Fischer W.B. Viral Membrane Proteins: Structure, Function, and Drug Design
Подождите немного. Документ загружается.

in the genes coding the hemagglutinin and neuraminidase which lead to amino acid sequence
changes in the antibody binding sites (epitopes) on these virus proteins.
The major shifts, on the other hand, involve complete replacement of the genes for one
or both of the surface antigens as a result of reassortment between human and animal (or
avian) influenza viruses, or by mutations in one of these latter viruses which results in their
ability to infect humans (Garman and Laver, 2003).
Nine serologically distinct subtypes of Type A influenza have been discovered in
nature. Of these, N1 and N2 have been found in viruses infecting humans. All of the nine sub-
types have been found in viruses infecting wild water birds.
Neuraminidase of subtype N9 was isolated from a white-capped noddy tern on North
West Island of Australia’s Great Barrier Reef in 1975 (Downie et al., 1977). Crystals of N9
neuraminidase (Figure 17.7) are of particularly high quality and this enzyme has been used
to investigate the antigenic topology of flu neuraminidase and the way the antibody binding
sites (epitopes) change during antigenic drift.
Until recently, the structure of epitopes on protein molecules was a matter of some
controversy. Attempts to characterize the sites on proteins which bound antibodies involved
a plethora of diverse methods (Laver et al., 1990).
These included the use of protein fragments to absorb antisera, the production of
antipeptide antibodies and their reaction with intact proteins, and proteolytic digestion of
protein–antibody complexes in an attempt to discover protected peptide bonds. One claim
was also made that the complete and precise determination of all the antigenic sites on
lysozyme had been achieved (Atassi, 1980). It was stated that there were three precisely
defined antigenic sites on the lysozyme molecule and that each comprised six to seven amino
acids contained within sharp boundaries. However, when crystals of antibodies bound to
lysozyme were analyzed by X-ray crystallography, none of the predicted sites was found to
be involved in the binding (Davies et al., 1989).
Furthermore X-ray crystallography showed that about sixteen amino acids on the sur-
face of lysozyme were in contact with about the same number on the antibody, a far cry from
the four to seven residues in the epitopes described by various authors (Laver et al., 1990).
A number of complexes of antibodies bound to influenza virus neuraminidase have now
been crystallized and the structures determined by X-ray crystallography. The structure of
one of these complexes, N9 neuraminidase–NC 41 Fab, determined at 2.9 Å resolution,
showed that the epitope on the neuraminidase is discontinuous, being composed of five
separate peptide segments involving about 17 amino acid residues (Figure 17.9) which were
in contact with a similar number of amino acid residues on the antibody molecule (Colman
et al., 1987). It has subsequently been shown that only about three or four of these residues
in the epitope contribute to the energy of binding, the others simply having to show
complementarity with residues on the antibody.
3.2. Antigenic Drift in Influenza Virus Neuraminidase
Antibodies to flu neuraminidase do not directly neutralize virus infectivity, but if the
cells in which the virus is growing are bathed in antisera to the neuraminidase, most of the
virus is prevented from exiting the cells and the infection is effectively terminated. However,
if the cells are bathed in a monoclonal antibody to the neuraminidase, mutant virus particles
with changes in the epitope recognized by the antibody will “escape” from the inhibiting
effect of the antibody and continue to grow unhindered.
Structure, Function, and Inhibition of Influenza Virus Neuraminidase 257
258 Elspeth Garman and Graeme Laver
(a)
(b)
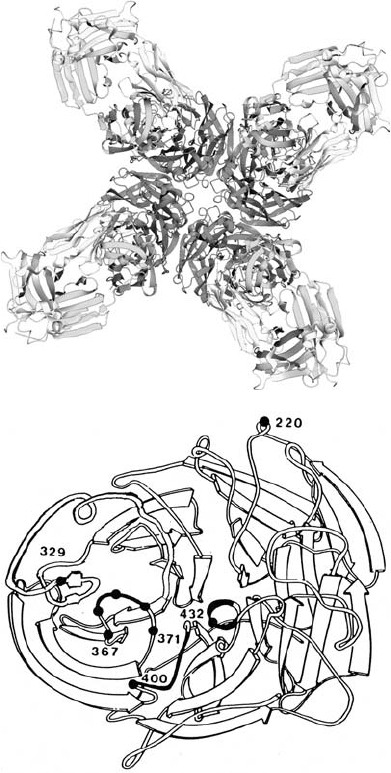
Figure 17.9. (a) Three-dimensional structure of tetrameric influenza neuraminidase subtype N9 complexed with
an Fab fragment from the monoclonal antibody NC41 consisting of F
c
and F
v
from heavy and light chains which
recognize an antigenic determinant (epitope) on the neuraminidase tetramer [PDB entry 1NCA] (Tulip et al., 1992).
