Geckeler K.E., Nishide H. (Eds.) Advanced Nanomaterials
Подождите немного. Документ загружается.


Amphiphilic Poly(Oxyalkylene) - Amines Interacting with Layered
Clays: Intercalation, Exfoliation, and New Applications
Jiang - Jen Lin , Ying - Nan Chan , and Wen - Hsin Chang
459
Advanced Nanomaterials. Edited by Kurt E. Geckeler and Hiroyuki Nishide
Copyright © 2010 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim
ISBN: 978-3-527-31794-3
14
14.1
Introduction
During recent years, organic – inorganic hybrid materials have attracted a great deal
of research interest due to their promising industrial applications [1 – 3] . The suc-
cessful development of Nylon - 6/ montmorillonite ( MMT ) nanocomposites by
the Toyota research group [4, 5] , refl ects the importance of utilizing mineral clays
for improving polymer properties [6 – 8] , including fi re retardation and gas barrier.
One of the crucial issues for preparing these advanced polymers is to overcome
the problem of incompatibility between the hydrophilic smectite - clays and hydro-
phobic polymers. Organic modifi cation is the common method to convert
the hydrophilic clay into an organophile [9 – 13] . Fine dispersion of the silicate
particles in polymer matrices was then achieved for a variety of hydrophobic nano-
composites including polypropylene [14] , polystyrene ( PS ) [15] , polyurethane [16] ,
polyester [17] , epoxy [18 – 20] , and polyimide [21] .
In the past, many reviews have been devoted to the subject of clay nanocompos-
ites [1 – 5, 21 – 25] . Signifi cant progress in polymer engineering and the limitations
of polymer compatibility to the nanoscale clays in intercalated and exfoliated forms
are already well reviewed. Recent research developments on utilizing the layered
silicate clays have also diversifi ed into the areas of biotechnology [26] , such as the
use of anionic clays for gene therapy. Actually, recent advances in biomedical
applications involve different types of nanomaterial, which can be generally clas-
sifi ed into three categories according to their geometric dimensions: spherical
(e.g., silver and titanium oxide particles) [27, 28] ; fi bril - shape (e.g., carbon nano-
tube) [29] ; and sheet - like (e.g., natural and synthetic clays) nanomaterials. With at
least one dimension that falls into the nanometer scale, all of these inorganic
materials possess a high - aspect ratio surface area relative to their weight. For the
sheet - like clays, each thin layer platelet with a thickness of approximately 1 nm
(almost at the molecular level) and the lateral dimension of approximately 50 nm
to several micrometers provides an extremely large specifi c surface ( > 500 m
2
g
− 1
).
Hence, the primary structure of the thin - layer clays is unique with respect to its

460 14 Amphiphilic Poly(Oxyalkylene)-Amines Interacting with Layered Clays
geometric shape as well as surface ionic charge, which could be important
when interacting with biological materials such as proteins, nucleic acids, and
microorganisms.
In this chapter, we review the synthetic aspects of organic modifi cations of
smectite clays, with particular attention being paid to reports on the uses of various
intercalation agents for the polymer – clay composite applications, without empha-
sizing their composite performance. Recent efforts on using high - molecular -
weight poly(oxyalkylene) - diamines to tailor the layered - silicate spacing is
particularly addressed [30 – 33] . The reaction profi le of the intercalation via ion
exchange by different equivalents of organic salt to clay exchange capacity, as well
as the unconventional mechanisms involving metal - ion chelation and hydrogen
bonding, are also reviewed. With the incorporation of hydrophobic amine - salts,
the resulting organoclays may exhibit unusual colloidal properties [34, 35] as well
as an ability to self - assemble into rigid - rod nanoarrays [36 – 38] . Furthermore, the
method of exfoliating the layered - silicate clays into random platelets has been
recently reported [39 – 41] . This process involved the use of polymeric amines via
zigzag conformation or phase inversion mechanisms for the platelet randomiza-
tion. The tailored organoclays are found to be suitable for embedding biomaterials
such as protein [42, 43] . The aim of this chapter is to summarize the literature
activities in clay utilization, with emphasis placed on the organic modifi cations
and the emerging research in the areas of biomedical applications.
