Geckeler K.E., Nishide H. (Eds.) Advanced Nanomaterials
Подождите немного. Документ загружается.

16.4 Nanocoatings and Surface Modifi cations 539
For these studies, the coral was obtained from the Australian Great Barrier Reef
and contained micropores of 100 to 300 μ m size (Figure 16.6 ). The coral was
shaped in the form of a block, and treated with boiling water and 5% NaClO solu-
tion. A hydrothermal conversion was carried out in a Parr reactor (Parr Instrument
Company, USA) with a Tefl on liner at 250 ° C and 3.8 MPa pressure with excess
(NH
4
)
2
HPO
4
. A total conversion to HAp was achieved in this way.
Nanocrystalline ceramic coatings were produced using the sol – gel process. For
this, the precursor solution was formed using the previously reported method.
Coatings were formed using these solutions, followed by subsequent heat treat-
ments. Mechanical testing involved a standard, four - point bend test according to
ASTM C1161, to measure the fl exural strength and fl exural modulus of the natural
coral. Comparative compression and biaxial strength tests were also carried out.
Fracture surfaces were then viewed using SEM, which was performed on a LEO -
Supra55VP instrument. Samples were analyzed using X - ray diffraction ( XRD ;
Siemens D - 5000, Karlsruhe, Germany), with scans being carried out from 20.0 to
60.0 in 0.020 steps at a step time of 2.0 s. A combined thermogravimetric analysis
( TGA )/ differential thermal analysis ( DTA ) was performed using a TA Instruments
SDT 2960, at a heating rate of 10 ° C min
− 1
.
Characterization studies of the natural and converted corals using XRD, SEM,
DTA/TGA, nuclear magnetic resonance ( NMR ) and Raman spectroscopy have
been reported previously.
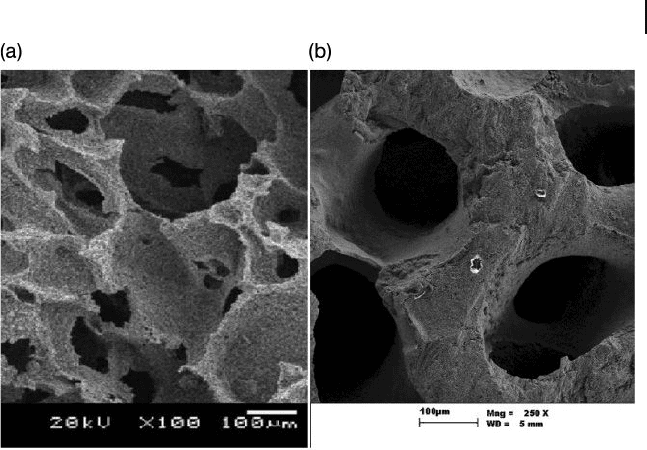
Figure 16.6 (a) Structural differences and morphology of (a) a
synthetic tricalcium phosphate and (b) a natural Australian
coral skeleton, showing pore size, distribution, and
interconnectivity.

540 16 Nanoceramics for Medical Applications
These results showed a large increase in all mechanical properties, specifi cally
the compression strength, due to hydrothermal conversion and nanocoating
methods. The bioactivity was enhanced through the nanocrystalline formation,
due to the HAp nanocoating.
16.4.3
Surface Modifi cations
The surfaces of nanostructured materials can be modifi ed and functionalized with
different reagents, using a variety of physical, chemical, and/or biological methods.
An enhanced solubility or stability of nanosized materials in aqueous media, as
well as new material functions and properties, can be achieved via the surface
modifi cation of nanostructured materials.
A variety of physical, chemical, and biological surface modifi cations to increase
bioactivity or mechanical properties have been proposed and investigated by many
groups. Molecular coating, surface entrapment, and physical treating with plasma,
ozone, or ultraviolet ( UV ) light have emerged as the leading strategies for surface
modifi cations of nanostructured materials using physical methods. Through
physical modifi cations, a range of functional molecules and entities, varying
charges or active chemical groups can be introduced onto the surfaces of nanos-
tructured materials, leading to functionalization and activation of the surfaces
of materials.