(b) Schematic diagram showing a monomer of neuraminidase viewed down the 4-fold axis. The epitope recognized
by NC41 antibody involves the three loops shown in heavy black and also part of the 329 (N2 numbering) loop
(Laver et al., 1987). The side chains of amino acids 368–370 point towards the viewer, while that of Arg 371 (an
active-site residue) points away and into the catalytic site located above and to the right of C
␣
371. Mutations at
positions 367, 369, 370, 372, 400, and 432 abolish the binding of NC41 antibody to neuraminidase, whereas
mutations at 368 and 329 reduce binding. A mutation at residue 220 (outside the NC41 epitope) has no effect on
binding of NC41 to neuraminidase.
Many such neuraminidase escape mutants of N2 and N9 have been analyzed and in
each case single amino acid sequence changes were found in the neuraminidase polypeptide
(Air and Laver, 1986). These single changes were enough to completely abolish binding of
the monoclonal antibody which was used to select the particular escape mutant analyzed.
Most of these sequence changes occurred on the top of the neuraminidase “head” on the
rim surrounding the active-site crater, suggesting that neutralizing epitopes were situated in
this region. Other epitopes almost certainly exist at the base of the tetramer, but escape
mutants of these have never been obtained, presumably because antibodies binding in this
region do not “neutralize” infectivity.
How do single amino acid sequence changes totally abolish antibody binding when
only one out of about seventeen contact residues in the epitope is altered? This question was
addressed structurally by Tulip et al. (1991) who determined the structure of five N9 antibody
escape mutants. The mutations were all situated within 5–10 Å of the N9 catalytic site. Only
local structural changes associated with the site of the amino acid substitution or residues
on either side of it were found; no large scale rearrangements were observed. Although the
precise basis for the abolition of antibody binding is still not clear, changes in charge and
shape complementarity between the two interacting surfaces no doubt play a part.
4. Inhibition of Influenza Virus Neuraminidase
4.1. Design and Synthesis of Novel Inhibitors of
Influenza Virus Neuraminidase
4.1.1. Relenza
Relenza was the first inhibitor to be synthesized which specifically inhibited the
neuraminidase of both Type A and Type B influenza viruses and was effective in controlling
influenza infections in people. Its design was based on the crystal structure of flu
neuraminidase and a sialic acid scaffold. Sialic (neuraminic) acid (Figure 17.10a) is itself a
mild inhibitor of flu neuraminidase, but the dehydrated derivative, deoxy dehydro N-acetyl
neuraminic acid, DANA, Neu5Ac2en (Figure 17.10b) the transition state analog, is a much
better inhibitor. This was convincingly demonstrated by Peter Palese and his colleagues in the
1970s (Palese et al., 1974b). DANA inhibited influenza virus replication in tissue culture but
failed to prevent disease in flu infected animals (Palese et al., 1977).
In using the three-dimensional structure of flu neuraminidase for the rational design
of antiviral drugs, manual inspection of the active site with the aid of computer graphics
was complemented by probing the active-site interactive surfaces with various chemical
substituents using the computer software program GRID (Goodford, 1996) to calculate
energetically favorable substitutions on the sialic acid scaffold.
The following precise account of the design of Relenza by Mark von Itzstein and his
colleagues is given by Dr. Wen Yang Wu.
“Structural studies where sialic acid was soaked into flu neuraminidase crystals showed
that there was a negatively charged zone in the neuraminidase active site which aligned
with the 4-position of the bound sialic acid (Colman et al., 1983). This led to the
suggestion that the introduction of a positively charged group, such as an amino group, to the
4-position of sialic acid should enhance its binding to the active site.
