Geckeler K.E., Nishide H. (Eds.) Advanced Nanomaterials
Подождите немного. Документ загружается.

15.2 Synthesis of Mesoporous Alumina 489
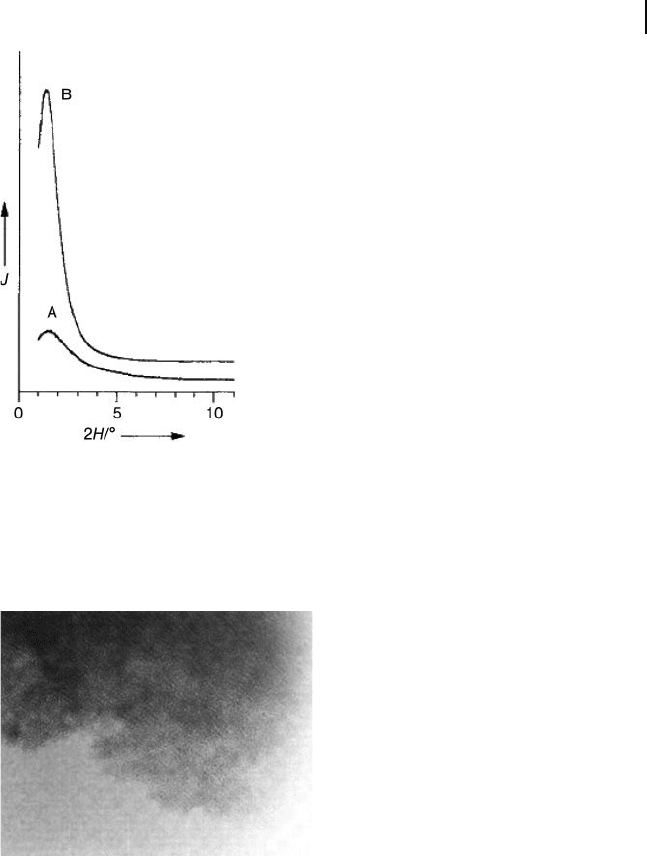
Figure 15.3 Powder X - ray diffraction patterns of the MSU - 3
alumina prepared with a surfactant of Pluronic
®
L64. The
intensity I is in arbitrary units. Spectrum A represents the
as - synthesized sample after air drying at room temperature
for 16 h. Spectrum B represents the sample calcined at 500 ° C
in air for 6 h. Reprinted with permission from Ref. [5a] .
Figure 15.4 Transmission electron microscopy image of the
MSU - 1 alumina prepared with a surfactant of Tergitol
®
15 - S - 9
and calcined at 500 ° C. Scale bar = 60 nm. Reprinted with
permission from Ref. [5a] .
basal spacings, and wormhole TEM images of the rare earth - stabilized MSU - X
aluminas were equivalent to those observed for the pure MSU - X aluminas, which
demonstrated that the incorporation of rare earth ions had not altered the original
mesopore structures. However, the surface area after calcination at 500 or 600 ° C
was 35% higher for the 5 mol% Ce
3+
- stabilized MSU - 1 alumina than for the pure
MSU - 1 alumina. In addition, the 1 mol% La
3+
- stabilized mesostructures formed
490 15 Mesoporous Alumina: Synthesis, Characterization, and Catalysis
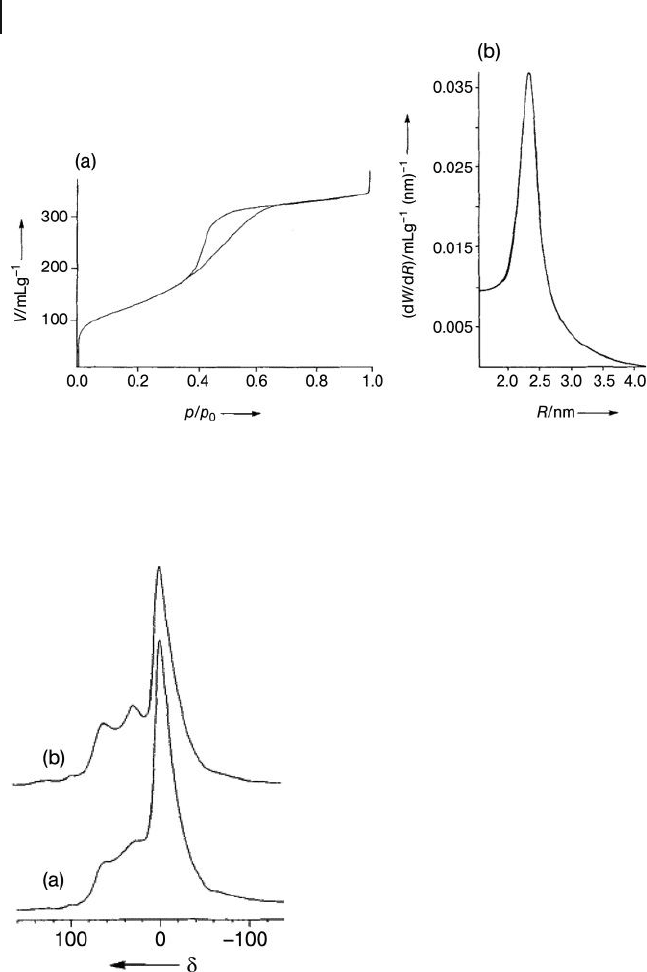
Figure 15.5 (a) N
2
adsorption – desorption isotherms for the
MSU - 3 alumina prepared with a surfactant of Pluronic
®
L64
and calcined at 500 ° C in air for 4 h; (b) BJH pore - size
distribution determined from the N
2
adsorption isotherm.
Reprinted with permission from Ref. [5a] .
Figure 15.6
27
Al MAS NMR spectra of the MSU - 3 alumina
prepared with a surfactant of Pluronic
®
L64. Spectrum A
represents the as - synthesized sample after air drying at room
temperature for 16 h. Spectrum B represents the sample
calcined at 500 ° C in air for 4 h. The chemical shifts are
referenced to external [Al(H
2
O)
6
]
3+
. Reprinted with permission
from Ref. [5a] .

15.2 Synthesis of Mesoporous Alumina 491
by the Pluronic surfactants also exhibited high surface areas after calcination
at 500 ° C. The authors suggested that the incorporation of rare earth cations
into the oxide framework took place during the assembly process of the mesostruc-
