Geckeler K.E., Nishide H. (Eds.) Advanced Nanomaterials
Подождите немного. Документ загружается.


15.2 Synthesis of Mesoporous Alumina 499
and calcined at 600 ° C to afford the mesoporous alumina (denoted Al - TUD - 1). One
of the most important parameters in the synthesis was the water : aluminum ratio.
The wide - angle XRD patterns of the alumina prepared with an Al : H
2
O molar ratio
of 1 : 10 – 20 indicated that the bulk structure of the alumina contained crystalline
phases of δ - or θ - Al
2
O
3
. Even though this alumina also gave an intensive diffraction
line at about 1.0 ° , the corresponding TEM image did not show any regular
mesopores in the alumina particles. On the other hand, decreasing the amount of
water to an Al : H
2
O molar ratio of 1 : 2 afforded the amorphous alumina with a
sponge - or worm - like randomly three - dimensionally connected mesoporous
network. The N
2
adsorption – desorption measurement of the latter alumina
displayed the isotherm of type IV, with a hysteresis loop that indicated the
presence of ink - bottle - like pores. In addition to the water content, the drying and
heating conditions were also important, as they signifi cantly affected the surface
area and pore size of the fi nal product. For instance, increasing the heating time
of the solid gel resulted in a monotonous increase and decrease in pore size and
surface area, respectively. Moreover, these two structural properties also could be
tuned by changing the drying and heating temperature, although few examples
were shown in the report and thus the tendencies remain unclear. The highlight
of the Al - TUD - 1 mesoporous alumina is its high stability toward high - temperature
treatment. The alumina synthesized under the optimal conditions possessed a
high surface area of 528 m
2
g
− 1
after calcination at 600 ° C, and of 414 m
2
g
− 1
even
after calcination at 700 ° C, indicating the high potential of this material for practical
uses.
Xu et al . synthesized mesoporous alumina in an aqueous medium using glucose
as a structure - directing agent [5k] . To an aqueous solution of aluminum isopro-
poxide and glucose was added diluted aqueous nitric acid to adjust the pH value.
After removal of the water by evaporation, the resulting solid material was calcined
at 600 ° C to give the mesoporous alumina (denoted Al
2
O
3
- X; X = 1 – 3). The pH
value of the starting solution had a major effect on the structural property of the
resultant alumina. The best alumina, Al
2
O
3
- 2, was obtained when the solution pH
was adjusted to 5.0, and showed a surface area, total pore volume, and BJH pore
diameter of 422 m
2
g
− 1
, 0.66 cm
3
g
− 1
, and 5.1 nm, respectively. On the other hand,
Al
2
O
3
- 1 and 3, which were obtained from solutions at pH 4.5 and 5.5, exhibited
smaller values of these parameters. The N
2
adsorption – desorption isotherm of
Al
2
O
3
- 2 was type IV, as typically observed for mesoporous materials; the isotherm
contained a hysteresis loop, the shape of which was typical for ink - bottle - like pores.
The most remarkable property of Al
2
O
3
- 2 was its high thermal stability, with a high
surface area of 225 m
2
g
− 1
even after calcination at 800 ° C. Such excellent thermal
stability and simple preparation method imply a high potential for this material
as a catalyst.