Functional molecules may also be linked to the surfaces of nanostructured
materials via certain chemical reactions. Compared to certain physical methods,
a chemical modifi cations can be used not only to activate the surfaces of nanos-
tructured materials to a greater extent, but also to offer stronger interactions
between the linking molecules and the material surfaces through stable chemical
bonds. A number of different chemical reagents and methods can be used for the
surface modifi cation of nanosized materials.
The biological modifi cation of nanoparticle surfaces is often necessary for nano-
particle functionality. By employing chemical or physical methods, biospecifi c
molecules and devices can be incorporated into the nanoparticles, thereby offering
biospecifi c sites for the further immobilization of ligands specifi c to these mole-
cules. The immobilizations of specifi c ligands can be performed through biologi-
cally specifi c reactions, for example antibody – antigen and receptor – ligand [21] .
Different biomedical devices and applications require different properties and
functions of materials. Therefore, methods to modify nanostructured materials in
order to meet the needs of various biomedical systems will vary. A brief summary
of the basic methods and technologies used to modify nanostructured materials
for biomedical devices is presented below.
Today, highly porous scaffolds with an open structure represent the best candi-
dates for cancellous bone substitution. In addition to natural and ceramic materi-
als, many polymers have been proposed for medical applications. Each of these
presents different biological and mechanical properties, allowing a choice of the
correct polymer for the correct application. However, polymers usually present low

16.5 Simulated Body Fluids 541
elastic modulus values, creep resistance and chemical constituents, compared to
bone, and this is the major reason that limits their clinical use for hard - and soft -
tissue substitution.
One example was that reported by Peroglio and coworkers [11] , who produced
PCL - coated alumina scaffolds that were then characterized to validate the concept
of polymer – ceramic composites with an increased fracture resistance. The alumina
scaffolds were processed using a classical foam replication technique, and then
sintered to produce an open - porous structure with ∼ 70% porosity and a mean pore
size of 150 μ m. The polymer coating was obtained by infi ltrating the scaffold with
either a PCL solution or PCL nanodispersion. An emulsion – diffusion technique,
using a nonionic surfactant, was used for the latter process. Subsequently, after
infi ltration with PCL, and irrespective of which quantity or infi ltration technique
was used, no change was seen in the Young ’ s modulus. However, this was to be
expected as the elastic modulus of PCL is negligible compared to that of alumina.
Nonetheless, the addition of PCL completely altered the mechanical behavior of
the scaffold during a four - point bending test, with a 10 – 20 vol% addition of PCL
to the alumina scaffold leading to seven - to 13 - fold increases in the apparent frac-
ture energy. A further examination of the material, using SEM, indicated that the
toughening was the result of the polymer fi brils bridging the cracks.
In recent years, nanoparticle systems have attracted increasing attention for
use as potential drug - delivery systems. Despite the advantages of nanoparti-
cles – such as their small size, which allows them to penetrate small capillaries
and be taken up by cells – a number of problems, including a relatively short
blood circulation time, have limited their clinical application. The effi ciency and
targeting ability of a nanoparticle drug - delivery system are often hampered by
the rapid recognition of the carrier system by the body. As the main concern for
nanoparticle drug carriers is a long circulation time in the blood, numerous
approaches to the design and engineering of long - circulating - time carriers have
been investigated. Among these, the surface modifi cation of nanoparticles with
a range of nonionic surfactant or polymeric macromolecules has proved to be
the most successful for maintaining nanoparticle presence in the blood for pro-
longed periods [41] . Suitable and effective modifi cations of the nanoparticles are
also required, however, to overcome a number of technical problems and possible
issues of toxicity.
16.5
Simulated Body Fluids
Artifi cial materials implanted into bone defects might be encapsulated from time
to time by a fi brous tissue, leading to their isolation from the surrounding bone.
Any improvement in bone - implant bonding to the tissues requires the correct
implant surface morphology and chemistry to generate a mechanical interlock and
good surface activity. Interactions between the bone and the implant will be con-
trolled with appropriate biological interactions.

542 16 Nanoceramics for Medical Applications
During the past three decades, many investigators have proposed that the essen-
tial requirement for an artifi cial material to bond to living bone is the formation
of a bone - like apatite on its surface when implanted in the living body. In 1991,
Kokubo et al . proposed that in vivo apatite formation on the surfaces of many
biomedical materials could be reproduced in a SBF with ion concentrations almost
equal to those of human blood plasma. In essence, this means that the in vivo
bone bioactivity of a material can be predicted from the apatite formation on its
surface in SBF (Figure 16.7 ) [42] .