tures (but not during the calcination process), and that the site substitution of
aluminum cations by rare earth cations reduces the lability of the oxide framework.
These successes in increasing the thermal stability should greatly extend the
potential applications of the MSU - X aluminas to catalytic reactions. Gonz á lez -
Pe ñ a et al . later reported that thermally stable mesoporous aluminas could also be
obtained by adding dipropylamine during formation of the surfactant – alumina
mesophase [5f] .
The synthesis of γ - Al
2
O
3
with mesostructured forms (denoted MSU - γ ) was also
accomplished with the neutral surfactants by the group led by Pinnavaia [5i] . The
key to synthesizing these mesostructured aluminas composed of γ - Al
2
O
3
walls was
the formation of a mesostructured surfactant/boehmite precursor (denoted MSU -
S/B). The MSU - S/B (the structure of which was elucidated by XRD) could be
formed by the hydrolysis of [Al
13
O
4
(OH)
24
(H
2
O)
12
]Cl
7
, AlCl
3
, or Al(O - sec - Bu)
3
in
the presence of a PEO/PPO copolymer surfactant such as Pluoronic
®
P84, P123,
and L64 with a formula of [PEO]
19
[PPO]
39
[PEO]
19
, [PEO]
20
[PPO]
69
[PEO]
20
, and
[PEO]
13
[PPO]
30
[PEO]
13
, respectively. Calcination of the MSU - S/B precursors at
550 ° C afforded the MSU - γ aluminas, and their γ - Al
2
O
3
walls were well - character-
ized by XRD, TEM, electron diffraction ( ED ), and
27
Al MAS NMR analysis. On the
other hand, the XRD patterns in the small - angle region, N
2
adsorption – desorption
isotherms, and BJH pore - size distributions of the MSU - γ aluminas were those
typically observed for mesoporous materials. A TEM image of the MSU - γ alumina
obtained through the hydrolysis of Al(O - sec - Bu)
3
in 2 - butanol as solvent indicated
that the nanoparticles were assembled into a scaffold - like array, whereas for those
aluminas prepared from the Al
13
oligomer and AlCl
3
in aqueous media, parallel
arrays were observed which gave a hierarchical, sheet - like morphology. The MSU - γ
alumina from the alkoxide had thicker and longer nanoparticles and a larger pore
size and pore volume compared to those from the other aluminum sauces. The
pore size of the MSU - γ aluminas was also correlated with the size of surfactant,
indicative of the presence of the surfactant - induced assembly process of nanopar-
ticles during the synthesis.
