Fowler A. Mathematical Geoscience
Подождите немного. Документ загружается.

820 D Melting, Dissolution, and Phase Changes
is the strain rate. We can write
σ
ij
˙ε
ij
=−p∇.u +τ
ij
˙ε
ij
, (D.16)
where τ
ij
is the deviatoric stress tensor, and using the conservation of mass equation
(D.12)
1
, we find
ρ
de
dt
+p
dv
dt
=τ
ij
˙ε
ij
−∇.q ≡R. (D.17)
The right hand side R of this equation consists of the viscous dissipation and the
heat transport. Using (D.11), this leads to
ρT
ds
dt
=R. (D.18)
Using the relation in (D.11), the energy equation can also be written in the form
ρc
p
dT
dt
−βT
dp
dt
=R, (D.19)
and using the definition of (specific) enthalpy, it takes the form
ρ
dh
dt
−
dp
dt
=R. (D.20)
These different forms are variously of use depending on the material properties.
In particular, for a perfect gas one can show (see Question D.12) that
dh =c
p
dT, de =c
v
dT. (D.21)
The second of these also applies to an incompressible fluid.
D.3 Phase Change: Clapeyron Equation
The use of the free energies G (Gibbs free energy) and F (Helmholtz free energy)
is that they describe thermodynamic equilibrium conditions. Specifically, they take
minimum (and thus stationary) values at equilibrium. The difference between them
resides in the external conditions. At constant temperature and pressure, the Gibbs
free energy is a minimum, while at constant temperature and volume, the Helmholtz
free energy is a minimum. Of course, we are never really interested in systems
which are at equilibrium. Implicitly, thermodynamics is useful because we typically
assume that in systems away from equilibrium (pretty much everything), there is
a rapid relaxation of some parts of the system towards equilibrium. For example,
it is common to assume that in melting or freezing, the solid–liquid interface is at
the melting point. This is often a good assumption, but not always. One needs to be
aware that in practice, we assume thermodynamic relations in a quasi-equilibrium

D.4 Phase Change in Multi-component Materials 821
manner. If there is a gradient in the Gibbs free energy, then transport will occur to
try to minimise the free energy. A gradient in temperature causes heat transport; a
gradient in pressure causes fluid flow. A gradient in chemical potential (discussed in
Sect. D.4) causes Fickian diffusion.
A simple use of the Gibbs free energy is in determining the Clapeyron relation,
which relates melting temperature (or any phase change temperature) to pressure.
The Gibbs free energy is G = H − TS, and using (D.2), we find (for intensive
variables)
dg =vdp−sdT. (D.22)
Suppose now that we have a phase boundary between, say, solid and liquid (of the
same material), denoted by subscripts s and l. At the phase boundary, equilibrium
dictates that g
s
=g
l
, where these are the free energies in the solid and liquid phase.
Inequality would cause transport, as we have said. Suppose the melting temperature
is T
M
, and the system moves to a different temperature and pressure. At the new
equilibrium, the perturbations to the free energies must be equal, thus g
s
= g
l
,
and thus
v
s
p −s
s
T =v
l
p −s
l
T , (D.23)
whence
T
M
p
=
v
s
, (D.24)
where
v =v
l
−v
s
(D.25)
is the change of specific volume on melting, and
s =s
l
−s
s
(D.26)
is the change of specific entropy on melting. We define the latent heat to be
L =T
M
s, (D.27)
so that (D.24) takes the form of the Clapeyron equation,
LT
M
T
M
=
1
ρ
l
−
1
ρ
s
p . (D.28)
This relation, or its differential equivalent, describes the form of the phase transition
curves which, for ice-water-water vapour, have been drawn in Fig. 2.7.
D.4 Phase Change in Multi-component Materials
Now we consider materials, such as alloys or aqueous solutions, which contain more
than one substance. In a sense, we have already introduced this by considering two
822 D Melting, Dissolution, and Phase Changes
different phases of a pure material. If we suppose that we have n
i
moles of substance
i (these are thus extensive variables), then each substance has its own Gibbs free
energy, and these contribute additively to the total free energy. The free energy of
each phase is called its chemical potential, and the chemical potential μ
i
of phase i
is defined more precisely by asserting that the total Gibbs free energy satisfies
dG =Vdp−SdT +
i
μ
i
dn
i
, (D.29)
thus
μ
i
=
∂G
∂n
i
, (D.30)
where the derivative is evidently at constant temperature and pressure. The chemical
potential is thus an intensive variable. Suppose we have a solid in equilibrium with
a liquid. Since the differential increments in (D.29) are all independent, we can
imagine a change of solid i to liquid i, such that dn
L
i
=−dn
S
i
. The consequent
change in Gibbs free energy is (μ
L
i
− μ
S
i
)dn
L
i
, and in equilibrium this must be
zero. Thus we must have
μ
L
i
=μ
S
i
(D.31)
at equilibrium, in each component. Just as heat flows down a temperature gradient,
so substance is transported down a chemical potential gradient.