Hydroxyapatite layers can be easily produced on various organic and inorganic
substrates when submerged in SBF and indeed, in 1989, Kokubo and Takadama
[42] showed that, after immersion in SBF, a wide range of biomaterial surfaces
initiated very fi ne crystallites of carbonate ion - containing apatite. Subsequently,
many reports have described the ability of osteoblasts to proliferate and differenti-
ate on this apatite layer. Based on these fi ndings, other SBFs have been produced
in order to provide insight into the reactivity of the inorganic component of blood
plasma, and to predict the bioactivity of implants and bone scaffolds, as well as
other novel biomaterials. SBF has also been used to prepare bioactive composites
by forming HAp on various types of substrate.
The SBF solutions have been shown to induce apatitic calcium phosphate forma-
tion on any metal, ceramic, or polymer soaked in them. The SBF solutions, which
closely resemble Hank ’ s balanced salt solution ( HBSS ) [39] , are prepared with the
aim of simulating the ion concentrations present in human plasma. Hence, the
solutions are prepared with relatively low calcium and phosphate ion concentra-
tions (i.e., 2.5 and 1.0 m M , respectively), while the pH is adjusted to a physiological
value of 7.4 by using organic buffers (e.g., Tris or HEPES).
Typically, a SBF solution will have ionic concentrations of 142.0 m M Na
+
, 5.0 m M
K
+
, 1.5 m M Mg
2+
, 2.5 m M Ca
2+
, 147.8 m M Cl
−
, 4.2 m M HCO
3 −
, 1.0 m M HPO
4
2−
,
and 0.5 m M
SO
4
2−
, with a pH of 7.4, all of these values being almost equal to those
of human blood plasma at 36.5 ° C. The SBF is usually prepared by dissolving
reagent - grade NaCl, NaHCO
3
, KCl, K
2
HPO
4
· 3H
2
O, MgCl
2
· 6H
2
O, CaCl
2
and
Na
2
SO
4
in distilled water and buffering at pH 7.4 with Tris(hydroxymethyl)ami-
nomethane ((CH
2
OH)
3
CNH
3
) and 1.0 M hydrochloric acid at 36.5 ° C.
Since their ionic composition is more or less similar to that of human blood
plasma, the HBSS or SBF formulations have only limited power with regards to
the precipitation of apatitic calcium phosphates. As a direct consequence, the
nucleation and precipitation of calcium phosphates from HBSS or SBF solutions
is rather slow. The time taken to achieve total surface coverage of a 10 × 1 0 × 1 mm
titanium or titanium alloy substrate immersed in a 1.5 × or 2 × SBF solution is
typically two to three weeks, with frequent (every 36 – 48 h) replenishment of the
solution [43] .
Among the metallic oxide gels prepared using a sol – gel method, those consist-
ing of SiO
2
, TiO
2
, ZrO
2
, and Ta
2
O
5
were found to have apatite formation on their
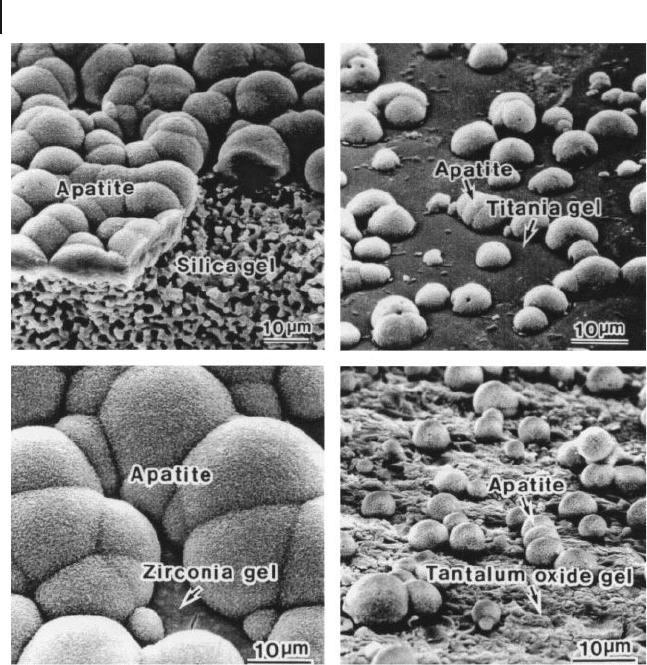
surfaces in SBF, as shown in Figure 16.7 . These results indicated that the Si – OH,
Ti – OH, Zr – OH, and Ta – OH groups on the surfaces of these gels were effective
in inducing apatite formation on their surfaces within the body environment
(Figure 16.8 ).