Zhao et al . investigated the effect of the addition of poly(ethylene glycol) ( PEG )
on the traditional precipitation synthesis using aluminum nitrate or sulfate and
ammonium carbonate as an aluminum source and a precipitator, respectively
[5m] . PEG 1540 was fi rst mixed with an aqueous solution of aluminum nitrate or
sulfate, followed by the slow addition of ammonium carbonate, an adjustment of
the pH of the mixture using ammonia, and aging. Subsequent fi ltration, washing
with water, and drying yielded the as - made material, which was calcined at 550 ° C
in air to give the mesoporous alumina denoted DL12. A comparison of the
TEM image of DL12 with that of the alumina synthesized in the absence of PEG
showed that both materials exhibited a similar wormhole channel motif without
any long - range order in their pore structure. The authors concluded that the PEG
had not acted as a template in the synthesis process, and that the mesophase of

492 15 Mesoporous Alumina: Synthesis, Characterization, and Catalysis
DL12 was formed by a self - assembly process of the inorganic precursor, which
was more strongly affected by the ammonium carbonate precipitator. The main
role of PEG as a surfactant in the synthesis was to create a slight enlargement of
the mesopore size.
15.2.2.2 Anionic Surfactant Templating
The anionic surfactant templating mechanism involves the coordination of nega-
tively charged surfactant molecules with aluminum sites on freshly formed alu-
minum hydroxides, as well as cylindrical assemblies of the surfactant alkyl groups
to form the corresponding mesophases. Anionic surfactants of types RCOO
−
,
RXSO
3
–
−
(R = long alkyl chain; X = O, C
6
H
4
, CH
2
), and ROPO
3
–
2
−
are plausible
candidates as structure - directing agents, although to date the successful synthesis
(including complete removal of the surfactant) has only been achieved with car-
boxylic acid surfactants.
Vaudry and Davis, in 1996, reported that carboxylic acids with long alkyl chains
could be used as templates for the alumina mesostructure assemblies [5b] . The
important parameters were the type of solvent, the synthesis temperature, and
the thermal history of the as - made material (i.e., temperature and atmosphere
for calcination), in addition to the type of alkyl group in the templates. Branched
or normal carboxylic acids from C
3
to C
18
were found to afford mesophases, while
suitable solvents were branched or normal alcohols, from C
1
to C
9
. The best
mesoporous alumina (denoted L
A383
) was obtained by hydrolyzing aluminum sec -
butoxide in 1 - propanol solvent under the infl uence of lauric acid (C
11
H
23
CO
2
H),
followed by heating at 110 ° C in a glass jar under static conditions. The solid was
then fi ltered, washed with ethanol, and dried at room temperature to give the as -
synthesized material, which was subsequently calcined at 430 ° C to give the L
A383
alumina. An N
2
adsorption – desorption isotherm and a BJH pore - size distribution
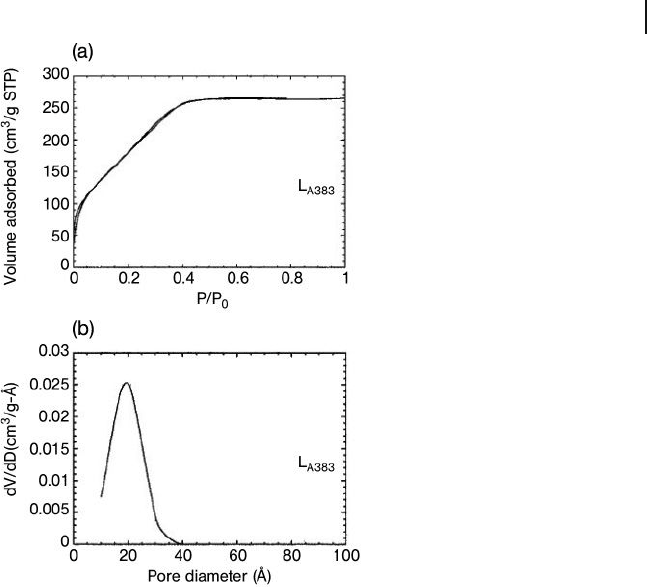
of this alumina are shown in Figure 15.7 .