For a perfect gas, the specific Gibbs free energy g(T,p) satisfies
∂g
∂p
T
=v =
RT
p
(D.32)
(since G =ng and pV =nRT ,forn moles of the gas), and thus
g =g
0
+RT lnp. (D.33)
In a mixture of gases, the partial pressure of each component gas is that pressure
it would have if the other gases were removed. Dalton’s law says that the partial
pressures are additive, so that their sum is the total pressure of the gas mixture. If
we suppose in a mixture that the analogue of (D.33) holds for partial energies and
pressures, i.e.,
g
i
=g
0
i
+RT ln p
i
, (D.34)
then since p
i
V =n
i
RT and g
i
is the chemical potential of gas i, we can write
μ
i
=μ
0
i
+RT lnc
i
, (D.35)
where c
i
is the molar fraction of phase i (=
n
i
i
n
i
). This relation more generally
characterises an ideal mixture, whether it be of gases, liquids or solids.
Now let us consider an interface (we will think of it as a solid-liquid interface)
between the melt and solid of a two component mixture containing substances A
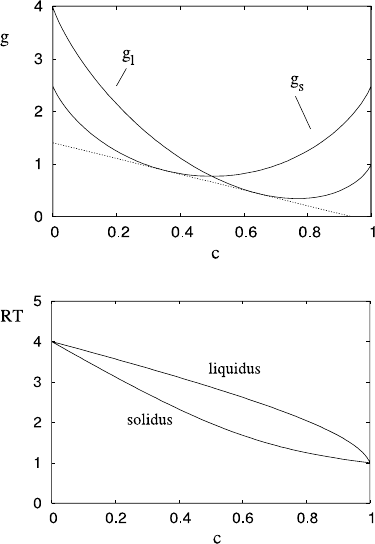
D.4 Phase Change in Multi-component Materials 823
Fig. D.1 The double tangent
construction for c
S
and c
L
.
The curves are the graphs of
the functions g
S
and g
L
defined by (D.38), in which
we define (the units are
arbitrary)
μ
0
A
(L) =μ
0
B
(L) =RT ,
μ
0
A
(S) =1, μ
0
B
(S) =4. The
figure shows the construction
at RT =2.5. c denotes the
concentration as mole
fraction of A
Fig. D.2 Typical phase
equilibrium for an ideal
solution. The same formulae
are used as in constructing
Fig. D.1, with the range
corresponding to 1 ≤RT ≤4
and B. We will suppose the mixture is ideal. At the interface, the chemical potentials
of each component must be equal, thus
μ
L
A
=μ
S
A
,μ
L
B
=μ
S
B
, (D.36)
and these will determine the interfacial concentrations as functions of temperature.
To be specific, let c denote the molar fraction of component A, so that 1 − c is the
molar fraction of B. Then the bulk Gibbs free energies (one in each phase) are
g =μ
A
c +μ
B
(1 −c), (D.37)
and for an ideal solution, we have
g =μ
0
B
(1 −c) +μ
0
A
c +RT
c ln c +(1 −c) ln(1 −c)
. (D.38)
The two functions g
S
and g
L
are thus convex upwards functions, and the criterion
for equilibrium as in (D.36) is obtained by drawing a common tangent to g
S
and g
L
,
as indicated in Fig. D.1, and done in Question D.3; this gives the solid and liquid
concentrations in equilibrium for a particular temperature; as the temperature varies,
we obtain the typical phase diagram shown in Fig. D.2.
Although our discussion is motivated by gases, the concept of an ideal solution
applies equally to liquids and solids. Indeed, Fig. 9.4 shows a phase diagram essen-
tially the same as that in Fig. D.2, for the solid solution of albite and anorthite. As
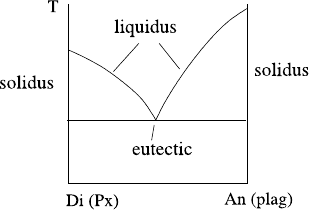
824 D Melting, Dissolution, and Phase Changes
Fig. D.3 A typical phase
diagram for a mixture
(pyroxene–plagioclase) with
a eutectic point. Such
diagrams are common for
aqueous solutions
for liquids and gases, ideal solutions occur when there is no penalty for introducing
molecules of different substance. In the case of solids, this means replacing atoms
in the crystal lattice.