16.5 Simulated Body Fluids 543
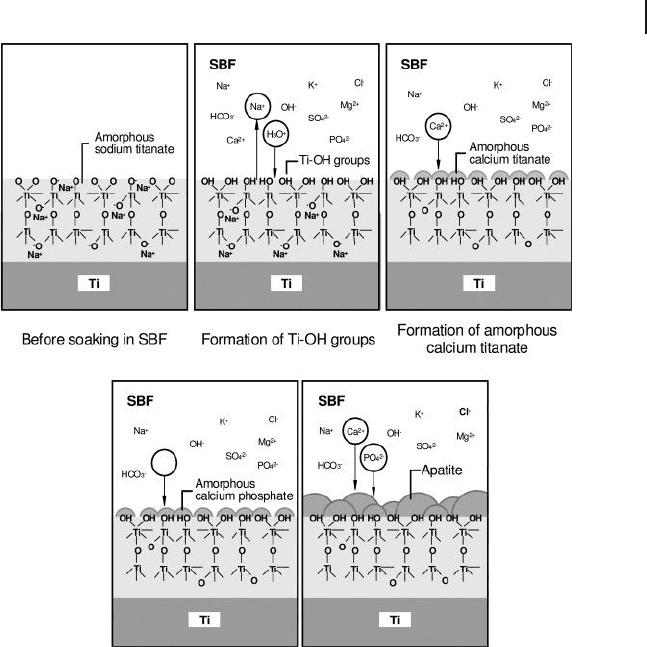
Figure 16.7 Treatment of a titanium alloy surface with NaOH
to produce a bioactive surface on which hydroxyapatite
particles nucleate and grow quite readily within the SBF
solution [40] .
A variety of studies have been conducted using SBF solutions to deposit
apatite on both two - dimensional ( 2 - D ) and 3 - D scaffolds. For example, Wu and
coworkers [41] developed a novel bioactive, degradable and cytocompatible bredig-
ite (Ca
7
MgSi
4
O
16
) scaffold with a biomimetic apatite layer ( BTAp ) for bone - tissue
engineering. For this, porous bredigite scaffolds were fi rst prepared using the
polymer sponge method. A BTAp was then applied to the scaffolds by soaking
them in SBF (pH 7.4) at 37 ° C for 10 days, with a solution volume - to - scaffold mass
ratio of 200 ml g
− 1
. After soaking, the scaffolds were dried at 120 ° C for one day,
such that bredigite scaffolds with BTAps were obtained. The porosity and in vitro
degradability of the BTAp scaffolds were investigated. Likewise, the osteoblast - like
cell morphology, proliferation and differentiation on BTAp scaffolds were evalu-
ated and compared with those of β - tricalcium phosphate ( β - TCP ) scaffolds. The
results showed that the bredigite scaffolds possessed a highly porous structure
with a large pore size (300 – 500 μ m). This biomimetic process mimics biominer-
alization and leads to the formation of a bone - like apatite layer on the scaffold
544 16 Nanoceramics for Medical Applications
surface. The obtained BTAp scaffolds also possessed a high porosity (90%) and
pore interconnectivity. When compared to β - TCP scaffolds, the cells on BTAp
scaffolds showed a higher proliferation rate and differentiation level.
Cromme and coworkers [44] investigated the activation of regenerated cellulose
2 - D model thin fi lms and 3 - D fabric templates with calcium hydroxide, Ca(OH)
2
.
For this, the Langmuir – Blodgett ( LB ) fi lm technique was applied to manufacture
model thin fi lms using a trimethylsilyl derivative of cellulose (TMS - cellulose).