14.2
Chemical Structures of Clays and Organic - Salt Modifi cations
14.2.1
Natural Clays and Synthetic Layered - Double - Hydroxide ( LDH )
Smectic clays are naturally abundant, with a well - characterized lamellar structure
of multiple inorganic plates, a high surface area, and ionic charges on the surface
[9 – 11] . The phyllosilicate clays of the 2 : 1 type, or smectites such as MMT, ben-
tonite, saponite, and hectorite, are conventionally utilized as catalysts [44 – 46] ,
adsorbents [47, 48] , metal - chelating agents [49] , fi llers for polymer composites
[1, 3, 21] , and so on. The generic structure is composed of multiple layers of
silicate/aluminum oxide, for example, with layers of two tetrahedron sheets sand-
wiching an edge - shared octahedral sheet [21] . Counter metal ions populate the
composition with variation in isomorphic substitution of silicon or aluminum by
divalent metal ions such as Mg
2+
, Ca
2+
, or Fe
2+
. These ionic charges are potentially
exchangeable through further ionic exchange with alkali metal ions such as Na
+
or Li
+
, as well as with organic ions. Although these layered silicates are hydrophilic
and swell in water, they often exist as aggregates in micrometer sizes from their
primary stack units. In the case of MMT, the primary stack structure possesses
multiple aluminosilicate plates of irregular polygonal shapes at average dimension
of ca. 100 nm × 100 nm × 1 nm for individual platelets [50] . For the ionic - exchange

14.2 Chemical Structures of Clays and Organic-Salt Modifi cations 461
reaction, the divalent counter - cations in most natural clays could be exchanged
into different ions, including Na
+
, Cu
2+
, Zn
2+
, Mg
2+
, Ca
2+
and an acidifi ed H
+
form
[51] . The replacement priority for these cations are: Al
3+
> C a
2+
> M g
2+
> K
+
=
NH
4
+
> N a
+
[46, 52] . According to this exchange order, organic quaternary ammo-
nium salts can replace Na
+
ions, but not with divalent cations in Mg
2+
- MMT and
Ca
2+
- MMT. Therefore, for most natural clays, the sodium ion exchange is neces-
sary to facilitate the subsequent organic ion intercalation.
Synthetic fl uorine mica , which structurally is similar to sodium tetrasilicic
micas, is prepared from the Na
2
SiF
6
treatment of talc at high temperature [53, 54] .
The synthetic mica (Na
+
- Mica) is water - dispersible and generally used as an inor-
ganic thickener. This synthetic fl uorinated mica has an average dimension of
300 – 1000 nm in 80 – 100 nm for MMT. Another class of synthetic clays, layered -
double - hydroxide d ( LDH s), can be prepared from the coprecipitation of inorganic
salts. The chemical structure is described as [Mg
6
Al
2
(OH)
16
]CO
3
· 4H
2
O] in the
example of magnesium/aluminum hydroxides. Various metal hydroxides, includ-
ing Ni, Cu, or Zn for divalent and Al, Cr, Fe, V, or Ga for trivalent metal ions, and
anions such as CO
3
2−
, Cl
−
, SO
4
2−
, NO
3
−
, or other various organic anions, have been
reported [55 – 58] . These LDHs are classifi ed as anionic clays that can be organically
modifi ed through an anionic - exchange reaction, using substances such as carboxy-
lic acids, anionic polymers, organic phosphoric acids, and so on [58] . These syn-
thetic clays may have various applications, including heterogeneous catalysts,
optical materials, biomimetic catalysts, separation agents, and DNA reservoirs
[59 – 63] . Recently, Mg – Al LDHs were incorporated with poly(oxypropylene) -
bis - amindocarboxylic acid salt s ( POP - acid ) to result in a wide basal spacing of
92 Å [64] . This wide spacing, as well as the introduction of a hydrophobic POP
backbone, may open up new applications for this class of anionic clays.
