Fisher John P. e.a. (ed.) Tissue Engineering
Подождите немного. Документ загружается.

mikos: “9026_c003” — 2007/4/16 — 21:46 — page 18 — #18
3-18 Tissue Engineering
[36] Ikonomou, L., Schneider, Y.J., and Agathos, S.N., Insect cell culture for industrial production of
recombinant proteins, Appl. Microbiol. Biotechnol. 62, 1–20, 2003.
[37] French, M.M. et al., Chondrogenic activity of the heparan sulfate proteoglycan perlecan maps to
the N-terminal domain I, J. Bone Miner. Res. 17, 48–55, 2002.
[38] Sodoyer, R., Expression systems for the production of recombinant pharmaceuticals, BioDrugs 18,
51–62, 2004.
[39] Bulleid, N.J., John, D.C., and Kadler, K.E., Recombinant expression systems for the production of
collagen, Biochem. Soc. Trans. 28, 350–3, 2000.
[40] Knox, S., Melrose, J., and Whitelock, J., Electrophoretic, biosensor, and bioactivity analyses of
perlecans of different cellular origins, Proteomics 1, 1534–41, 2001.
[41] Patrick, C.W., Jr., Adipose tissue engineering: the future of breast and soft tissue reconstruction
following tumor resection, Semin. Surg. Oncol. 19, 302–11, 2000.
[42] Heilshorn, S.C. et al., Endothelial cell adhesion to the fibronectin CS5 domain in artificial
extracellular matrix proteins, Biomaterials 24, 4245–52, 2003.
[43] Yurchenco, P.D., Smirnov, S., and Mathus, T., Analysis of basement membrane self-assembly and
cellular interactions with native and recombinant glycoproteins, Meth. Cell Biol. 69, 111–44, 2002.
[44] Hohenester, E. and Engel, J., Domain structure and organisation in extracellular matrix proteins,
Matrix Biol. 21, 115–28, 2002.
[45] Hulmes, D.J., Building collagen molecules, fibrils, and suprafibrillar structures, J. Struct. Biol. 137,
2–10, 2002.
[46] Matese, J.C., Black, S., and McClay, D.R., Regulated exocytosis and sequential construction of the
extracellular matrix surrounding the sea urchin zygote, Dev. Biol. 186, 16–26, 1997.
[47] Ottani, V. et al., Hierarchical structures in fibrillar collagens, Micron 33, 587–96, 2002.
[48] Gelse, K., Poschl, E., and Aigner, T., Collagens — structure, function, and biosynthesis, Adv. Drug
Deliv. Rev. 55, 1531–46, 2003.
[49] Myllyharju, J. and Kivirikko, K.I., Collagens, modifying enzymes and their mutations in humans,
flies and worms, Trends Genet. 20, 33–43, 2004.
[50] Brown, P.J. and Juliano, R.L., Expression and function of a putative cell surface receptor for
fibronectin in hamster and human cell lines, J. Cell Biol. 103, 1595–603, 1986.
[51] Hersel, U., Dahmen, C., and Kessler, H., RGD modified polymers: biomaterials for stimulated cell
adhesion and beyond, Biomaterials 24, 4385–415, 2003.
[52] Shin, H. et al., Attachment, proliferation, and migration of marrow stromal osteoblasts cultured
in biomimetic hydrogels modified with an osteopontin-derived peptide, Biomaterials 25, 895–906,
2004.
[53] Colognato, H., Winkelmann, D.A., and Yurchenco, P.D., Laminin polymerization induces a
receptor-cytoskeleton network, J. Cell Biol. 145, 619–31, 1999.
[54] Grimpe, B. and Silver, J., The extracellular matrix in axon regeneration, Prog. Brain Res. 137,
333–49, 2002.
[55] Mukaratirwa, S. and Nederbragt, H., Tenascin and proteoglycans: the role of tenascin and
proteoglycans in canine tumours, Res. Vet. Sci. 73, 1–8, 2002.
[56] Sage, E.H., Regulation of interactions between cells and extracellular matrix: a command
performance on several stages, J. Clin. Invest. 107, 781–3, 2001.
[57] Sid, B. et al., Thrombospondin 1: a multifunctional protein implicated in the regulation of tumor
growth, Crit. Rev. Oncol. Hematol. 49, 245–58, 2004.
[58] Murphy-Ullrich, J.E., The de-adhesive activity of matricellular proteins: is intermediate cell
adhesion an adaptive state? J. Clin. Invest. 107, 785–90, 2001.
[59] Esko, J.D. and Selleck, S.B., Order out of chaos: assembly of ligand binding sites in heparan sulfate,
Annu. Rev. Biochem. 71, 435–71, 2002.