Regenerated cellulose fi lms were obtained by treating the TMS - cellulose LB - fi lms
with hydrochloric acid vapors. For the 3 - D templates, regenerated cellulose fabrics
were used, and the templates activated with a Ca(OH)
2
- suspension and subse-
quently exposed to 1.5 × SBF to induce the in situ formation of calcium phosphate
phases. The calcium phosphates were identifi ed using Fourier transform infrared
( FTIR ) and Raman spectroscopy as highly carbonated apatite s ( CA ) lacking
hydroxyl ions. Such 3 - D fabric templates of regenerated cellulose covered with a
Figure 16.8 Apatite formation on silica, titania, zirconia, and
tantalum oxide gel - covered substrates within the SBF solution
[40] .

16.5 Simulated Body Fluids 545
biomimetic coating of apatite might be of particular interest for novel scaffold
architectures in bone repair and tissue engineering.
Lim and coworkers [45] noted that bone - like apatite could be more effi ciently
coated onto the scaffold surface by using polymer/ceramic composite scaffolds
rather than polymer scaffolds, and by using an accelerated biomimetic process to
enhance the osteogenic potential of the scaffold. The creation of a bone - like,
apatite - coated polymer scaffold was achieved by incubating the scaffolds in SBF.
Apatite growth on the porous poly( D , L - lactic - co - glycolic acid)/nanohydroxyapatite
( PLGA/HAp ) composite scaffolds was signifi cantly faster than on the porous
PLGA scaffolds. In addition, the distribution of coated apatite was more uniform
on the PLGA/HAp scaffolds than on the PLGA scaffolds. After a fi ve - day incuba-
tion period, the mass of apatite coated onto the PLGA/HAp scaffolds incubated
in 5 × SBF was 2.3 - fold higher than on the PLGA/HAp scaffolds incubated in
1 × SBF. Furthermore, when the scaffolds were incubated in 5 × SBF for fi ve
days, the mass of apatite coated onto the PLGA/HAp scaffolds was 4.5 - fold higher
than on the PLGA scaffolds. These results indicated that the SBF - initiated apatite
coating could be accelerated by using a polymer/ceramic composite scaffold and
concentrated SBF. It was reported that, when seeded with osteoblasts, the apatite -
coated PLGA/HAp scaffolds exhibited signifi cantly higher cell growth, alkaline
phosphatase ( ALP ) activity, and mineralization in vitro compared to the PLGA
scaffolds coated only with HAp. In conclusion, the biomimetic apatite coating
could be accelerated by both introducing nucleation sites into polymer scaffolds
and using concentrated SBF. When seeded with osteoblasts, scaffolds with acceler-
ated apatite coating signifi cantly enhanced cell growth, ALP activity, and miner-
alization in vitro .
The SBF method was used by Kolos et al . [46] to fabricate calcium phosphate
fi bers for biomedical applications. A natural cotton substrate was fi rst pretreated
with phosphorylation and a Ca(OH)
2
- saturated solution, and then soaked in SBF
of two different concentrations, namely 1.5 × and 5.0 × the ion concentration of
blood plasma. The cotton was then burned out by sintering the ceramic coating
at 950 ° C, 1050 ° C, 1150 ° C, and 1250 ° C, such that hollow calcium phosphate fi bers
approximately 25 μ m in diameter and with a 1 μ m wall thickness were success-
fully manufactured. However, the 5.0 × SBF produced a thicker and more crystal-
line coat of greater uniformity. Kolos et al . further reported that osteoblastic cells
were able to cover the entire surface of the cotton fi bers; more surprisingly, the
cell coverage seemed to be independent of the surface roughness and the fi bers ’
Ca : P ratio.
Although of micron size rather than nanosize, a bioactive CHAp layer on cel-
lulose fabrics was developed by Hoffman et al . [47] . Nonwoven cellulose (regener-
ated, oxidized) fabrics were coated with CHAp using a procedure based on the
SBF method. For this, SBF with a high degree of supersaturation (5 × SBF) was
applied to accelerate the biomimetic formation of bone - like apatite on the cellulose
fabrics. After creating calcium phosphate nuclei on the cellulose fi bers in an initial
5 × SBF with high Mg
2+
and
HCO
3
−
concentrations, the cellulose fabrics were
additionally soaked in a second 5 × SBF which was optimized with regards to

546 16 Nanoceramics for Medical Applications
accelerated crystal growth by reduced Mg
2+
and HCO
3
−
concentrations. The car-
bonated apatite layer thickness was increased from 6 μ m after a 4 h soaking in the
latter solution, to 20 μ m after 48 h. The amount of CO
3
2−
substituting
PO
4
3−
in the
HAp lattice of the precipitates could be varied by changing the soaking time.