14.2.2
Low - Molecular - Weight Intercalating Agents and X - Ray Diffraction d - Spacing
The common strategy for utilizing smectite clays is to alter their inherent
hydrophilic nature so that they become hydrophobic and organically compatible
with polymers. For the synthesis of polymer – clay nanocomposites, organic oniums
such as alkyl ammonium salts [22] are commonly used to intercalate the layered
minerals. The resultant organoclays are then suitable for the consequent process
of melt - blending with polymers and in situ polymerization. For example, sodium
montmorillonite (Na
+
- MMT), consisting of sodium ions on the silicate surface
( ≡ Si – O
−
Na
+
), can be intercalated with organic onium salts. The quaternary alkyl
ammonium (R
4
N
+
X
−
) or alkyl phosphonium (R
4
P
+
X
−
) salts are the common inter-
calating agents because of their commercial availability. The incorporation of
organic intercalating agents also resulted in a silicate gallery expansion. For
example, the C
18
- alkyl quaternary salts may intercalate Na
+
- MMT, causing a layer
space expansion to 20 − 30 Å basal spacing from the pristine 12 Å clay gallery. It is
noteworthy that the same organic quaternary salt may not exchange with natural
clays with divalent counter ions such as M
2+
- MMT [65] (where M
2+
= Mg
2+
or Ca
2+
).

462 14 Amphiphilic Poly(Oxyalkylene)-Amines Interacting with Layered Clays
A large number of intercalating agents for modifying the cationic smectite clays
have been described, and it is possible to classify these according to their chemical
structures and organic functionalities (see Table 14.1 ). Low - molecular - weight alkyl
ammonium and phosphonium salts are the common intercalating agents, and
normally these may result in a widening of the basal spacing in the range of 13
to 50 Å . Thermally stable and reactive surfactant types of imidazolium salts (with
C
12
, C
16
and C
18
alkyl groups) were reported for the preparation of polystyrene/
MMT nanocomposites [93] . The general idea here is that the presence of alkyl
imidazolium may contribute to the thermal stability of the composites. Other
reactive cationic surfactants, such as vinylbenzyl dimethyldodecyl ammonium
chloride and surfactants with 2 - methacryloyl functionalities, have been reported
for poly(methylmethacrylate) ( PMMA ) and PS – clay nanocomposites. The purpose
of introducing a reactive functionality to the intercalating agent is to facilitate not
only a layer exfoliation but also fi ne dispersion in the polymer matrices.
14.3
Poly(Oxyalkylene) - Polyamine Salts as Intercalating Agents,
and Their Reaction Profi les
14.3.1
Poly(Oxyalkylene) - Polyamine Salts as Intercalating Agents
The uses of poly(oxyalkylene) - amines of M
w
2000 − 4000 g mol
− 1
for the intercalation
of Na
+
- MMT to prepare organoclays with a large d spacing was reported in 2001
[30] . Poly(oxyalkylene) - diamine s ( POA - diamine s) are commercially available poly-
ether amines that are produced from the amination of polyols such as polyethylene
glycols, polypropylene glycols, and their mixed poly(oxyethylene - oxypropylene) [99,
100] . Both, hydrophobic and hydrophilic types of POA - diamines, poly(oxypropylene) -
amine s ( POP - amine s) and poly(oxyethylene) - amine s ( POEamine s), respectively,
are available. By using the hydrophobic POP - diamines of 230, 400, 2000, and
4000 g mol
− 1
, Na
+
- MMT was intercalated into organoclays at varied X - ray diffraction
( XRD ) basal spacings (15.0, 19.4, 58.0, and 92.0 Å , respectively). The lamellar
expansion was found to be proportionally correlated with the POP molecular
weights, through the POP tethering with silicate surfaces or the ionic - exchange
reaction of the quaternary ammonium ions. Within the silicate confi nement,
the POP backbones may aggregate through a hydrophobic phase separation
and consequently stretch out the basal spacing of the silicate interlayer gallery
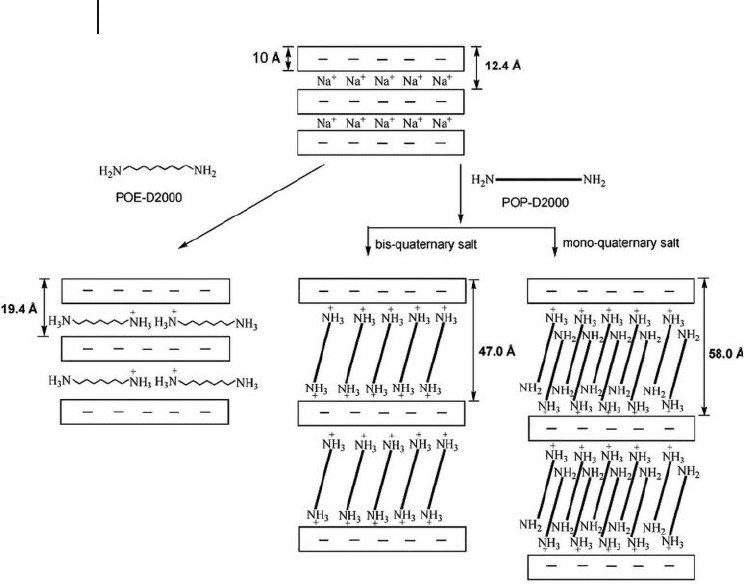
(Figure 14.1 ).