The isotherm has no hysteresis loop and the distribution is centered at 1.9 nm.
It was concluded, from the shape of distribution and the low - pressure hydrocarbon
adsorption data (which showed that there was minimal difference between n -
heptane uptake and neopentane uptake) that the mesoporous alumina L
A383
pos-
sessed neither “ zeolitic ” micropores nor pores larger than 4.0 nm. Characterization
of the L
A383
alumina by XRD also showed a diffraction line in the small - angle
region, which was attributed to the regular mesoporosity (Figure 15.8 ).
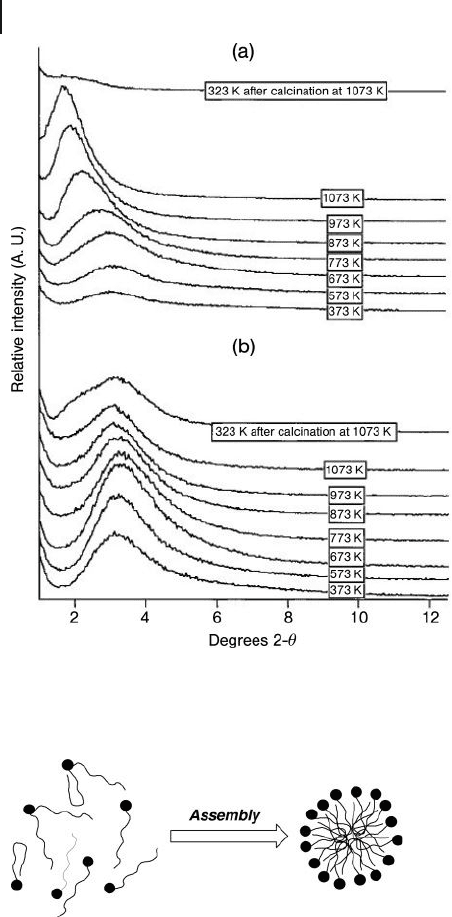
In addition, these fi gures show that the alumina is stable up to 800 ° C, even
under an air/water atmosphere. The shift to a higher d - spacing was, however,
striking for the calcination in the presence of water, and indicated that sintering
had taken place with increasing Al – OH groups to collapse the mesoporous struc-
ture. The authors referred to a mechanism of the alumina mesophase formation,
in which very small AlO(OH) clusters with coordinated carboxylate ligands were
fi rst formed, followed by their rapid aggregation to produce the alumina mes-
ophase (Scheme 15.1 ).
It should be noted that the alkyl chain of coordinated carboxylate is not in the
straight - form but rather in bent - form. This is because the observed d - spacing of
15.2 Synthesis of Mesoporous Alumina 493
Figure 15.7 (a) N
2
adsorption – desorption isotherm and
(b) BJH pore - size distribution of the L
A383
alumina calcined
at 430 ° C for 1.5 h. Reprinted with permission from Ref. [5b] .
the as - made material (2.9 nm) is much shorter than the sum of the estimated
aggregate diameter, assuming a straight alkyl - chain model (3.1 nm) and the wall
average width (ca. 1.0 nm). On the other hand, the diameter assuming a curved
alkyl - chain model (1.9 nm) is equal to the d - spacing by adding the wall width. The
minimal effect of surfactant size on pore diameter can be seen more clearly in the
calcined samples (see Table 15.1 , II). The factor which controls the porous proper-
ties involving pore diameter is the extent of dehydration of the inorganic walls by
calcination, which should also prevent pore - size control by the choice of surfactant
size. Dehydration through calcination also drastically lowered the local order and
symmetry of the aluminum, increasing the amount of four - and fi ve - fold coordi-
nated aluminum at the expense of the six - fold type, as demonstrated by
27
Al MAS
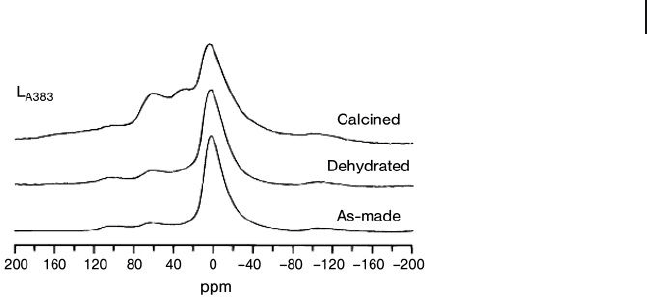
NMR studies (Figure 15.9 ; see Section 15.2.1.2 for the peak assignments).