16.6
Nano - and Macrobioceramics for Drug Delivery and Radiotherapy
16.6.1
Nanobioceramics for Drug Delivery
For drug delivery, the primary aim is to target drugs to specifi c sites within the
body, and to release them in a controllable fashion. However, for many current
delivery systems the guest molecules are often released upon dispersion of the
carrier/drug composites in water. This type of premature release is particularly
undesirable and problematic when the guest molecule (e.g., an anti - tumor drug)
is cytotoxic and might potentially harm healthy cells and tissues before being
delivered to the affected sites [48] .
In the case of ceramics, the critical pore and grain size may be varied from a
few nanometers up to microns in order to control the ease of delivery and disper-
sion of a material to the targeted area. A variety of nanoceramic drug - delivery
systems are currently undergoing clinical evaluation. In addition to reducing
toxicity to nondiseased cells, these systems have the potential to increase drug
effi ciency, which translates to signifi cant cost savings for the expensive drug treat-
ments that currently are being engineered. On the basis of their physical size,
nano drug - delivery systems also have the extraordinary characteristic of being
able to target and control drug release with very high precision.
Mesoporous silica nanoparticle ( MSN ) materials, such as mobile composition
of material s ( MCM ) - 41/48, can be synthesized by utilizing surfactants as structure -
directing templates to generate a range of mesoporous structures with high surface
areas ( > 900 m
2
g
− 1
), tunable pore sizes in the range of 2 to 20 nm, and uniform
pore morphologies. Recent breakthroughs in terms of morphology control and the
surface functionalization of MSN materials have resulted in a range of new materi-
als that can be used as stimuli - responsive, controlled - release delivery - carriers for
many biotechnological and biomedical applications. As with some other conven-
tional drug - delivery agents with high loading capacities (e.g., polymer and lipo-
somes), MSNs can encapsulate large quantities of drugs with various sizes, shapes,
and functionalities [46] . In contrast to many biodegradable polymeric delivery
systems, in which the loading of drug molecules requires organic solvents, the
molecules of interest can be encapsulated inside the porous framework of the
MSN by capping the openings of the mesoporous channels covalently with size -
defi ned “ caps, ” which physically block the drugs from leaching out. Drug mole-
cules loaded into the pores may then be released by the introduction of “ uncapping
triggers, ” with the rate of release being controlled by the concentration of the

16.6 Nano- and Macrobioceramics for Drug Delivery and Radiotherapy 547
trigger molecules. Prior to uncapping, the capped MSN system exhibits a negligi-
ble release of drug molecules. This “ zero - release ” feature of a capped MSN delivery
system, along with an ability to tune the rate of release by varying stimulant con-
centrations, are important prerequisites for developing delivery systems with
many site - specifi c applications, such as highly toxic anti - tumor drugs, hormones,
and neurotransmitters to certain cells types and tissues [48] .
An MCM - 41 - type MSN - based, controlled - release delivery system has been syn-
thesized and characterized using surface - derivatized cadmium sulfi de (CdS)
nanocrystals as chemically removable caps to encapsulate several drug molecules
and neurotransmitters inside the organically functionalized MSN mesoporous
framework. Lai and coworkers [49] studied the stimuli - responsive release profi les
of vancomycin - and adenosine triphosphate ( ATP ) - loaded MSN delivery systems
by using disulfi de bond - reducing molecules, such as dithiothreitol ( DTT ) and
mercaptoethanol ( ME ), as release triggers. The biocompatibility and delivery effi -
ciency of the MSN system with neuroglial cells (astrocytes) in vitro was demon-
strated. In contrast to many current delivery systems, the molecules of interest
were encapsulated inside the porous framework of the MSN by capping the open-
ings of the mesoporous channels with size - defi ned CdS nanoparticles, so as to
physically prevent the drugs/neurotransmitters from leaching out.