[60] Iozzo, R.V., Perlecan: a gem of a proteoglycan, Matrix Biol. 14, 203–8, 1994.
[61] Bernfield, M. et al., Biology of the syndecans: a family of transmembrane heparan sulfate
proteoglycans, Annu.Rev.CellBiol.8, 365–93, 1992.
mikos: “9026_c003” — 2007/4/16 — 21:46 — page 19 — #19
Extracellular Matrix: Structure and Function 3-19
[62] Fransson, L.A., Glypicans, Int. J. Biochem. Cell Biol. 35, 125–9, 2003.
[63] Knudson, C.B. and Knudson, W., Cartilage proteoglycans, Semin. Cell Dev. Biol. 12, 69–78,
2001.
[64] Roberts, S. et al., Matrix metalloproteinases and aggrecanase: their role in disorders of the human
intervertebral disc, Spine 25, 3005–13, 2000.
[65] Sodek, J. et al., Novel functions of the matricellular proteins osteopontin and osteonectin/SPARC,
Connect. Tissue Res. 43, 308–19, 2002.
[66] Denhardt, D.T., The third international conference on osteopontin and related proteins,
San Antonio, Texas, May 10–12, 2002, Calcif. Tissue Int. 74, 213–19, 2004.
[67] Gritli-Linde, A. et al., The whereabouts of a morphogen: direct evidence for short- and graded
long-range activity of hedgehog signaling peptides, Dev. Biol. 236, 364–86, 2001.
[68] Cadigan, K.M., Regulating morphogen gradients in the Drosophila wing, Semin. Cell Dev. Biol. 13,
83–90, 2002.
[69] Hirano, Y. and Mooney, D.J., Peptide and protein presenting materials for tissue engineering, Adv.
Mater. 16, 17–25, 2004.
[70] Drury, J.L. and Mooney, D.J., Hydrogels for tissue engineering: scaffold design variables and
applications, Biomaterials 24, 4337–51, 2003.
[71] Shin, H., Jo, S., and Mikos, A.G., Biomimetic materials for tissue engineeering, Biomaterials 24,
4353–64, 2003.
[72] Sakiyama-Elbert, S.E. and Hubbell, J.A., Functional biomaterials: design of novel biomaterials,
Ann. Rev. Mater. Res. 31, 183–201, 2001.
[73] Seal, B.L., Otero, T.C., and Panitch, A., Polymeric biomaterials for tissue and organ regeneration,
Mat. Sci. Eng. Res. Rep. 34, 147–230, 2001.
[74] Biran, R. et al., Surfactant-immobilized fibronectin enhances bioactivty and regulates sensory
neurite outgrowth, J. Biomed. Mater. Res. 55, 1–12, 2001.
[75] Healy, K.E., Rezania, A., and Stile, R.A., Designing biomaterials to direct biological responses,
Ann. NY Acad. Sci. 875, 24–35, 1999.
[76] Barber, T.A. et al., Peptide-modified p(AAm-co-EG/AAc)IPNs grafted to bulk titanium modulate
osteoblast behavior in vitro, J. Biomed. Mater. Res. A. 64A, 38–47, 2003.
[77] Lutolf, M.P. and Hubbell, J.A., Synthesis and physicochemical characterization of end-linked
poly(ethylene glycol)-co-peptide hydrogels formed by Michael-type addition, Biomacromolecules
4, 713–2, 2003.
[78] Seal, B.L. and Panitch, A., Physical polymer matrices based on affinity interactions between
peptides and polysaccharides, Biomacromolecules 4, 1572–82, 2003.
[79] Heggli, M. et al., Michael-type addition as a tool for surface functionalization, Bioconj. Chem. 14,
967–73, 2003.
[80] Hermanson, G.T., Bioconjugate Techniques. Academic Press, New York, 1996.
[81] Rezania, A. and Healy, K.E., The effect of peptide surface density on mineralization of a matrix
deposited by osteogenic cells, J. Biomed. Mater. Res. 52, 595–600, 2000.
[82] Rowley, J.A. and Mooney, D.J., Alginate type and RGD density control myoblast phenotype,
J. Biomed. Mater. Res. 60, 217–23, 2002.
[83] Hern, D.L. and Hubbell, J.A., Incorporation of adhesion peptides into nonadhesive hydrogels
useful for tissue resurfacing, J. Biomed. Mater. Res. 39, 266–76, 1998.
[84] West, J.L. and Hubbell, J.A., Polymeric biomaterials with degradation sites for proteases involved
in cell migration, Macromolecules 32, 241–4, 1999.
[85] Schense, J.C. et al., Enzymatic incorporation of bioactive peptides into fibrin matrices enhances
neurite extension, Nat. Biotechnol. 18, 415–9, 2000.