For non-ideal solutions, the logarithmic terms such as lnc in the free energy
are replaced by corresponding quantities ln a, where a is a function of c called the
activity. One typical effect is to make the free energy curves g
S
and g
L
have multiple
minima, and this allows for more than one pair of liquidus and solidus values at a
given temperature. A typical such consequent phase diagram is shown in Fig. D.3,
which is actually that for pyroxene and plagioclase shown in Fig. 9.12. Here there
are two liquidus curves, which meet at the eutectic point. The solidus curves in
this diagram are vertical, thus on freezing, one forms either pure pyroxene or pure
plagioclase, depending on which side of the eutectic the liquid composition lies.
Below the eutectic point only solid can exist in equilibrium.
D.5 Melting and Freezing
In discussing phase change, we have mostly referred to melting and freezing. In
terms of pure materials, there is no distinction to be made between this, boiling and
condensation (of liquid and gas), and sublimation and condensation (of solid and
gas). A point we will now make is that there is similarly no distinction between the
different corresponding situations which refer to multi-component phase change.
The melting and freezing of an alloy is familiar in industrial contexts (in forming
solid castings) as well as the environment. The simplest example is the case of an
iceberg, consisting of fresh water ice in equilibrium with a slightly salty ocean. Ice-
bergs are of course not formed by freezing the ocean (but sea ice is), but the principle
will serve. Freezing of salty sea water occurs on a diagram similar to Fig. D.3;fora
sufficiently dilute solution, freezing forms more or less pure water ice, with the salt
being rejected into the water. We routinely refer to this as freezing.
D.6 Precipitation and Dissolution
Suppose, however, that we take a salty solution at high temperature. Better, think
of sugar dissolved in water (or tea) at high temperatures. The solubility is greater at

D.7 Evaporation and Boiling 825
higher temperatures, and if we cool the tea (a lot), eventually the sugar will come
out of solution; it precipitates, while at high temperature it dissolves. We do not nor-
mally think of this as melting and freezing, but the process is exactly the same. The
only difference to the iceberg is that we are on the other side of the eutectic. Now,
when we take our saline solution at high salt concentration and high temperature,
and lower the temperature, we reach a liquidus on the other side of the eutectic to
that of the iceberg; solid salt is frozen (but we say it is precipitated), and the rem-
nant water becomes purer. Or, if we pour salt into water when we cook, it dissolves
as we heat the water; we aid the dissolution by stirring, which increases the avail-
able surface area for dissolution. We do not think that the salt is melting; but it is.
There is no distinction between the processes of melting and freezing of alloys and
precipitation and dissolution of solutes.
D.7 Evaporation and Boiling
Surely, however, evaporation and boiling are not the same at all? Evaporation occurs
continually at temperatures below the boiling point: we sweat; boiling occurs at a
fixed temperature. For water, boiling occurs at 100°C at sea level. But evaporation
occurs from oceans at their much lower temperatures. Certainly, on the top of Mount
Everest, boiling temperature is reduced, but this is because the pressure is lower, and
occurs through the Clapeyron effect.
So then, what is evaporation? The saturation vapour pressure of water vapour,
p
sv
, is a function of temperature, given by the solution of (2.56), and it increases
to a pressure of one bar (sea level atmospheric pressure) at a temperature of 100°C,
where boiling occurs spontaneously.
It is all, in fact, the same story. The ocean, let us say, is pure water (ignore salt).
The atmosphere is a two component mixture (let us say) of water vapour and air;
it is an alloy. If we take a hot atmosphere and reduce its temperature, condensa-
tion occurs at a temperature which depends on atmospheric composition. The molar
fraction of water vapour in the atmosphere is just p
v
/p
a
, the vapour pressure di-
vided by the atmospheric pressure. On what would be the liquidus (but now must
be the vaporus
2
), the vapour pressure has its saturation value, the molar fraction of
water vapour is p
sv
/p
a
=c
sv
, and the saturation temperature T
s
is a function of c
sv
.
What has boiling to do with this? Not much! Evaporation is boiling. What we nor-
mally call boiling refers to the position of the vaporus when c
sv
=1, i.e. p
sv
=p
a
.