Structure, Function, and Inhibition of Influenza Virus Neuraminidase 259
260 Elspeth Garman and Graeme Laver
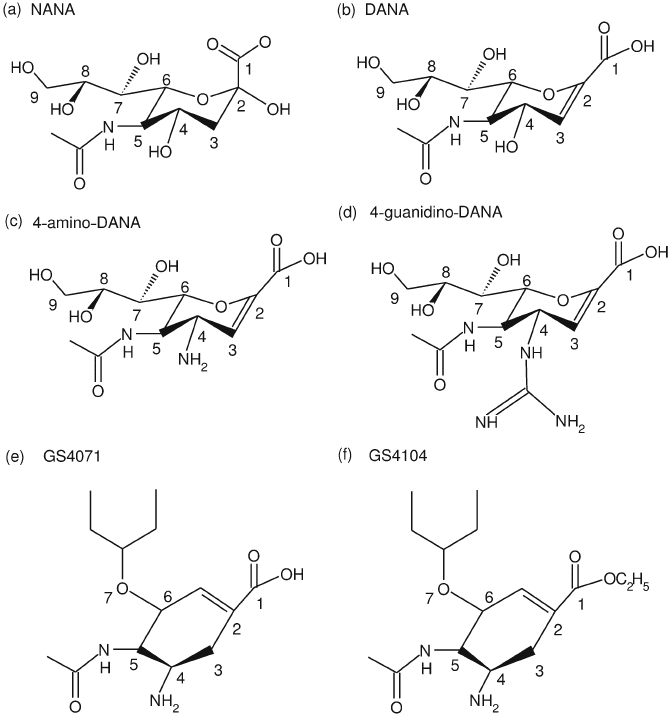
Figure 17.10. Chemical structure of (a) N-acetyl neuraminic acid, NANA, (b) 2-deoxy 2,3-dehydro-N-acetyl
neuraminic acid, DANA, (c) 4-amino-DANA, (d) 4-guanidino-DANA, (Relenza, zanamivir), (e) (3R,4R,5S)-4-
acetamido-5-amino-3-(1-ethylpropoxyl)-1-cyclohexane-1-carboxylic acid (GS4071), and (f) ethyl ester derivative of
(GS4104, tamiflu).
4-Amino DANA was therefore synthesized and, as predicted, bound more tightly to the
active site. There was a 100-fold increase in inhibitory activity for flu neuraminidase of
4-amino DANA compared to the unsubstituted DANA.
Furthermore, the 4-amino DANA was specific for influenza virus neuraminidase and did
not inhibit mammalian neuraminidases. This strong inhibitory activity and high specificity
suggested that the approach being used might lead to a safe and effective anti-influenza drug.
Further modifications at the 4-position were therefore explored. In this, the synthetic
chemistry focused on the introduction of additional positive charges at the 4-position. A num-
ber of 4-substituted amino-DANA analogs were therefore prepared. All showed good
inhibitory activity and specificity.
It was then proposed to synthesize an analogue with a guanidino group at the 4-position
of DANA, because of its increased positive charge and bigger size, compared to the amino
group. Although it appeared that the bulky 4-guanidino-DANA (Figure 17.10c) would not fit
into the neuraminidase active site, after one water molecule was expelled from the active site,
it would fit in perfectly.
After a few synthetic chemistry challenges were overcome, the 4-guanidino-DANA
analogue was prepared and tested. It was found to be 1,000-fold better inhibitor of flu neu-
raminidase than DANA and did not inhibit mammalian neuraminidases (Von Itzstein et al.,
1993). Although subsequently many derivatives of 4-guanidino-DANA were prepared
and tested, ultimately the 4-guanidino-DANA analogue (Figure 17.10d) was chosen for clin-
ical trials. It is now marketed as “Relenza” by Glaxo-Smith Kline Ltd.”
However, because of the guanidino group, Relenza is not orally bioavailable and is
given as a powder which is puffed into the lungs.
A second generation Relenza is being developed. This is a dimer in which two mole-
cules of 4-guanidino-DANA are linked via their 7-hydroxyl groups by an appropriate spacer
such as a benzene ring or aliphatic chain. The dimer exhibits cooperativity in binding so that
the inhibitory activity for flu neuraminidase is 100-fold greater than that of Relenza.
Moreover, after administration, the dimer remains in the respiratory secretions for up to
a week. This suggests that one dose of the dimer every 5 days should be effective, compared
to the therapeutic regime for Relenza and Tamiflu of 2 doses/day for a period of 5 days
(Tucker, 2002).