[86] Sakiyama-Elbert, S.E. and Hubbell, J.A., Development of fibrin derivatives for controlled release
of heparin-binding growth factors, J. Control. Release 65, 389–402, 2000a.
[87] Sakiyama-Elbert, S.E., Panitch, A., and Hubbell, J.A., Development of growth factor fusion proteins
for cell-triggered delivery, FASEB J. 15, 1300–2, 2001.
mikos: “9026_c003” — 2007/4/16 — 21:46 — page 20 — #20
3-20 Tissue Engineering
[88] Dalsin, J.L. et al., Mussel adhesive protein mimetic polymers for the preparation of nonfouling
surfaces, J. Am. Chem. Soc. 125, 4253–8, 2003.
[89] Tjia, J.S. and Moghe, P.V., “Cell-internalizable” ligand microinterfaces on biomaterials, in Biomi-
metic Materials and Design, Dillow, A.K. and Lowman, A.M. (Eds.), Marcel Dekker, Inc., New York,
2002, pp. 335–73.
[90] Hartgerink, J.D., Beniash, E., and Stupp, S.I., Self-assembly and mineralization of peptide-
amphiphile nanofibers, Science 294, 1684–8, 2001.
[91] Silva, G.A. et al., Selective differentiation of neural progenitor cells by high-epitope density
nanofibers, Science 303, 1352–5, 2004.
[92] Ferrari, F.A. and Cappello, J., Biosynthesis of protein polymers, in Protein-Based Materials,
McGrath, K. and Kaplan, D. (Eds.), Birkhauser, Boston, 1997, pp. 37–60.
[93] Urry, D.W. et al., Transductional elastic and plastic protein-based polymers as potential medical
devices, in Drug Targeting and Delivery, Handbook of Biodegradable Polymers, Domb, A.J., Kost, J.,
and Wiseman, D.M. (Eds.), Harwood Academic Publ., Amsterdam, 1997, pp. 367–86.
[94] Panitch, A. et al., Design and biosynthesis of elastin-like extracellular matrix proteins containing
periodically spaced fibronectin CS5 domains, Macromolecules 32, 1701–3, 1999.
[95] Liu, J.C., Heilshorn, S.C., and Tirrell, D.A., Comparative cell response to artificial extracellu-
lar matrix proteins containing the RGD and CS5 cell-binding domains, Biomacromolecules 5,
497–504, 2004.
[96] Welsh, E.R. and Tirrell, D.A., Engineering the extracellular matrix: a novel approach to polymeric
biomaterials. I. Control of the physical properties of artificial protein matrices designed to support
adhesion of vascular endothelial cells, Biomacromolecules 1, 23–30, 2000.
[97] Di Zio, K. and Tirrell, D.A., Mechanical properties of artificial protein matrices engineered for
control of cell and tissue behavior, Macromolecules 36, 1553–8, 2003.
[98] Fertala, A., Han, W.B., and Ko, F.K., Mapping critical sites in collagen II for rational design of
gene-engineered proteins for cell-supporting materials, J. Biomed. Mater. Res. 57, 48–58, 2001.
[99] Hayashi, M., Tomita, M., and Yoshizato, K., Production of EGF-collagen chimeric protein which
shows the mitogenic activity, Biochim. Biophys. Acta 1528, 187–95, 2001.
[100] Halstenberg, S. et al., Biologically engineered protein-graft-poly(ethylene glycol) hydrogels: a cell
adhesive and plasmin-degradable biosynthetic material for tissue repair, Biomacromolecules 3,
710–23, 2002.
[101] Kapur, T.A. and Shoichet, M.S., Immobilized concentration gradients of nerve growth factor guide
neurite outgrowth, J. Biomed. Mater. Res. A 68A, 235–43, 2004.
[102] Dertinger, S.K.W. et al., Gradients of substrate-bound laminin orient axonal specification of
neurons, Proc. Natl Acad. Sci. USA 99, 12542–7, 2002.
[103] Kramer, S. et al., Preparation of protein gradients through the controlled deposition of protein-
nanoparticle conjugates onto functionalized surfaces, J. Am. Chem. Soc. 126, 5388–95, 2004.
[104] Ravenscroft, M.S. et al., Developmental neurobiology implications from fabrication and analysis
of hippocampal neuronal networks on patterned silane-modified surfaces, J. Am. Chem. Soc. 120,
12169–77, 1998.
[105] Chang, J.C., Brewer, G.J., and Wheller, B.C., Modulation of neural network activity by patterning,
Biosensors Bioelectron. 16, 527–33, 2001.
[106] Mrksich, M. et al., Controlling cell attachment on contoured surfaces with self-assembled
monolayers of alkanethiolates on gold, Proc. Natl Acad. Sci. USA 93, 10775–8, 1996.