Zhang and coworkers reported the use of hydroxy carboxylic acids as structure -
directing agents [5l] . Here, boehmite sol was fi rst prepared by peptizing a water –
boehmite suspension with nitric acid. To this sol was added a hydroxy carboxylic
acid, and the mixture was then stirred at 30 ° C. The resultant homogeneous
mixture was dried and then calcined at 500 ° C in air to give the corresponding

500 15 Mesoporous Alumina: Synthesis, Characterization, and Catalysis
mesoporous alumina (denoted ID 1 – 11). The textural properties of the mesopo-
rous alumina depended heavily on the content of hydroxy carboxylic acid and the
drying temperature before calcination. When citric acid was used as a structure -
directing agent, a citric acid : Al
3+
molar ratio in the range of 0.5 to 2.0, and a drying
temperature of 100 ° C, were found to be the optimal synthesis conditions. The use
of excess citric acid decreased the surface area and pore volume of the mesoporous
alumina, and led to a deterioration of the intrinsic crystalline structure of boehmite
in the as - synthesized material. This effect was caused by the formation of larger
aggregates of free citric acid molecules, as well as the enhancement of dissolution
of boehmite particulates by the increased acidity of the synthetic mixture. The
drying temperature, on the other hand, affected the pore structure by altering the
coordination interaction between the citric acid molecules and boehmite. Raising
the drying temperature increased the amount of hydroxy carboxylic acids coordi-
nated with boehmite, but decreased the size of the aggregation of free hydroxy
carboxylic acid molecules, owing to the decrease in free molecules. The size of the
aggregation appeared to be crucial for the fi nal structure of pores. Thus, the N
2
adsorption – desorption isotherm of the sample obtained by drying at 30 ° C indi-
cated the presence of larger mesopores, while that at 150 ° C was analogous to the
type I isotherm that is characteristic of microporous materials. Only the mesopo-
rous alumina dried at 100 ° C gave the type IV isotherm with a hysteresis loop that
indicated the presence of ink - bottle - type pores. Unfortunately, the XRD patterns
in the low - angle region were not reported, which makes the quality of the aluminas
ambiguous.
15.3
Mesoporous Alumina in Heterogeneous Catalysis
Mesoporous alumina is superior to conventional alumina in that it possesses a
larger surface area and organized mesopores that are not easily plugged by coke
and other high - molecular - weight byproducts. The ideal structural properties of
mesoporous alumina, however, are not always crucial factors when determining
catalytic performance; the surface reactivity such as acidity and basicity is, in most
cases, much more important. The reactivity of mesoporous alumina surface
should depend heavily on the synthetic method, including the fi nal calcination
temperature, as is usually observed for most oxide catalysts. Unfortunately, most
studies on mesoporous alumina have focused only on the synthesis and quality of
mesoporous structure, and have not referred to the surface reactivity, which
renders the potential for mesoporous alumina in catalysis ambiguous. At present,
therefore, reliance must be placed on rather primitive trial - and - error methods to
determine which mesoporous alumina is most suitable for the catalytic reaction
under investigation. Nevertheless, continuous efforts are being made to utilize
mesoporous alumina as a catalyst and catalyst support. In fact, the use of mesopo-
rous alumina has in some cases led to better results than with conventional
alumina, a topic which is introduced in the following sections (see Table 15.2 ).
15.3 Mesoporous Alumina in Heterogeneous Catalysis 501
Table 15.2 Survey of reactions catalyzed by mesoporous alumina and modifi ed mesoporous alumina.
Catalyst
a
Reaction equation Conditions (Reactor type;
solvent; reaction
temperature; atmosphere)
Reference(s)
I. Base - catalyzed reactions
A. Tishchenko reaction
meso Al
2
O
3
;
mesoAl O SO
23 4
2−
Batch; scCO
2
(8 MPa); 40 ° C [18]
B. Knoevenagel reaction
meso Al
2
O
3
Batch; scCO
2
(8 MPa); 40 ° C [19]
C. Double - bond migration
Na/ meso Al
2
O
3
Batch; neat; 2 ° C; in vacuo [20]
Na/ meso Al
2
O
3
Batch; neat; rt; in vacuo [20]

502 15 Mesoporous Alumina: Synthesis, Characterization, and Catalysis
II. Epoxidation
Au/ meso Al
2
O
3
Batch; benzene; 82 – 83 ° C [21]
Batch; CH
3
CN; 60 ° C [22]
III. Hydrodechlorination
Ni/ meso Al
2
O
3
Continuous fl ow; 300 ° C [23, 24]
Ni – Mg/ meso Al
2
O
3
b
Continuous fl ow; 300 ° C [25]
Catalyst
a
Reaction equation Conditions (Reactor type;
solvent; reaction
temperature; atmosphere)
Reference(s)
Table 15.2 Continued.