The synthesis of mesoporous alumina using sulfate or sulfonate surfactants has
been attempted by several groups, but has usually proved unsuccessful owing to
the low thermal stability and diffi culties of surfactant removal. Vaudry and Davis
added sodium dodecylbenzenesulfonate dissolved in formamide to an aqueous
solution of hydrolyzed aluminum sec - butoxide, followed by aging of the mixture
494 15 Mesoporous Alumina: Synthesis, Characterization, and Catalysis
Figure 15.8 Variation of the X - ray diffraction patterns with
increasing temperature under (a) an air/water atmosphere
and (b) an air atmosphere. Reprinted with permission from
Ref. [5b] .
Scheme 15.1 Mechanism of the mesophase formation as
proposed by Vaudry and Davis [5b] . The fi lled circles represent
small clusters of AlO(OH), while the lines attached to the
circles represent coordinated carboxylates.
at 110 ° C for 2 days [5b] . The resultant solid was fi ltered, washed with deionized
water, and dried at room temperature. The as - made material thus obtained exhib-
ited a low thermal stability, losing the XRD line at a high d - spacing above a calci-
nation temperature of 510 ° C. In addition, the calcined solid still contained
15.2 Synthesis of Mesoporous Alumina 495
Figure 15.9
27
Al MAS NMR spectra of the as - made,
dehydrated, and calcined solid L
A383
. Reprinted with
permission from Ref. [5b] .
substantial amounts of sulfur components that might have reduced the stability
to high - temperature treatment. The sulfonate surfactant also could not be removed
from the as - synthesized material by extraction with HCl/ethanol, HCl/water, or
AcOH/water solutions.
The use of sodium dodecyl sulfate as a surfactant template was reported by
Yada and coworkers [10] , who noted that an aluminum - based dodecyl sulfate
mesophase possessing a hexagonal structure such as MCM - 41 could be synthe-
sized by the homogeneous precipitation method, using aluminum nitrate nonahy-
drate and urea as an aluminum source and a pH - controlling agent, respectively.
The as - made solid obtained from the mixture, of which the components were
Al : surfactant : urea : water (1 : 2 : 30 : 60), exhibited an XRD line at a high d - spacing
even after calcination at 600 ° C, if it was heated up at a slow rate of 1 ° C min
− 1
.
However, the content of sulfur impurities in the calcined sample was not reported,
and thus the purity remained doubtful. In addition, the surface area – which was
in the range 93 to 365 m
2
g
− 1
depending on the calcination conditions – was much
smaller than that of the mesoporous alumina synthesized with carboxylic acid
surfactants.
The above two examples indicate that sulfate and sulfonate surfactants are not
appropriate as structure - directing agents if the aim is to obtain pure mesoporous
alumina with a high thermal stability. However, this approach may open the pos-
sibility that sulfated mesoporous alumina, which is an important material in the
chemistry of strong acid catalysis, could be synthesized via a one - step process by
using sulfate or sulfonate surfactants.