[107] Kane, R.S. et al., Patterning proteins and cells using soft lithography, Biomaterials 20, 2363–76,
1999.
[108] Bernard, A. et al., Microcontact printing of proteins, Adv. Mater. 12, 1067–70, 2000.
[109] Patel, N. et al., Printing patterns of biospecifically adsorbed protein, J. Biomater. Sci. Polym. Ed.
11, 319–31, 2000.
[110] McDevitt, T.C. et al., Spatially organized layers of cardiomyocytes on biodegradable polyurethane
films for myocardial repair, J. Biomed. Mater. Res. A 66A, 586–95, 2003.
mikos: “9026_c003” — 2007/4/16 — 21:46 — page 21 — #21
Extracellular Matrix: Structure and Function 3-21
[111] Lee, K.B. et al., Pattern generation of biological ligands on a biodegradable poly(glycolic acid) film,
Langmuir 20, 2531–5, 2004.
[112] Schmalenberg, K.E., Buettner, H.M., and Uhrich, K.E., Microcontact printing of proteins on
oxygen plasma-activated poly(methyl methacrylate), Biomaterials 25, 1851–7, 2004.
[113] Roda, A. et al., Protein microdeposition using a conventional ink-jet printer, Biotechniques 28,
492–6, 2000.
[114] Park, A., Wu, B., and Griffith, L.G., Integration of surface modification and 3D fabrication tech-
niques to prepare patterned poly(l-lactide) substrates allowing regionally selective cell adhesion,
J. Biomater. Sci. Polym. Ed. 9, 89–110, 1998.
[115] Roth, E.A. et al., Inkjet printing for high-throughput cell patterning, Biomaterials 25, 3707–15,
2004.
[116] Wilson, D.L. et al., Surface organization and nanopatterning of collagen by dip-pen nanolitho-
graphy, Proc. Natl Acad. Sci. USA 98, 13660–4, 2001.
[117] Lee, K.B. et al., Protein nanoarrays generated by dip-pen nanolithography, Science 295, 1702–5,
2002.
[118] Lee, K.B., Lim, J.H., and Mirkin, C.A., Protein nanostructures formed via direct-write dip-pen
nanolithography, J. Am. Chem. Soc. 125, 5588–9, 2003.
[119] Maheshwari, G. et al., Cell adhesion and motility depend on nanoscale RGD clustering, J. Cell Sci.
113, 1677–86, 2000.
[120] Koo, L.Y. et al., Co-regulation of cell adhesion by nanoscale RGD organization and mechanical
stimulus, J. Cell Sci. 115, 1423–33, 2002.
[121] Lee, K.Y. and Mooney, D.J., Controlled growth factor delivery for tissue engineering, in Advances in
Controlled Drug Delivery: Science, Technology, and Products, American Chemical Society, NewYork,
2003, pp. 73–83.
[122] Peters, M.C. et al., Release from alginate enhances the biological activity of vascular endothelial
growth factor, J. Biomater. Sci. Polym. Ed. 9, 1267–78, 1998.
[123] Richardson, T.P. et al., Polymeric system for dual growth factor delivery, Nat. Biotechnol. 19,
1029–34, 2001.
[124] Yang, W.D. et al., Perlecan domain I promotes FGF-2 delivery in collagen I fibril scaffolds, Tissue
Eng., 11, 76–89, 2005.
[125] Wissink, M.J.B. et al., Improved endothelialization of vascular grafts by local release of growth
factor from heparinized collagen matrices, J. Control. Release 64, 103–14, 2000.
[126] Liu, L.S. et al., Hyaluronate-heparin conjugate gels for the delivery of basic fibroblast growth factor
(FGF-2), J. Biomed. Mater. Res. 62, 128–35, 2002.
[127] Matsuda, T. and Magoshi, T., Preparation of vinylated polysaccharides and photofabrica-
tion of tubular scaffolds for potential use in tissue engineering, Biomacromolecules 3, 942–50,
2002.
[128] Chinen, N. et al., Action of microparticles of heparin and alginate crosslinked gel when used as
injectable artificial matrices to stablilize basic fibroblast growth factor and induce angiogenesis by
controlling its release, J. Biomed. Mater. Res. A 67A, 61–8, 2003.
[129] Yamaguchi, N. and Kiick, K.L., Polysaccharide-poly(ethylene glycol) star copolymers as scaffolds
for the production of bioactive hydrogels, Biomacromolecules 6, 1921–30, 2005.
[130] Sakiyama-Elbert, S.E. and Hubbell, J.A., Controlled release of nerve growth factor from
a heparin-containing fibrin-based cell ingrowth matrix, J. Control. Release 69, 149–58,
2000b.
[131] Lee, A.C. et al., Controlled release of nerve growth factor enhances sciatic nerve regeneration, Exp.