In contrast, the hydrophilic POE - diamine of M
w
2000 g mol
− 1
( POE2000 ) resulted
in a spacing of 19.4 Å . The conclusion has been drawn that the intercalating agent
requires a hydrophobic nature in order to expand the ionically charged platelets.
The hydrophilic POE backbones or the – (CH
2
CH
2
O)
x
– structure, resulted in
the organics associating tightly with the silicate surface. Only the hydrophobic
POP backbone – (CH
2
CH(CH
3
)O)
x
– has shown an ability to generate a new
“ supporting ” phase for widening the gallery space.

14.3 Poly(Oxyalkylene)-Polyamine Salts as Intercalating Agents, and Their Reaction Profi les 463
Table 14.1 Representatives of the various intercalating agents described in the literature.
Functionality Intercalating agent Clay XRD ( Å ) Reference(s)
POA - diamine
salt
Poly(oxypropylene) - diamine (2000 M
w
),
Poly(oxypropylene) - diamine (POP -
amines, 4000 M
w
),
Poly(oxypropylene) - diamine (POP -
amines, 5000 M
w
)
Li
+
-
fl uorohectorite,
Na
+
- MMT,
Na
+
- Mica
46 – 92 [12, 30, 35]
Polymeric
amine
Amine - terminated PS surfactants,
Amine - terminated butadiene
acrylonitrile copolymers
Na
+
- MMT 10 – 155 [66 – 68]
Alkyl - amine
salt
1 - Hexadecylamine and octadecylamine Na
+
- MMT 16 – 37 [69 – 76]
Amino acid 6 - Aminohexanoic acid,
12 - Aminododecainoic acid
Na
+
- MMT 17 – 49 [12, 77]
Acid C
18
carboxylic acids and other acids,
2 - Acrylamido - 2 - methyl - 1 -
propanesulfonic acid
Mg
2+
- or
Ca
2+
- MMT,
Na
+
- MMT
35,
Exfoliation
[65, 78 – 80]
Ammonium
salt
Hexadecyltrimethylammonium
bromide,
Dioctadecyldimethyl ammonium
bromide,
Vinylbenzyldimethyldodecylammonium
chloride,
Dialkyldimethylammonium salt from
hydrogenated tallow
Na
+
- MMT 19 – 40 [69, 70, 81 – 86]
Phosphonium
salts
10 - [3,5 - bis(methoxycarbonyl)phenoxy]
decyltriphenylphosphonium bromide
Dodecyltriphenyl phosphonium
bromide,
Hexadecyltributyl phosphonium
bromide,
Tetraoctyl phosphonium bromide
Na
+
- Mica,
Li
+
-
fl uorohectorite,
Na
+
- MMT
24 – 32 [72, 87 – 92]
Imidazolium
salt
1,2 - Dimethyl - 3 - (benzyl ethyl isobutyl
polyhedral oligomeric silsesquioxane)
imidazolium chloride ( DMIPOSS ),
Dioctadecyl imidazolium,
1,2 - Dimethyl - 3 - hexadecyl imidazolium,
vinyl - alkyl - imidazolium (C
12
, C
16
and
C
18
)
Na
+
- MMT 36 [93 – 96]
Stibonium
salt
Triphenylhexadecyl stibonium
trifl uoromethyl sulfonate
Na
+
- MMT 20 [97]
Poly(ethylene
glycol)
PEG400 vs. POP - amine
a
N a
+
- MMT 17.7 [98]
a POP - amines: poly(oxyalkylene) - amines of M
w
230 – 4000.