4.1.2. Tamiflu
In order to produce a neuraminidase inhibitor which was orally bioavailable and which
flu sufferers could swallow as a pill, Choung Kim and his associates at Gilead Sciences in
California synthesized a carbocyclic compound which fulfilled this requirement (Kim et al.,
1997). They noticed the presence of a large hydrophobic pocket in the active site region of flu
neuraminidase that accommodated the glycerol side chain of the substrate, sialic acid, and
exploited this pocket in the synthesis of carbocyclic sialic acid analogs with hydrophobic
alkyl side chains. These carbocyclic compounds are not sugars and have no oxygen in
the ring.
X-ray crystallography of flu neuraminidase with DANA bound in the catalytic site
showed that the C7 position of the glycerol side chain had no interactions with any of the
amino acids in the neuraminidase catalytic site. This suggested that the C7 hydroxyl could be
eliminated from the glycerol side chain of the carbocyclic system without losing binding
affinity to the neuraminidase.
The CHOH group at the C7 position of the glycerol side chain was therefore replaced
by an oxygen atom. Then, in order to create a molecule with hydrophobic groups which
would interact well with the amino acids Glu 276, Ala 246, Arg 224, and Ile 222 (N9 subtype
numbering) in the large hydrophobic pocket occupied by the glycerol side chain of DANA,
various lipophilic side chains were attached to the oxygen linker that had been introduced at
the 7 position.
Structure, Function, and Inhibition of Influenza Virus Neuraminidase 261
The carboxylate and acetamido groups corresponding to the same groups on DANA
were retained on the new carboxylic compound and an amino group was introduced at posi-
tion C4. An amino rather than a guanidino group was chosen at C4 since the latter, while giv-
ing a more tightly binding inhibitor, would create a molecule having the same disadvantage
as Relenza, in that it would not be orally bioavailable.
The final compound chosen, GS4071, with a 3-pentyl side chain (Figure 17.10e) was a
potent and specific inhibitor of Type A and Type B influenza virus neuraminidase with an
IC50 of 1–2 nM. The X-ray crystallographic structure of GS4071 bound in the catalytic site
of flu neuraminidase is shown in Figure 17.11.
Because of the zwitterionic nature of GS4071 imposed on the molecule by the
carboxylate and amino groups, GS4071 was not orally bioavailable. This problem was over-
come by converting the carboxylate to the ethyl ester. The resulting compound, GS4104
(Figure 17.10f) now marketed as “Tamiflu”, can be swallowed as a pill. Following absorption
of this prodrug from the gut, the ester is hydrolyzed in the liver and the resulting active neu-
raminidase inhibitor finds its way into the respiratory tract. It is not clear why GS4071 is able
to cross membranes in the respiratory tract when it was unable to cross membranes in the gut.
Following clinical trials Relenza and Tamiflu are now being used worldwide for the
treatment of influenza. They are safe and effective drugs, provided they are used correctly.
They need to be given very soon after infection and they are effective against influenza only,
and not against any other respiratory pathogens, viral or bacterial. Their effectiveness in
preventing death in cases of severe influenza has not been established, but anecdotal evidence
suggests that this may indeed be an important property of these drugs.
4.1.3. Other Inhibitors of Influenza Virus Neuraminidase
Two other potent and specific inhibitors of flu neuraminidase have been developed.
One, invented at BioCryst, is BCX-1812 (1S,2S,3R–4R,1S)-3-(1-acetylamino-2-ethyl)
butyl-4-[(aminoimino)-methyl]amino-2-hydroxycyclopentane-1-carboxylic acid) (Babu
et al., 2000).
The second inhibitor, made by Abbott Labs, is A315675 ()-(2R,4S,5R,1R,2S)-5-
(1-acetylamino-2-methoxy-2-methyl-pentyl)-4-propenyl-pyrrolidine-2-carboxylic acid (Kati
et al., 2001). So far, neither of these drugs has been approved for human use.
The way these two compounds, as well as Relenza and Tamiflu, bind in the catalytic
site of flu neuraminidase is shown in Figure 17.11.
4.2. Drug Resistance
One of the unanswered questions is; if Relenza and Tamiflu are used widely in the
community to treat influenza, how easily will drug resistant mutants of influenza virus arise?