Neurol. 184, 295–303, 2003.
[132] Kuhl, P.R. and Griffith-Cima, L.G., Tethered epidermal growth factor as a paradigm for growth
factor-induced stimulation from the solid phase, Nat. Med. 2, 1022–7, 1996.
[133] Mann, B.K., Schmedlen, R.H., and West, J.L., Tethered-TGF-beta increases extracellular matrix
production of vascular smooth muscle cells, Biomaterials 22, 439–44, 2001a.
mikos: “9026_c003” — 2007/4/16 — 21:46 — page 22 — #22
3-22 Tissue Engineering
[134] Mann, B.K. et al., Smooth muscle cell growth in photopolymerized hydrogels with cell adhesive and
proteolytically degradable domains: synthetic ECM analogs for tissue engineering, Biomaterials
22, 3045–51, 2001b.
[135] Zisch, A.H. et al., Covalently conjugated VEGF-fibrin matrices for endothelialization, J. Control.
Release 72, 101–13, 2001.
[136] Zisch, A.H. et al., Cell-demanded release of VEGF from synthetic, biointeractive cell-ingrowth
matrices for vascularized tissue growth, FASEB J. 17, 2260–2, 2003.

mikos: “9026_c004” — 2007/4/9 — 15:50 — page1—#1
4
Mechanical Forces on
Cells
Yan-Ting Shiu
University of Utah
4.1 Introduction.............................................. 4-1
4.2 The Role of Cytoskeletal Tension in
Anchorage-Dependent Cells ............................ 4-2
4.3 The Role of ECM Scaffolds in Regulating Cellular
Tension ................................................... 4-4
Effects of the Compliance of ECM Scaffolds • Effects of the
Spatial Distribution of ECM Ligands • Physicality of ECM
Scaffolds in Tissue Engineering
4.4 The Role of Externally Applied Mechanical Forces in
Cell Function............................................. 4-6
Devices and Methodology Used for Mechanical Stimulation of
Cells In Vitro • Responses of Cells to Mechanical Stimulation
In Vitro • Mechanosensing of Cultured Cells to Externally
Applied Mechanical Forces • Applications of Externally
Applied Mechanical Forces in Tissue Engineering
4.5 Concluding Remarks .................................... 4-12
Acknowledgments............................................... 4-13
References ....................................................... 4-13
4.1 Introduction
All cells in the body are subjected to mechanical forces that are either self-generated or originate from the
environment. Depending on their location within the body, cells may be selectively exposed to various
forces such as pressure, fluid shear stress, stretch, and compression. These externally applied mechanical
forces play a significant role in normal tissue homeostasis and remodeling. For example, gravitational
compressive forces control bone deposition, mechanical loads on skeletal muscle determine muscle mass,
and blood flow-associated mechanical forces regulate the homeostasis of vascular walls [1–3]. All external
forces that impinge on cells are imposed on a dynamic backdrop of various internally generated forces
necessary for carrying out fundamental cellular events (e.g., cell division and migration). When cells sense
a change in their net external loading, they actively alter their internal forces to counteract external forces.
There is growing recognition that the balance between internally generated forces and externally applied
forces is a key determinant of cell fate [1,4].
The importance of mechanical forces in tissue engineering applications is clear. The main goal of tis-
sue engineering is the fabrication of artificial tissues for replacing damaged body structures. To produce
4-1

mikos: “9026_c004” — 2007/4/9 — 15:50 — page2—#2
4-2 Tissue Engineering
functional tissues outside the body, it is necessary to create an in vitro culture environment that embod-
ies the basic parameters of a physiological setting. Enormous strides have been made to understand the
biochemical aspects of the in vivo microenvironment. However, the same level of understanding does
not exist for the mechanical contributions. The mechanical environment of mammalian cells in the
body is defined by an intricate balance between external loading and intracellular tension. The goal of
this chapter is to examine the characteristics of each component within the mechanical microenviron-
ment and highlight their implications in tissue engineering applications. The discussion will focus on
anchorage-dependent, nonsensory cells.