464 14 Amphiphilic Poly(Oxyalkylene)-Amines Interacting with Layered Clays
Figure 14.1 Schematic representation of Na
+
- MMT
intercalation by hydrophilic and hydrophobic
poly(oxyalkylene) - diamine salts of the same molecular
weight [31] .
It was noted that formation of the hydrophobic POP phase in the silicate gallery
could be understood by estimating the theoretical length of the fully stretched POP
backbone. Based on calculations of the bond lengths (1.54 Å for C – C and 1.43 Å
for C – O) and bond angles (109.6 ° and 112 ° , respectively), the theoretical length
(77 Å ) for POP2000 was longer than the value of 58.0 Å observed for those mole-
cules in confi nement. Apparently, the POPs were aggregated into hydrophobic
phase but not fully extended at the molecular level, nor arranged in a tilting ori-
entation in the gallery confi nement.
14.3.2
Critical Conformational Change in Confi nement During the Intercalating Profi le [31]
The intercalation profi le was studied by varying the equivalent ratios (from 0.2 to
1.0) of POP2000 salt to the clay CEC (120 mEq 100 g
− 1
). Critical intercalation points
were apparent, as shown in Figure 14.2 , for the clay expansion with respect to the
POP intercalants. Before the critical point, the basal spacing expansion was similar
(19.0 − 20.2 Å ) and in the range of amine addition, from 0.2 to 0.8 CEC equivalent.
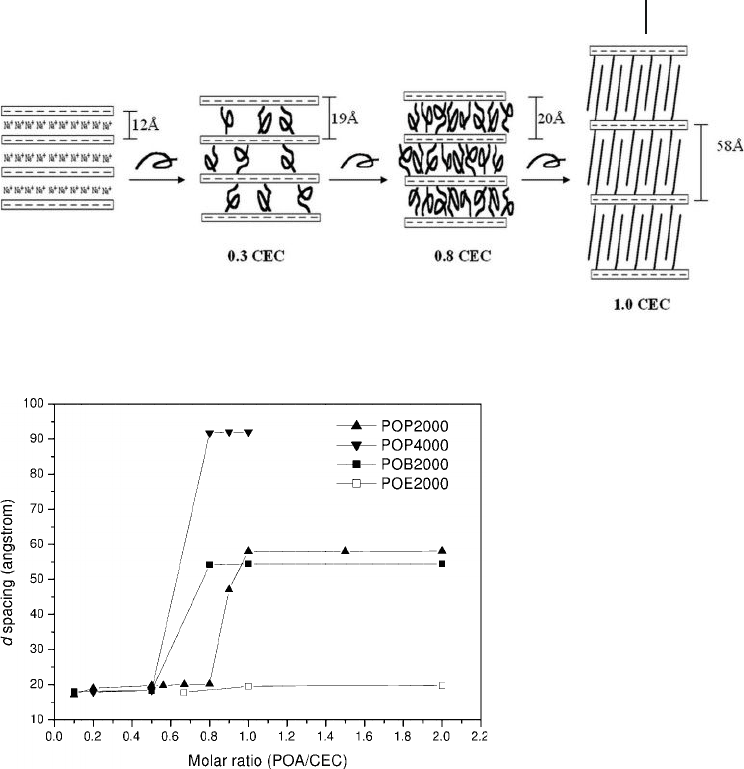
14.3 Poly(Oxyalkylene)-Polyamine Salts as Intercalating Agents, and Their Reaction Profi les 465
Figure 14.2 Schematic representation of POP - diamine
forming a hydrophobic phase in a clay gallery [31] .
Figure 14.3 Critical points of poly(oxyalkylene) - diamine salts
intercalating montmorillonite at different CEC ratios [31] .