Porous aluminosilicate ceramics were investigated by Byrne and Deasy [50] for
their potential to act as extended - release drug - delivery systems. The aluminosili-
cate pellets were obtained either commercially, produced by extrusion - spheroiza-
tion, or by cryopelletization. It was reported that each product had a highly
interconnected porous microstructure, with the porosity and pore - size distribution
being product - dependent. Drugs were loaded into the pellets using a vacuum
impregnation technique, with the concentration of the drug loading solution and
pellet porosity infl uencing the loading obtained. Each product provided an extended
release of the incorporated drug, with the rate - determining step of release being
the diffusion of the drug from the porous pellet interior into the bulk dissolution
medium. Byrne and Deasy [50] showed that this rate was infl uenced by the pellet
size, its porosity, pore - size distribution and porous microstructure, and by electro-
static interactions between the pellet surfaces and the drug. The solubility of the
drug in the dissolution medium and its molecular weight also infl uenced the
release rate. It was concluded that porous aluminosilicate pellets represent a par-
ticularly versatile class of extended - release drug - delivery system, as the drug is
incorporated into the pellets after their production.
A new TiO
2
nanostructured bioceramic device was synthesized by L ó pez and
coworkers [51] , using a sol – gel process in order to control the pore - size distribution
and particle size. The objective was to obtain a constant drug release rate for anti -
epileptic drugs directly into the central nervous system ( CNS ). This method of
drug delivery, using small reservoirs, is very important in pharmaceutical applica-
tions, as it offers advantages such as the elimination of secondary effects, a long
duration of pharmacological activity, and protection of the drug against enzymatic
degradation or pH variations. Among the best - developed and most studied materi-
als, Titania has been shown to be an excellent candidate because of the possibility

548 16 Nanoceramics for Medical Applications
to manipulate both the structure and the number of OH groups. Point defects
were generated in the Titania network in order to obtain the desired interaction
between a highly polar drug and the Titania device. The device contained an anti-
convulsant drug, valproic acid ( VPA ), which could be released directly into the
temporal lobe of the brain, at a constant rate. During the initial stage of the syn-
thesis, VPA was added until a completely homogeneous solution was created; this
solution was then maintained under constant stirring until a gel was formed.
Under these conditions, the titanium dioxide underwent nucleation, whilst at the
same time it was restructuring around the VPA in such a way that the chemical
and polar properties of the anticonvulsant were preserved. The reaction was seen
to take place under well - controlled conditions of pressure and temperature, which
were purposely kept low. During the gelation period, both the electronic and
molecular properties of the material were preserved. Finally, a dry gel was obtained,
in which the anticonvulsant was occluded within the pore structure. The release
of VPA into the temporal lobe of the brain, and its effect on epileptic rats, was
observed by using the Kindling method. Several important conclusions were
drawn from these studies, notably that a Titania device charged with an anticon-
vulsant drug could be successfully implanted in the rat ’ s temporal lobe. Pore -
blocking with higher concentrations of VPA led to a fall in the initial rate of drug
release, while insertion of the device caused a drastic reduction in the animal ’ s
epileptic activity.
16.6.2
Microbioceramics for Drug Delivery
The particulate forms of ceramic materials have found application in both medical
and non - medical fi elds. Particles in the form of microspheres are especially appli-
cable when treating tumors located in organs that are supplied by a single afferent
arterial blood supply. More traditionally, microspheres and nanostructurally modi-
fi ed ceramics have been used in the targeted drug delivery of chemotherapeutic
and radiotherapeutic agents.
In recent years, the preparation of surface - modifi ed hollow microspheres has
attracted considerable attention because of their unusual properties, notably their
large specifi c surface area due to the nanolayer - modifi ed surfaces, their low density
and their encapsulation properties. Consequently, these materials should be very
useful for novel applications such as drug - and protein - delivery systems. In certain
applications, the effi cacy of microparticulate materials can be greatly improved if
they can act simultaneously as carriers for biologically active molecules. In this
sense, porous and surface - modifi ed materials have an advantage, as they present
an additional surface area that greatly infl uences the loading capacity and release
rates.
In dental clinical applications, such as the treatment of severe periodontitis,
where massive alveolar bone loss occurs, bone defect fi lling and intensive systemic
long - term antibiotics administration are often required. Nanohydroxyapatite
microspheres intended for use as injectable bone fi lling material or as enzyme -