4.2 The Role of Cytoskeletal Tension in
Anchorage-Dependent Cells
For anchorage-dependent cells, the ability to apply cytoskeletal forces against the extracellular matrix
(ECM) through integrin receptors is essential for cell survival and proliferation [4–6]. When a cell resides
on an ECM scaffold, its contractile bundles of actin and myosin filaments (i.e., stress fibers) pull on
an array of well-established connections between the cell and ECM known as focal adhesions (FAs). FAs
consist of clustered integrins that span the plasma membrane, interacting with specific ECM ligands on the
outside and with bundles of actin filaments via cytoskeletal-associated proteins (e.g., paxillin, α-actinin,
and vinculin) on the inside [7–9]. In this way, cytoskeletal forces are transmitted via integrins to the
underlying ECM, which acts as an external support for anchoring the cell and balancing the forces that
maintain cell shape (Figure 4.1a) [7,8]. Thus, the adherent cell is under tension due to the ECM’s resistance
to deformation. The tension residing in the cytoskeleton of a resting adherent cell (often referred to as
initial tension, resting tension, or prestress) is a major determinant of cell shape and functions such as
proliferation, differentiation, deformability, migration, signal transduction, and ECM remodeling (see
References 1,6, and 10–12 for review). Cytoskeletal tension is dynamic and can change without external
stimuli during specific fundamental cellular events such as cell division and migration. Cytoskeletaltension
also changes when the cell receives and responds to externally applied mechanical stimuli. Importantly,
cellular responses to an external load may differ depending on the level of the initial tension (or, the
“mechanical tone”) in the cell [4,10–12].
The amount of the initial tension in an adherent cell is collectively controlled by its own actomyosin
contractile machinery and its interaction with the ECM. Actomyosin contraction is driven by the motor
protein myosin II and is triggered by the phosphorylation of the myosin light chain (MLC) in nonmuscle
and smooth muscle cells [13]. The Rho GTPase (a member of the Rho family of GTP binding proteins)
and its effector Rho kinase (ROCK) are important regulators of myosin activity. Blocking cell contractility
by inactivating Rho or ROCK inhibits the formation of tension-dependent structures such as stress
fibers and FAs [14–17]. The intracellular contractile force exerted on the ECM (also called the traction
force) is essential for the assembly of fibronectin fibrils [18]. When the cell is cultured on a silicone
rubber membrane, traction forces distort the substrate, forming wrinkles that can be visualized using
phase-contrast microscopy [19].
Several techniques have been developed to characterize traction forces by measuring the deformation
of elastic substrates (see References 13, and 20–22 for review). These methods can be combined with
fluorescence imaging of FA proteins in living cells (e.g., green fluorescent protein [GFP]-tagged vinculin)
to examine the relationship between the size/shape of FAs and the forces transmitted through them over
time. Many studies have demonstrated conclusively that FAs transmit cytoskeletal forces in the range
of several nanonewtons per square micrometers to the underlying substrate [13]. In addition, it was
found that in stationary fibroblasts expressing GFP-tagged vinculin, the size of FAs is proportional to the
local transmitted force (Figure 4.1b) and the orientation of FAs is parallel to the direction of the force
applied at each FA (Figure 4.1c); the relaxation of the forces (induced by contractility inhibitors) and
the disassembly of FAs occurred simultaneously [23]. It is important to note that the traction force is
directly related, but not identical, to the intracellular contractile force, part of which could be dissipated
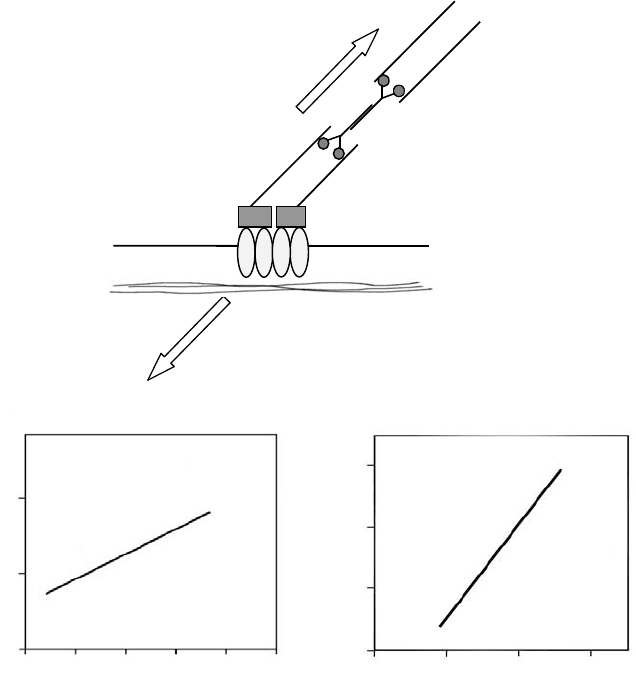
mikos: “9026_c004” — 2007/4/9 — 15:50 — page3—#3
Mechanical Forces on Cells 4-3
Force (nN)
Area of single focal adhesions (mm
2
)
0
2.5
5.0
Force orientation (rad)
Orientation of focal adhesions (rad)
3.0
2.01.0
0
Myosin
Actin
Extracellular matrix
Cell membrane
Cytoskeletal tension
Resistance
Integrins
a
b
a
b
0 5 10 15 20 25 0 1.0 2.0 3.0
(b)
(a)
(c)
FIGURE 4.1 Forces on focal adhesions (FAs). (a) FAs are specialized sites of adhesion that form between cultured
cells and a substrate. They contain clustered integrin receptors (heterodimeric transmembrane glycoproteins) whose
extracellular domain binds to specific ECM ligands and intracellular domain interacts with bundles of actin filaments
via cytoskeletal-associated proteins (the boxes between integrins and actin filaments). Myosin II-driven contractile
forces applied to a cluster of integrins can lead to the development of tension if the underlying ECM is sufficiently
rigid. (Adapted from Burridge, K. and Chrzanowska-Wodnika, M., Annu.Rev.CellDev.Biol.,12, 463, 1996; Geiger, B.