15.2.2.3 Cationic Surfactant Templating
Cabrera et al. , in 1999, fi rst reported mesoporous alumina (denoted ICMUV - 1)
synthesized with a cationic surfactant template [5d] . A mixture of aluminum
sec - butoxide and triethanolamine was added to cetyltrimethylammonium bromide
( CTAB ) dissolved in water, followed by aging of the resultant solution. The aged
solid was fi ltered, washed with ethanol, dried, and calcined at 500 ° C in air to give
496 15 Mesoporous Alumina: Synthesis, Characterization, and Catalysis
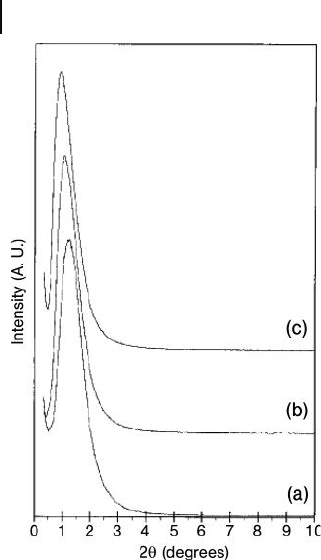
Figure 15.10 X - ray diffraction patterns of calcined ICMUV - 1
aluminas synthesized with water/triethanolamine ratios of 7.4
(pattern a, d = 6.9 nm), 22.1 (pattern b, d = 7.9 nm), and 44.5
(pattern c, d = 9.5 nm). Reprinted with permission from
Ref. [5d] .
the ICMUV - 1 alumina. The pore diameter and surface area of the alumina was
found to be tailored by changing the Al : water : triethanolamine molar ratio in the
solution from 2 : 111 : 15 to 2 : 195 : 4, while the Al : surfactant ratio was held at 2 : 1
for all the syntheses. Increasing the amount of water led to a monotonous increase
in pore diameter, from 3.3 to 6.0 nm, whilst monotonously decreasing the surface
area from 340 to 250 m
2
g
− 1
. The XRD patterns of some samples are shown in
Figure 15.10 .
Each sample exhibited an XRD line with a high d - spacing that was attributed to
the organized mesopore structures, and these peaks remained unaltered with
increasing temperature up to 900 ° C, indicative of the high thermal stability of the
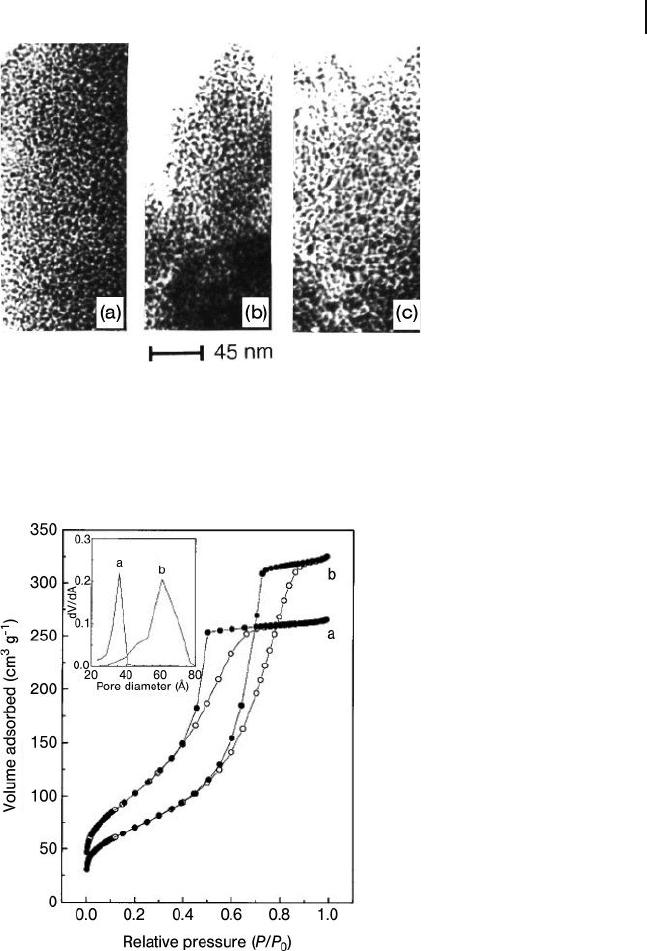
mesoporous aluminas. The TEM images of ICMUV - 1 aluminas showed the worm-
hole - like channel motif, without any apparent order in the pore arrangement
(Figure 15.11 ).
The increase in pore diameter with an increase in water : triethanolamine ratio
can be clearly seen in the images. The ICMUV - 1 aluminas were also characterized
using the N
2
adsorption – desorption method (Figure 15.12 ). The hysteresis loop in
the isotherm indicates the presence of ink - bottle - type pores, while the volume at
15.2 Synthesis of Mesoporous Alumina 497
Figure 15.11 Transmission electron microscopy images of
ICMUV - 1 aluminas showing the wormhole - like channel motif.