15.3 Mesoporous Alumina in Heterogeneous Catalysis 503
IV. Hydrodesulfurization
M o S
2
/ meso Al
2
O
3
b
Continuous fl ow;
280 – 400 ° C; 1 MPa
[26]
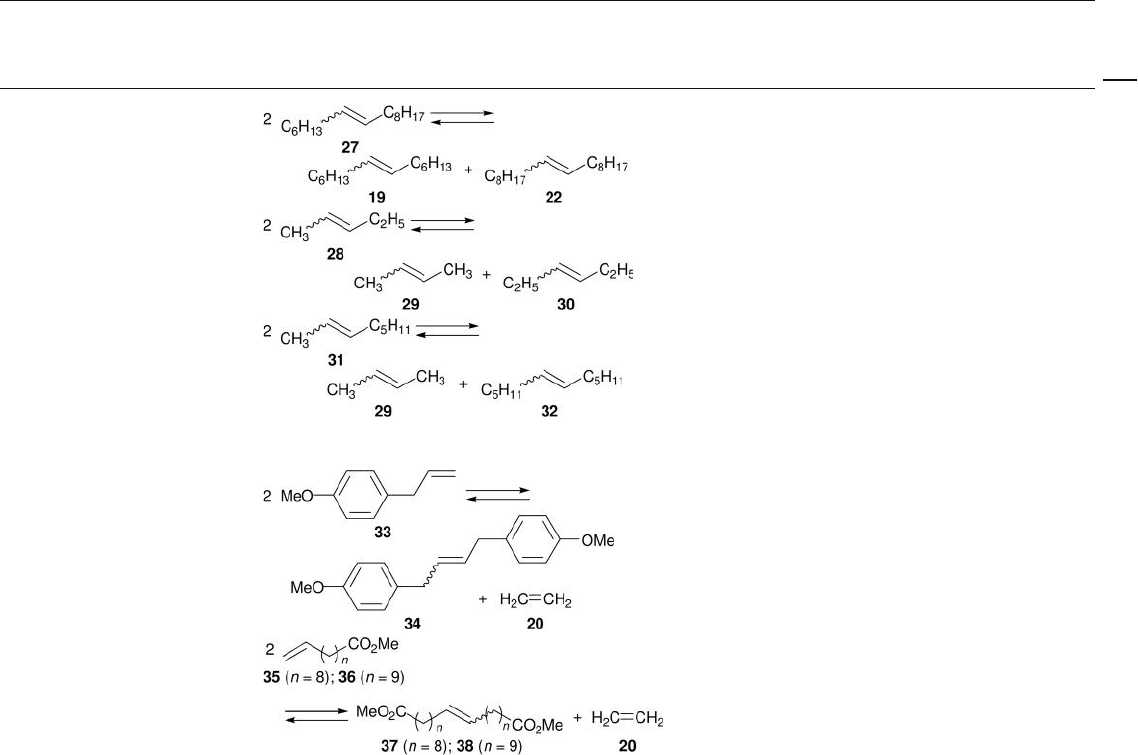
V. Olefi n metathesis
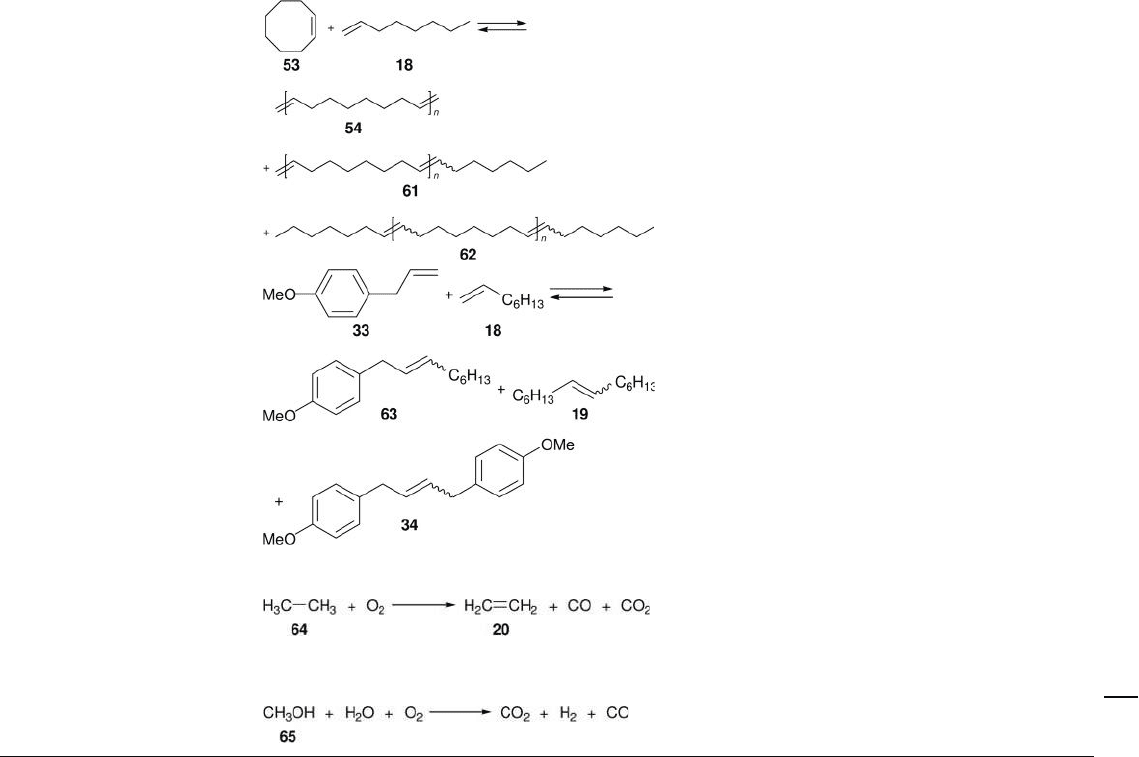
A. Terminal hydrocarbon olefi ns
R e
2
O
7
/ meso Al
2
O
3
Batch; n - heptane; – 1 ° C; N
2
Batch; neat; 40 or 60 ° C; Ar
[27 – 29]
MTO/ZnCl
2
// meso Al
2
O
3
Batch; CH
2
Cl
2
; rt; N
2
[30]
R e
2
O
7
/ meso Al
2
O
3
Batch; neat; 25, 40, or
60 ° C; Ar
[28 – 31]
R e
2
O
7
/ meso Al
2
O
3
Batch; neat; 60 ° C; Ar [28]
R e
2
O
7
/ meso Al
2
O
3
Batch; dodecane; 40 ° C; N
2
[32]
B. Internal hydrocarbon olefi ns
R e
2
O
7
/ meso Al
2
O
3
Batch; n - heptane; 50 ° C; N
2
[27]
504 15 Mesoporous Alumina: Synthesis, Characterization, and Catalysis
MTO/ZnCl
2
// meso Al
2
O
3
Batch; CH
2
Cl
2
; rt; N
2
[30]
R e
2
O
7
/ meso Al
2
O
3
Batch; neat; 25 ° C; Ar [29]
R e
2
O
7
/ meso Al
2
O
3
Batch; neat; 40 ° C; Ar [29]
C. Terminal functionalized olefi ns
R e
2
O
7
/ meso Al
2
O
3
+ Me
4
Sn
Batch; toluene or decane;
25 ° C; Ar
[31]
MTO/ZnCl
2
// meso Al
2
O
3
Batch; CH
2
Cl
2
; rt; N
2
[30]
Catalyst
a
Reaction equation Conditions (Reactor type;
solvent; reaction
temperature; atmosphere)
Reference(s)
Table 15.2 Continued.