and Bershadsky, A., Cell, 110, 139, 2002. With permission.) (b) and (c) Fibroblasts expressing GFP-tagged vinculin
were cultured on silicone elastomers imprinted with micropatterns of dots. The traction force applied by the cell on
the substrate was calculated to the precision of a single adhesion site based on the displacements of dots (markers)
and the locations of the FAs. In stationary fibroblasts, mature FAs were elongated structures; the size of vinculin-
containing FAs was proportional to the local transmitted force (b) and the main axis of this elongation is parallel to
the direction of the force applied at each FA (c). (Adapted from Balaban, N.Q., et al., Nat. Cell Biol., 3, 466, 2001.
With permission.)
due to cell deformation or other cellular processes [23,24]. Furthermore, the magnitude and direction
of traction forces vary among different regions of a cell [13,23,25,26]. Experimental evidence shows that
the average traction force magnitude over the entire cell area correlates with the state of cell contraction.
Therefore, quantification of traction forces provides insightful information on the state of cellular tension.
Finally, in addition to the ECM, other structures exist that may provide mechanical support for the
tensed actin network in the cytoplasm. Candidates include microtubules and cell–cell contacts. Several
studies have reported that microtubules are under compression in living cells, and that compressive loads

mikos: “9026_c004” — 2007/4/9 — 15:50 — page4—#4
4-4 Tissue Engineering
could be transferred from microtubules to the ECM based on the observation of an increase in traction
forces upon the disruption of microtubules [27–30]. Cells can also transmit forces to their neighbors
through cell–cell junctions. Cells in a confluent monolayer generally form fewer and smaller FAs with the
ECM than subconfluent cells [31,32], suggesting a decreased tension at the interface between the ECM
and a cell monolayer. The interactions between groups of cells and the ECM define “the resting stress
field” within a tissue and are essential for guiding tissue development, remodeling, repair, and maintaining
tissue homeostasis [6,33].
4.3 The Role of ECM Scaffolds in Regulating Cellular Tension
The effect of ECM molecules on cells is primarily mediated through integrins. It is well recognized that the
chemical composition of the ECM influences integrin-mediated signaling pathways. However, a number
of observations have shown that adhesion to the ECM (i.e., ligand occupation) alone is not sufficient to
elicit a complete integrin-mediated response unless the matrix proteins are immobilized and can physically
resist tension [34,35]. In vitro studies have demonstrated that although many integrin signaling events can
be induced in suspended cells by allowing the cells to bind to ECM-coated microbeads, these cells never
enter S phase and may even undergo apoptosis [36–38]. The tension-dependent control of cell growth is
attributed to ensure that only anchored cells can grow. Loss of this control (i.e., anchorage independence)
is a hallmark of cancerous cells [37].
While the chemical composition of the ECM determines whether a cell can bind to it or not, once
ligation is established, the development of tension is influenced by the physicality of the ECM.
4.3.1 Effects of the Compliance of ECM Scaffolds
Because the mechanical properties of the ECM determine its deformation under compressive loads, they
affect the level of tension that a cell can develop; a rigid surface can resist higher tension than a softer surface
and thus allow cells to carry more tension in the cytoskeleton [1,37]. Experimental observations have
confirmed this notion. Wang et al. [39,40] have developed ECM-coated polyacrylamide substrates that
allow the compliance to be varied while maintaining a constant chemical environment. When compared
with rigid substrates, fibroblasts grown on soft substrates exert smaller traction forces, indicating a decrease
in their intracellular tension (Figure 4.2a). This response to a soft substrate is accompanied by a decrease
in the cell spreading area, a decrease in the rate of DNA synthesis, and an increase in the rate of apoptosis
[40]. Similar phenomena have also been observed in three-dimensional cell cultures using stabilized and
freely floating collagen gels (i.e., stressed vs. relaxed gels). Fibroblasts grown in stabilized collagen gels
generate isometric stresses within the gels while those cultured on freely floating gels do not [41]. The
implication of these results for tissue engineering applications is clear; the compliance of the scaffolds for
cells is an important regulator of cell behavior through its influence on cell tension.