For given atmospheric pressure, we cannot raise the liquid temperature beyond the
vaporus temperature at vapour concentration of one. If we change atmospheric pres-
sure, then this temperature will change. Yes, because of Clapeyron, but also because
pressure dictates concentration. Gases are different because the amount of gas de-
pends on pressure. For liquids and solids, this is mostly not the case.
2
Solidus is a perfectly good Latin word, but liquidus is not; vaporus is invented here.
826 D Melting, Dissolution, and Phase Changes
D.8 Chemical Reactions
Surely chemical reactions are different? So it would appear. If we pour vinegar
(acetic acid) into a kettle furred up with limescale (calcium carbonate), the limescale
will dissolve, or react, forming carbon dioxide in the process. In a coal fire, the car-
bon in the coal reacts with oxygen, forming carbon dioxide. There is no equilibrium
surface or phase diagram here, surely?
But in fact the difference is only one of degree. When a salt M dissolves in water
to the point of saturation, the equilibrium that results is a consequence of a simple
reversible reaction
M
S
k
D
k
P
M
L
, (D.39)
where k
D
is the rate of dissolution and k
P
is the rate of precipitation. The fact that
there is an equilibrium is a consequence of the reversibility. The only effective dif-
ference between this and a chemical reaction is that the examples cited above are
almost irreversible. If we burn coal in a sealed environment, the carbon reacts with
the oxygen to form a mixed atmosphere of O
2
with CO
2
, just as when we evap-
orate water vapour in air. If the reaction is reversible, then an equilibrium will be
obtained. In practice (in this example) the backward reaction rate is negligible, and
so the equilibrium which obtains occurs when the coal is (almost) entirely used up.
Chemical reaction is thus the process describing the evolution towards thermody-
namic equilibrium.
D.9 Surface Energy
Interfaces between two materials, be they both fluids, fluid and solid, or any other
such combination, carry a surface energy per unit area, denoted γ . The existence of
a surface energy causes a pressure jump across the interface, and the requirement of
force balance (Newton’s third law) on the massless interface means that the interface
appears to carry a tension, the surface tension. To see how the surface energy induces
this pressure jump, we consider equilibrium of a system containing an interface. For
example, we may think of a box containing fluid with a gas bubble in it. To change
the surface area of the interface, we may alter the external pressure, and thus the
equilibrium is that associated with constant volume and temperature, for which the
relevant minimum is obtained by the Helmholtz free energy F . The basic recipe for
an increment of F for each phase follows from (D.1) and (D.2), and is
dF =−pdV −SdT; (D.40)
when the surface area of a phase interface has a surface energy per unit area γ , then
a change in surface area dA causes an additional contribution γdA, which must
also be included. Suppose the two sides of the interface are denoted by subscripts −
and +, and have corresponding pressures p
−
and p
+
. For an isothermal change at

D.10 Pre-melting 827
constant total volume, dV
−
=−dV
+
, and thus the increment of the total Helmholtz
free energy of the system is
dF =−p
−
dV
−
−p
+
dV
+
+γdA=−(p
−
−p
+
)dV
−
+γdA=0, (D.41)
and thus
p
−
−p
+
=γ
∂A
∂V
−
. (D.42)
This determines the pressure jump at the interface. It is a result of differential geom-
etry that
∂A
∂V
−
=2κ, where κ is the mean curvature of the surface (the average of the
two principal curvatures); for example the mean curvature κ of a spherical surface
measured from the side on which the centre of the sphere lies is just 1/R, where R
is the sphere radius.
D.9.1 The Gibbs–Thomson Effect
The curvature of an interface also has an effect on the melting temperature, and this
is known as the Gibbs–Thomson effect. For this we may go back to the Clapeyron
type argument and specific Gibbs free energy of each phase (i.e., their chemical
potentials). Denoting these as before as g
s
and g
l
, but now allowing solid and liquid
pressures to change independently, we have
v
s
p
s
−s
s
T =v
l
p
l
−s
l
T , (D.43)
and with L =T
M
s being the latent heat, we have the generalised Clapeyron rela-
tion
LT
M
T
M
=
1
ρ
l
−
1
ρ
s
p
l
−
(p
s
−p
l
)
ρ
s
, (D.44)
in which the first term on the right is the Clapeyron effect of changing pressure, and
the second is the Gibbs–Thomson effect, which describes change of melting tem-
perature with surface curvature, since p
s
−p
l
=2γκ, with the curvature measured
from the solid side of the interface.