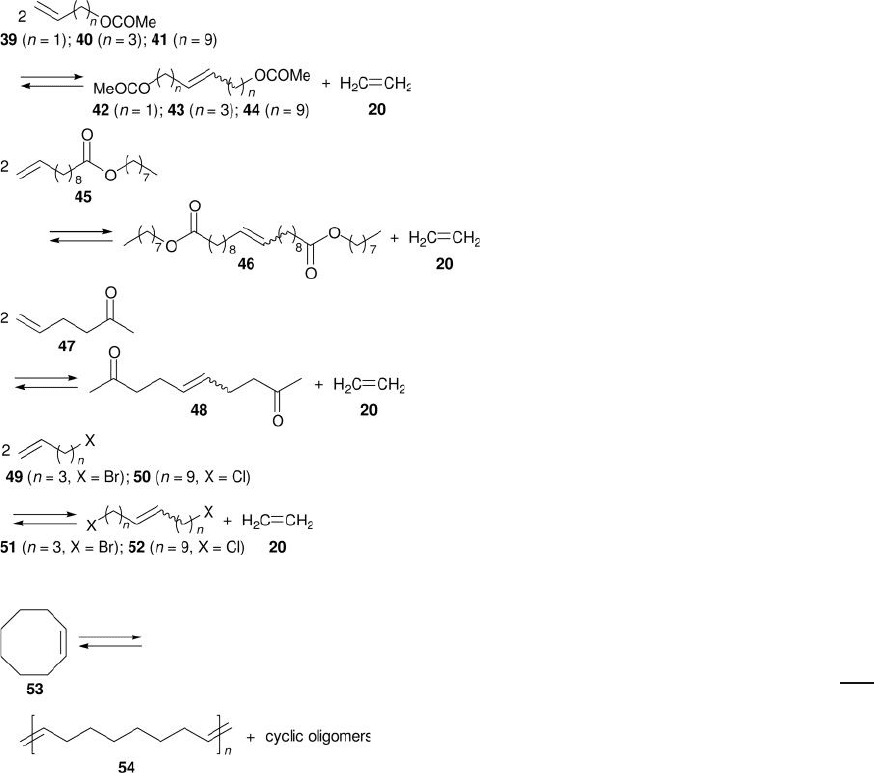
15.3 Mesoporous Alumina in Heterogeneous Catalysis 505
MTO/ZnCl
2
// meso Al
2
O
3
Batch; CH
2
Cl
2
; rt; N
2
[30]
MTO/ZnCl
2
// meso Al
2
O
3
Batch; CH
2
Cl
2
; rt; N
2
[30]
MTO/ZnCl
2
// meso Al
2
O
3
Batch; CH
2
Cl
2
; rt; N
2
[30]
R e
2
O
7
/ meso Al
2
O
3
Batch; CH
2
Cl
2
; rt; N
2
[30]
D. Ring - opening metathesis polymerization ( ROMP )
R e
2
O
7
/ meso Al
2
O
3
Batch; toluene; 40 ° C; Ar [29]
506 15 Mesoporous Alumina: Synthesis, Characterization, and Catalysis
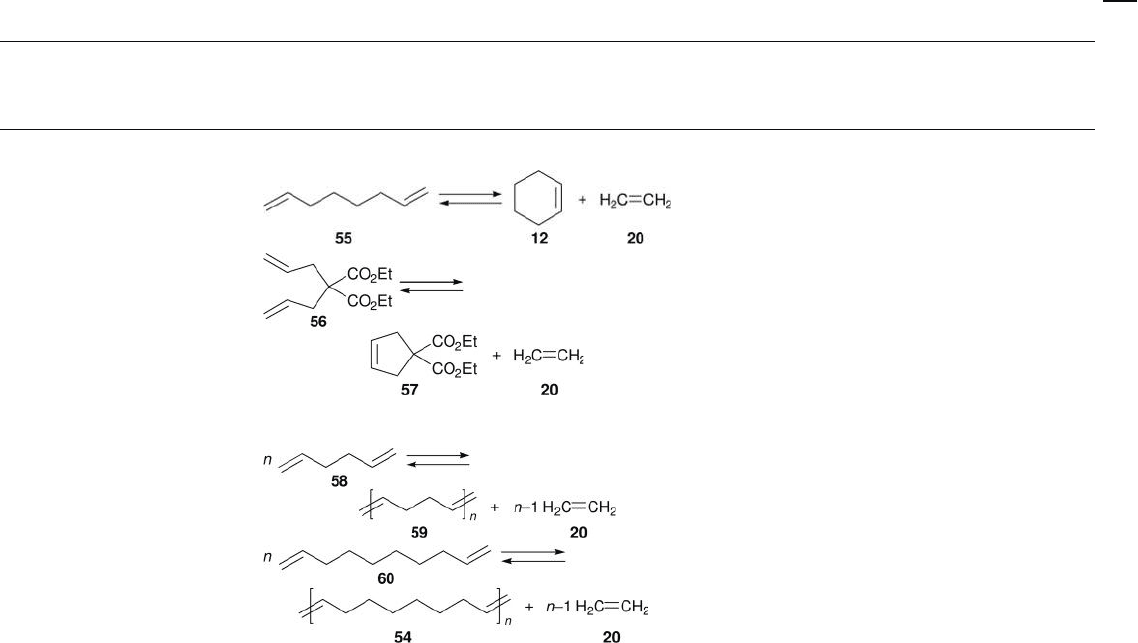
E. Ring - closing metathesis ( RCM )
R e
2
O
7
/ meso Al
2
O
3
Batch; neat or dodecane;
40 ° C; Ar
[29]
R e
2
O
7
/ meso Al
2
O
3
+ Me
4
Sn
Batch; toluene; 0 – 40 ° C; Ar [31]
F. Polymerization of terminal dienes
R e
2
O
7
/ meso Al
2
O
3
Batch; toluene; 25 ° C; Ar [29]
R e
2
O
7
/ meso Al
2
O
3
Batch; toluene; 40 ° C; Ar [29]
Catalyst
a
Reaction equation Conditions (Reactor type;
solvent; reaction
temperature; atmosphere)
Reference(s)
Table 15.2 Continued.
15.3 Mesoporous Alumina in Heterogeneous Catalysis 507
G. Cross metathesis
R e
2
O
7
/ meso Al
2
O
3
Batch; neat; 40 ° C; Ar [29]
R e
2
O
7
/ meso Al
2
O
3
+ Me
4
Sn
Batch; toluene; 40 ° C; Ar [31]
VI. Oxidative dehydrogenation
V / meso Al
2
O
3
c
Mo – V/ meso Al
2
O
3
c
Continuous fl ow; 520 –
600 ° C; atmospheric
pressure
[33, 34]
VII. Oxidative steam reforming of methanol
Pd – Zn/ meso Al
2
O
3
c
Continuous fl ow;
100 – 400 ° C:
[35]
a meso Al
2
O
3
represents mesoporous alumina.
b Products were not reported.
c Stoichiometries are not considered due to the complex reaction mechanism.
rt = room temperature.

508 15 Mesoporous Alumina: Synthesis, Characterization, and Catalysis
15.3.1
Base - Catalyzed Reactions