The aluminas were synthesized with water/triethanolamine
ratios of 7.4 (a), 22.1 (b), and 44.5 (c). Reprinted with
permission from Ref. [5d] .
Figure 15.12 N
2
adsorption – desorption isotherms of
ICMUV - 1 aluminas prepared with water : triethanolamine
ratios of 7.4 : 1 (isotherm a) and 44.5 : 1 (isotherm b). The
corresponding BJH pore - size distributions are shown in the
inset. Reprinted with permission from Ref. [5d] .

498 15 Mesoporous Alumina: Synthesis, Characterization, and Catalysis
the saturation vapor pressure demonstrates the absence of any macroporosity in
the aluminas. It is interesting to note that the pore wall widths were approximately
3.5 nm, regardless of the water : triethanolamine ratio. Unfortunately, the contents
of nitrogen and bromide impurities in the calcined solids were not reported.
The authors suggested that formation of the (HOCH
2
CH
2
)
3
N – Al[OCH(CH
3
)
CH
2
CH
3
]
3
complex lowered the rate of hydrolysis, which allows the partially hydro-
lyzed inorganic moieties to be organized together with the surfactant molecules.
In contrast, without triethanolamine, the hydrolysis of aluminum sec - butoxide
proceeded too rapidly to be infl uenced by the surfactant, yielding the lamellar - type
aluminum hydroxides. The fact that an increasing water : triethanolamine ratio
increased the pore diameter should be attributed to a shift towards the formation
of lamellar moieties.
Ž ilkov á and C
ˇ
ejka applied 1 - methyl - 3 - octylimidazolium chloride to the synthesis
of mesoporous alumina [5n] . To an aqueous solution of aluminum chlorohydrate
and the cationic surfactant was added ammonium hydroxide solution drop - wise,
followed by stirring, hydrothermal treatment, fi ltration of the precipitate, and
washing the recovered solid with ethanol. The as - synthesized sample thus obtained
was calcined at 560 ° C with a temperature ramp of 1 ° C min
− 1
to give the mesopo-
rous alumina (denoted I – III, depending on the Al : surfactant molar ratio). The
effect of modifying the Al : surfactant molar ratio on the structural parameters was
investigated by comparing the results of N
2
adsorption – desorption of the calcined
solids. The aluminas I and II prepared with a respective Al : surfactant ratio of 5.8
and 3.3 exhibited similar values of surface area, mesopore volume, and pore diam-
eter, but increasing the ratio to 12.0 (alumina III) drastically decreased the surface
area and increased the pore volume and pore diameter remarkably. The latter
exhibited an N
2
adsorption – desorption isotherm that is typically observed for dis-
persed materials composed of small particles, and also showed a broad pore - size
distribution without maximum, whereas a single XRD line at a low - angle region
was observed for this alumina. Thus, the authors referred to the risk of judging
the mesoporosity of materials based on XRD data alone. The aluminas I and II,
on the other hand, exhibited not only the XRD lines but also the BJH pore - size
distributions, with clear maxima centered at 3.8 and 3.9 nm, respectively. The XRD
peaks at higher - angle regions indicated that alumina I was composed of γ - Al
2
O
3
.
A wormhole framework structure was observed for the alumina I by TEM. The S
+
X
−
I
+
- type interaction, which has been discussed in detail elsewhere [6] , was pro-
posed for the alumina mesophase formation, where S
+
, X
−
, and I
+
represent the
CHN
12 23 2
+
, Cl
−
, and cationic aluminum species, respectively.
15.2.2.4 Nonsurfactant Templating
Shan et al. succeeded in synthesizing mesoporous alumina with a high thermal
stability by utilizing tetraethylene glycol as a nonsurfactant template [5j] . A con-
trolled amount of water was added stepwise to a mixture of aluminum iso propoxide
and tetraethylene glycol dissolved in ethanol and 2 - propanol to produce a suspen-
sion, which was subsequently aged at room temperature and then dried in air at
60 – 100 ° C. The solid gel thus obtained was heated at 120 – 190 ° C in an autoclave