It is worth noting that when cells are grown on a substrate containing a stiffness gradient, cells move
preferentially toward the rigid side (a phenomenon known as durotaxis) [42]. This finding indicates that
cells not only respond to but also actively probe substrate flexibility, most likely by applying contractile
forces to the substrate via adhesion sites and then responding to the feedback (i.e., counter-forces from
the substrate) via the same sites [39,40]. Hence, cell-substrate adhesion sites may act as mechanoprobing
devices, translating “external” mechanical input into intracellular signals [39,40]. Several lines of experi-
mental evidence strongly support a pivotal role for integrin-mediated adhesions in the mechanosensing
process (see References 5,8,9,13,43, and 44 for review). FAs are multimolecular complexes consisting of
more than 50 different proteins that link ECM-attached integrins to the actin cytoskeleton [7,8]. Enrich-
ment of signaling and structural proteins at FAs could facilitate intracellular signaling by bringing enzymes
and their substrates into close proximity, thereby enhancing rate and opportunity of the reaction [45].
It is hypothesized that external forces received by integrins may physically change the structure of specific
FA molecules and rearrange the relative positions of FA components, thereby affecting the function of

mikos: “9026_c004” — 2007/4/9 — 15:50 — page5—#5
Mechanical Forces on Cells 4-5
Young’s modulus of the substrate (Pa)
Traction force (Pa)
0 30,000
0 500 1,000
1,500
2,000
(b)(a)
0 1,000 2,000 3,000 4,000
Cell projected area (mm
2
)
Traction force (Pa)
0 20 40 60 80 100
10,000 20,000
25,000
FIGURE 4.2 Effects of the physicality of ECM substrates on traction forces. Cells wereculturedon polyacrylamide gels
embedded with 0.2 µm-diameter fluorescent beads. Cellular traction forces were estimated based on the displacements
of the beads (which reflected the deformation of the gel). The average traction force magnitude over the entire cell area
was reported here. (a) Fibroblasts were cultured on collagen-coated polyacrylamide gels with different stiffness. Cells
exerted larger traction forces on stiffer than softer gels. (Adapted from Wang, H.B., Dembo, M., and Wang,Y.L., Am.
J. Physiol. Cell Physiol., 279, C1345, 2000. With permission.) (b) Human airway smooth muscle cells were cultured
on various micro-sized adhesive islands on the surface of polyacrylamide gels. The Young’s modulus of the gels was
≈1,300 Pa. Promoting cell spreading resulted in increased traction forces. (Adapted from Wang, N. et al., Cell Motil.
Cytoskeleton, 52, 97, 2002. With permission.)
associated signaling molecules and triggering a cascade of signaling events [8,13]. Alternatively, forces
distributed along noncovalent bonds in a multimolecular FA complex may alter bond formation and
dissociation kinetics, thereby altering signal transduction events [3,46].
4.3.2 Effects of the Spatial Distribution of ECM Ligands
An apparent effect of the ECM distribution on a cell is the cell shape (or projected area in a planar culture).
It is well recognized that there is a close relationship between cell area and cell growth. Cells that are forced
to spread over a large surface area survive better and proliferate faster than cells that are more confined
[38,47]. Cell area may also affect the amount of cytoskeletal tension; a larger area underneath the cell
body may resist greater levels of traction, thereby increasing isometric tension inside the cell [26,37,48].
A common and simple way to control cell area is to control the density of the ECM molecules coated on
otherwise nonadhesive cell culture dishes. A higher ECM coating density allows cells to spread better and
form more FAs than a lower coating density. However, because ECM coating density also affects integrin
activation, it remained controversial whether the effect of increased cell area was due to the ECM density
or was separate from it. This issue has been recently resolved by advances in micropatterning techniques,
which allow the synthesis of surfaces on which different micron-sized islands are coated with the same
ECM density and surrounded by nonadhesive regions to constrain cell spreading. When ECM islands are
created on elastic substrates, traction forces can be estimated based on substrate deformation [26]. It was
found that cultured cells spread to take the size and shape of the islands, and that traction forces increased
as cell spreading was promoted (Figure 4.2b) [26]. These results indicate that larger cells carry greater
cytoskeletal tension, and demonstrate that it is the extent of cell spreading, rather than ECM density, that
influences cell tension. Furthermore, blocking the generation of actomyosin-based tension in well-spread
cells (with an inhibitor that does not alter cell shape) was found to inhibit cell growth; thus, cytoskeletal
tension is required for shape-dependent growth control [49].
It has been shown that myosin II-driven tension promotes cell spreading, and cell spreading stimulates
MLC phosphorylation, thereby further increasing cytoskeletal tension generation [26,50]. Hence, there
is an intimate crosstalk between the generation of cytoskeletal tension and the extent of cell distortion,