Thermally activated alumina has long been recognized as a solid base catalyst [7b] .
In fact, studies of the use of mesoporous alumina as a solid base catalyst were
initiated by Seki and Onaka in their attempt to develop heterogeneous basic cataly-
sis in supercritical CO
2
( scCO
2
). These authors found that mesoporous alumina
synthesized in a similar manner to L
A383
(see Table 15.1 , II) and activated at 500 ° C
under vacuum (10
− 4
Torr) exhibited a strong base catalysis for the Tishchenko
reaction, even in the Lewis acidic medium of scCO
2
(Table 15.2 , I, A) [18] . In addi-
tion, the activity could be increased by introducing
SO
4
2−
ions into the alumina
framework. Conventional γ - Al
2
O
3
and CaO, in contrast, showed quite low activities
in scCO
2
, even though they were very active in benzene as solvent under nitrogen
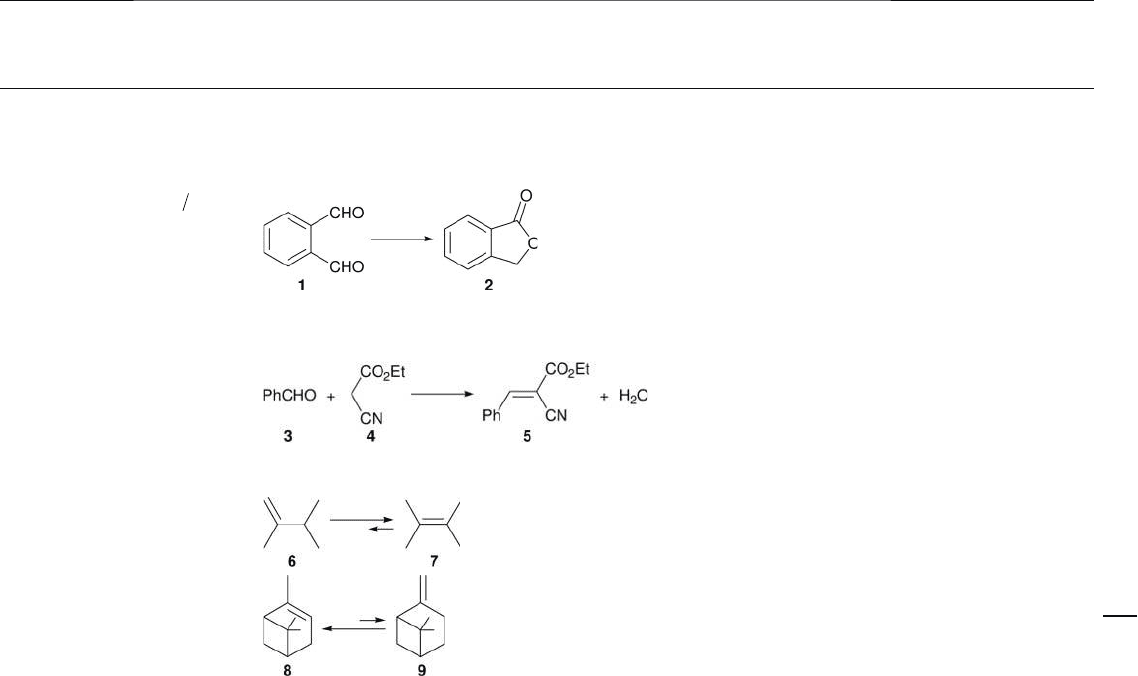
atmosphere. The phthalide 2 formation over the sulfated mesoporous alumina was
accelerated remarkably by adding a small amount of tetrahydrofuran ( THF ) as a
cosolvent, whereas the addition of acetic acid (or even much less - acidic methanol)
led to a fatal deactivation of the catalyst due to strong poisoning. The former result
was attributed to the improvement of solubility of phthalaldehyde 1 in scCO
2
,
while the latter result suggested that the strong base sites on sulfated mesoporous
alumina had promoted the reaction in scCO
2
. The infrared ( IR ) spectroscopy of
adsorbed pyrrole implied that the average strength of base sites on sulfated mes-
oporous alumina was weaker than on conventional γ - Al
2
O
3
. However, the poison-
ing by methanol indicated that it still had a small number of strong base sites that
functioned even in scCO
2