D.10 Pre-melting
It is commonly the case that a solid will maintain a thin liquid film of its melt at an
interface with, for example, a quartz grain, even at temperatures below the freezing
point. This phenomenon is known as ‘pre-melting’ (Dash et al. 2006; Wettlaufer and
Worster 2006), and is associated with an excess free energy manifested by very thin
films due to a variety of intermolecular forces, for example Van der Waals forces.
The scale on which these forces act is measured in molecular diameters, and so the

828 D Melting, Dissolution, and Phase Changes
film thicknesses over which these free energy effects are important are of the order
of nanometres. Just as for surface energy, pre-melting causes an excess pressure,
called the disjoining pressure, to occur in the film, and it causes a displacement of
the freezing temperature. A particular geophysical problem in which this disjoining
pressure is important is in the phenomenon of frost heave (Rempel et al. 2004),
wherein freezing soil is uplifted, causing the heave which can be very damaging to
roads and structures. The force generated in frost heave can be very large, of the
order of bars, and this force is due to the disjoining pressure in the thin water films
which separate the ice from the soil grains.
3
To understand the dynamic effects, we consider a thin film of thickness h sepa-
rating an ice surface from a foreign solid surface. In the absence of the film, the ice-
solid interface has a surface energy which we denote by γ
si
, while the interposition
of a liquid film creates two new surfaces, of interfacial energies γ
sw
(solid-water)
and γ
iw
(ice-water). In addition, the liquid film has a Gibbs free energy per unit area
of the form
G =ρ
l
μ
l
h +Φ(h), (D.45)
where μ
l
is the chemical potential energy of the bulk liquid, and Φ is the free energy
associated with intermolecular forces. In particular, we suppose
Φ(0) =γ
si
,Φ(∞) =γ
sw
+γ
iw
; (D.46)
the liquid film is energetically preferred if γ < 0, where
γ =γ
sw
+γ
iw
−γ
si
, (D.47)
and it is in this case that a positive disjoining pressure occurs. We write
Φ =γ
si
+γ φ(h), (D.48)
where φ increases monotonically from zero at h =0 to one at h =∞. For example,
Van der Waals forces lead to a form for φ of
φ =
1 −
σ
2
h
2
+
, (D.49)
where the constant σ is of the order of a molecular diameter. Clearly, if γ < 0,
then Φ is a monotonically decreasing function of h, while the bulk free energy is an
increasing function, and thus a minimum of G in (D.45) will be obtained when h is
finite, if |γ | is sufficiently large. This causes the wetting film.
3
This is perhaps an inverted way of looking at it. Heaving requires the maintenance of the film
between ice and soil grains; as long as the film is maintained, heave will occur. The presence of a
large overburden pressure will eventually suppress heave, but the necessary pressures are large.

D.11 Liesegang Rings 829
D.10.1 Disjoining Pressure
We consider the Helmholtz free energy of a film of thickness h. Following a small
perturbation to the film thickness,
dF =−p
w
dV
w
−p
i
dV
i
−SdT +AdΦ, (D.50)
where A is surface area. We have dV
w
=Adh; for an isothermal change at constant
volume dV
w
=−dV
i
=Adh, and therefore
p
i
−p
w
=−Φ
(h) =−γ φ
(h); (D.51)
this is the disjoining pressure. For (D.49), this leads to
p
i
−p
w
=−
A
6πh
3
, (D.52)
where A is the Hamaker constant
A= 12πσ
2
γ. (D.53)
D.10.2 Freezing Point Depression
Finally we consider the effect of a thin film on the freezing point. This simply fol-
lows from (D.44), which we write in the form
LT
T
M
=(v
w
−v
i
)p
w
−v
i
(p
i
−p
w
), (D.54)
and thus, from (D.51), (ignoring liquid pressure variations)
L(T −T
M
)
T
M
≈
γ φ
(h)
ρ
i
. (D.55)
For γ < 0, this represents the freezing point depression due to pre-melting only;
the Clapeyron and Gibbs–Thomson effects can be added to the right hand side.
Because φ
∝
1
h
3
, these thin films can be maintained to temperatures quite a way
below the normal freezing point.
D.11 Liesegang Rings
As discussed in Chap. 9, Liesegang rings can form when crystals are precipitated
in a dilute solution. Liesegang himself put some silver nitrate on a gel containing
potassium dichromate, and the resulting silver dichromate crystals precipitate in
