure results in improved mechanical
properties. Sol – gel processing is also unique in that it can be used to produce
different forms, such as powders, platelets, coatings, fi bers, and monoliths of the
same composition, merely by varying the chemistry, viscosity, and a number of
factors of a given solution.
The advantages of the sol – gel technique are numerous: it is of the nanoscale; it
results in a stoichiometric, homogeneous and pure product, owing to mixing on
the molecular scale; high purity can be maintained as grinding can be avoided; it
allows reduced fi ring temperatures due to its small particle sizes with high surface
areas; it has the ability to produce uniform fi ne - grained structures; it allows the
use of different chemical routes; and it is easily applied to complex shapes with a
range of coating techniques, including dip, spin, and spray deposition (Figure
16.4 ). Furthermore, sol – gel coatings have the added advantages that the costs of
precursors are relatively unimportant, owing to the small amounts of materials
required [31] .
The lower processing temperature has another advantage, namely that it avoids
the phase transition ( ∼ 1156 K) observed in titanium - based alloys used for biomedi-
cal devices.
Currently, companies are producing HAp nanoparticles with diameters in the
range of 15 to 20 nm. Various sol – gel routes have been used for the production of
synthetic HAp. A number of studies have been carried out on a range of precur-
sors to produce pure nanocrystalline apatites for medical applications. A coating
thickness in the range of 70 – 90 nm has been reported by some investigators
[31, 32] .
Figure 16.4 Schematic showing the stages of the sol – gel
dipping process and densifi cation stages of nanocrystalline
coating process.
538 16 Nanoceramics for Medical Applications
Ti metal forms a nanoscale sodium titanate hydrogel layer on its surface, when
it is soaked in 5 M NaOH solution at 60 ° C for 24 h. In Japan, a large number of
patients have been reported to receive artifi cial total hip joints of Ti alloy, modifi ed
with titanium beads subjected to NaOH treatments.
16.4.2
Sol – Gel Nanohydroxyapatite and Nanocoated Coralline Apatite
Current bone graft materials are mainly produced from coralline HAp. Due to the
nature of the conversion process, commercial coralline HAp has retained coral or
CaCO
3
and the structure possesses nanopores within the inter - pore trabeculae,
resulting in high dissolution rates. Under certain conditions, these features reduce
durability and strength, respectively, and are not utilized where high structural
strength is required. To overcome these limitations, a new double - stage conversion
technique was developed by Ben - Nissan and coworkers [23, 31, 32] .
The current technique involves a two - stage application route whereby, in the
fi rst stage, a complete conversion of coral to pure HAp is achieved. In the second
stage, a sol – gel - derived HAp nanocoating is applied directly to cover the meso - and
nanopores within the intra - pore material, while maintaining the large pores for
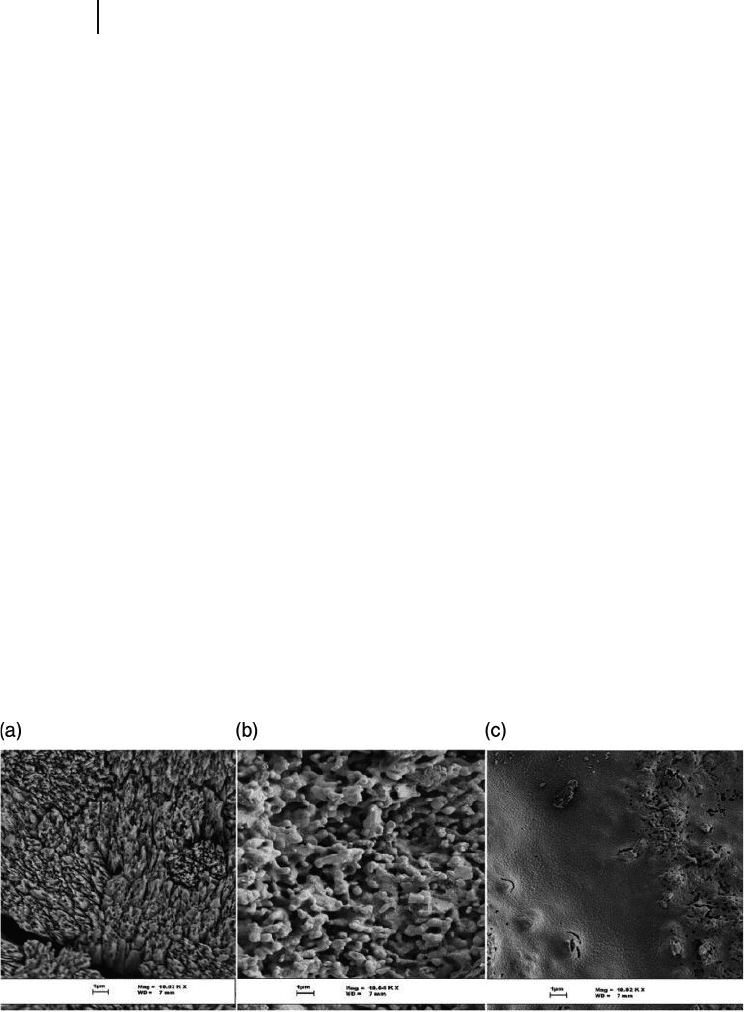
appropriate bone growth. The process is shown in Figure 16.5 .
The compression and biaxial strengths, fracture toughness, and Young ’ s
modulus were each improved as a result of this unique double treatment. Applica-
tion of the treatment method is expected to result in an enhanced bioactivity due
to the nanograin size – and hence large surface area – that increases the reactivity
of the nanocoating. It is anticipated that this new material could be applied
to load - bearing bone - graft applications where high - strength requirements are
pertinent.
Figure 16.5 Stages of nanocrystalline
hydroxyapatite - coated coralline apatite
formation. (a) An enlarged micrograph of a
coral skeleton spine area that has very sharp
meso - and nanopore platelet regions;
(b) Surface morphological changes of the
coral after conversion to hydroxyapatite with
the hydrothermal method; (c) Stage 2
covering of the mesopores and nanopores
with a nanohydroxyapatite coating.