. It was concluded that the CO
2
molecules adsorbed onto
the sulfated mesoporous alumina were less stable compared to those on conven-
tional γ - Al
2
O
3
, due to the weaker surface basicity of the former, and thus could
offer the active strong base sites to 1 much more easily through the surface diffu-
sion, which in turn made a noticeable difference in activity.
In a subsequent study, the same authors reported that mesoporous alumina also
exhibited a higher activity for the Knoevenagel reaction between benzaldehyde 3
and ethyl cyanoacetate 4 compared to conventional γ - Al
2
O
3
in scCO
2
(Table 15.2 ,
I, B) [19] . Although the surface area of the mesoporous alumina employed was
2.7 - fold larger than that of conventional form, the numbers of active base sites
on the aluminas were almost equal according to the results of temperature -
programmed desorption ( TPD ) of CO
2
. This indicated that the density of active
base sites was much smaller for mesoporous alumina than for conventional γ -
Al
2
O
3
. The authors thus suggested that the surface diffusion of CO
2
took place
smoothly on mesoporous alumina owing to the large vacancy around the sites,
while the CO
2
molecules adsorbed on the active base sites of conventional γ - Al
2
O
3
were jam - packed, thus retarding the surface diffusion with each other. As free
active base sites should appear by moving the adsorbed CO
2
, it was concluded that
the difference in facility of the surface diffusion of CO
2
accounted for the differ-
ence in activity between the two aluminas. In the same study, a careful examina-
tion was also made of the CO
2
- TPD in the high temperature range of 527 – 1000 ° C.
The results implied that mesoporous alumina had a small number of very strong