At 0.8 CEC equivalent, the POP addition expanded the d spacing suddenly to
58 Å in the case of POP2000 intercalation.
The critical concentration for the basal spacing expansion is generalized
for several hydrophobic polyether - amines, as summarized in Figure 14.3 .
The POP4000 and poly(oxybutylene)diamine of 2000 g mol
− 1
M
w
( POB2000 )
showed a critical point for the intercalation (Figure 14.3 ,
䉲
and
䊏
, respectively).
The increases in basal spacing, from 20 Å to 92 Å for POP4000, and from 20 Å to
54 Å for POB2000, were observed and attributed to the molecular length,
POP4000 > POP2000 > POB2000. The existence of a critical concentration was
explained by the POP hydrophobic phase generated in the layer gallery. For the
POE - backbone amine salt, there was no change in the critical basal spacing.

466 14 Amphiphilic Poly(Oxyalkylene)-Amines Interacting with Layered Clays
14.3.3
Correlation between MMT d - Spacing and Intercalated Organics [32, 33]
The basal spacing enlargement can only be achieved by a hydrophobic aggregation
of the intercalating agents in the gallery. By taking in consideration the molecular
end - to - end length in the confi nement, a linear correlation was found for the basal
spacing expansion. As the incorporated organics occupy the same volume as the
measured silicate spacing, the relationship between the interlayer spacing ( D ) and
the organic weight fraction ( R or w/w of POP - amine/MMT by weight) is expressed
by the following equation [32] :
DR
A
t=×
×
+
1
ρ
(14.1)
where A is the surface area of MMT, t is the plate thickness, and ρ is the density
of the intercalant in the gallery. The XRD measurement is a function of the organ-
ics occupied in the gallery, and the relationship of gallery distance, volume, and
organic fraction is predictable.
14.4
Amphiphilic Copolymers as Intercalating Agents
14.4.1
Various Structures of the Amphiphilic Copolymers [34, 40, 101, 102]
In order to tailor the hydrophobic nature for organoclays, several research groups
have used amphiphilic copolymers as intercalating agents. For example, the copol-
ymers of POP - amine grafting on poly(propylene) ( PP ) [34, 40] , poly( styrene -
ethylene/butadiene - styrene ) ( SEBS ) [101] and poly( styrene - maleic anhydride )
( SMA ) [102] copolymers were reported. These copolymers, which comprised mul-
tiple amines as the pendant groups, were comb - like in shape and amphiphilic in
nature (Figure 14.4 ). The copolymers, after treating with HCl, were able to form
stable emulsions in water and to intercalate with Na
+
- MMT. The generation of an
emulsion at 670 nm diameter for the amine - grafted PP copolymer at ambient
temperature, and at 560 nm at 75 ° C in toluene/water, has also been reported [34] .
The fi ne emulsion rendered the copolymers able to intercalate with Na
+
- MMT. The
pendant quaternary ammonium ions of the copolymers may undergo an ionic -
exchange reaction to afford the silicates of 19.5 Å spacing. The layered silicate
platelet gallery was widened and surrounded with hydrophobic copolymer back-
bones. The resultant silicate/copolymer hybrids resembled a single micelle struc-
ture containing a hydrophilic rigid silicate core and a hydrophobic organic corona.
The hybrids were dispersible in toluene and were found to be approximately
500 nm in size in the example of PP - POP2000/MMT.
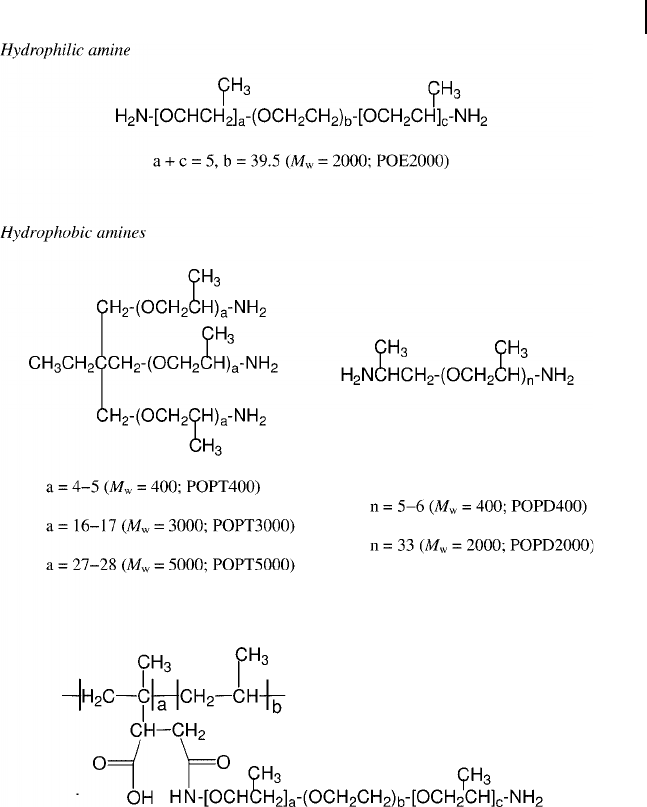
14.4 Amphiphilic Copolymers as Intercalating Agents 467
Figure 14.4 Chemical structures of POA - diamines and the grafted PP copolymers [34, 40] .
The POA - diamine grafting onto SEBS - g - MA generated another class of comb -
like and amphiphilic polyamines. The particular SEBS – POA copolymer, with an
average of nine amine pendants, is capable of forming a fi ne emulsion and inter-
calating with Na
+
- MMT to afford a wide range of XRD d spacing, from 17 Å to
52 Å . The high d spacing was due to SEBS backbone intercalation, while the POA
intercalation could result in only a low spacing. Thus, it was concluded that two
different intercalating modes were present, the conceptual description of which is
shown in Figure 14.5 .
The amphiphilic properties of the copolymers can be better controlled by using
poly( styrene - co - maleic anhydride s) ( SMA ) grafted with the POA - diamines. The
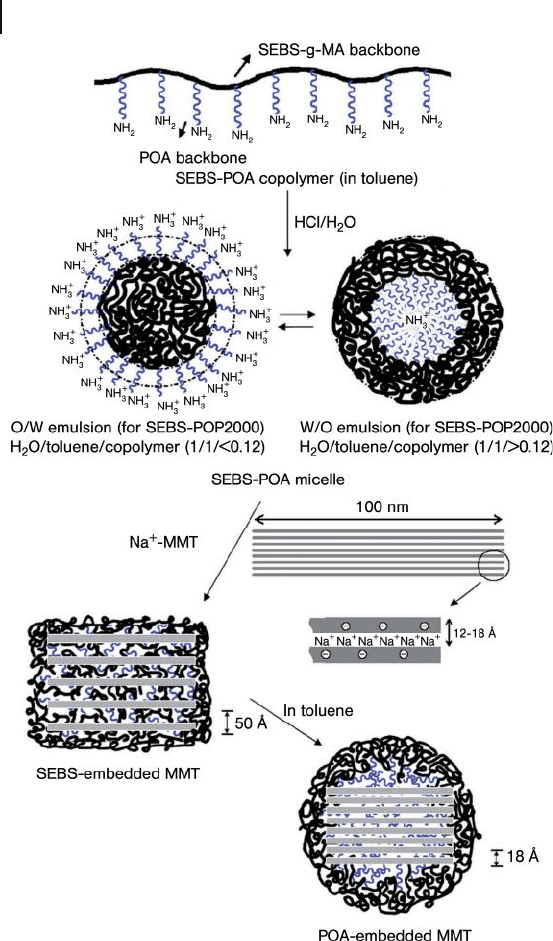
468 14 Amphiphilic Poly(Oxyalkylene)-Amines Interacting with Layered Clays
Figure 14.5 Schematic representation of copolymer/clay
hybrids in different forms of intercalation [101] .
copolymers with different ratios of styrene/MA monomers provided a wide range
of hydrophobic properties. The SMA - POP copolymers generated the intercalated
organoclays with 12.9 Å to 78.0 Å basal spacing. Two types of intercalation were
also observed for the SMA - POP intercalation.