Experiments so far have suggested that the virus might have difficulty in escaping from
the neuraminidase inhibitors. Influenza viruses resistant to the neuraminidase inhibitors have
been selected in vitro by growing virus in the presence of sublimiting concentrations of the
drugs. These experiments revealed the existence of two classes of resistant mutants (Roberts,
2001).
Some mutants had amino acid sequence changes in the hemagglutinin and none in the
neuraminidase, while others had changes in the neuraminidase but not in the hemagglutinin.
It is thought that the hemagglutinin mutants had a reduced capacity to bind to sialic acid
262 Elspeth Garman and Graeme Laver
Structure, Function, and Inhibition of Influenza Virus Neuraminidase 263
(a)
(b)
Figure 17.11. Continued
receptors, and so had little need for these to be destroyed by neuraminidase for the virus to
“escape.” The fact that the hemagglutinin mutants have so far been found to be as susceptible
to the neuraminidase inhibitors as the wild-type viruses in animal experiments, suggests
that the neuraminidase may play some vital role other than receptor destruction in the
infection process. Possibly the enzyme is required to facilitate the movement of virus
particles through respiratory secretions, and thus if it is blocked, the virus may be trapped and
immobilized.
264 Elspeth Garman and Graeme Laver
(c)
(d)
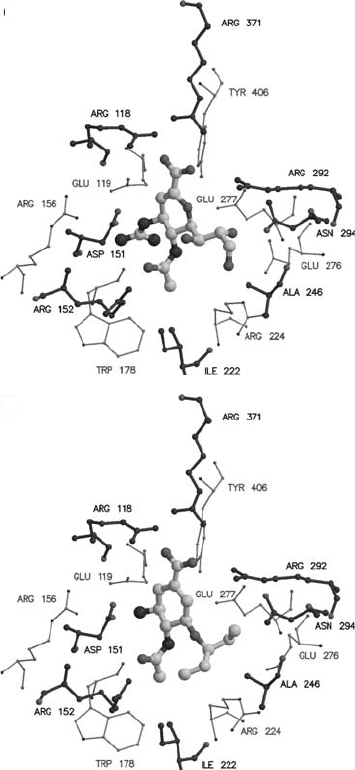
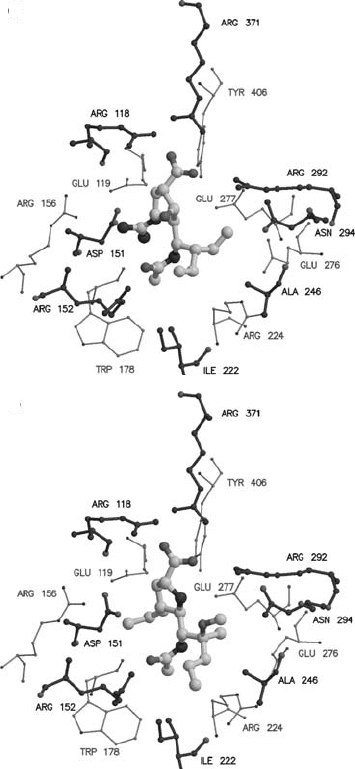
Figure 17.11. Crystallographic structures of influenza virus neuraminidase (N9 subtype) with four different
rationally designed inhibitors bound in the active site of the enzyme. The inhibitors are shown as atom-colored
ball and stick models. The catalytic site of the enzyme is shown with the closer carbon atoms shown darker than those
shown further away. This catalytic site is conserved across all flu neuraminidases. (a) and (b) are antiflu drugs
approved for use: (a) is Relenza (4-guanidino-Neu5Ac2en); (b) is de-esterified Tamiflu (4-acetamido-5-amino-3
(1-ethylpropoxyl)-1-cyclohexane-1-carboxylic acid); (c) and (d) are two further drugs which are being developed:
(c) is BCX-1812 [BioCryst] (1S,2S,3R,-4R,1S)-3-(1-acetylamino-2-ethyl)butyl-4-[(aminoimino)-methyl]amino-
2-hydroxycyclopentane-1-carboxylic acid), and (d) is A315675 [Abbott] ()-(2R,4S,5R,1R,2S)-5-(1-acetylamino-
2-methoxy-2-methyl-pentyl)-4-propenyl-pyrrolidine-2-carboxylic acid. The figures were drawn with Molscript and
rendered with Raster3d.
Sequence changes in the neuraminidase mutants selected by the neuraminidase
inhibitors occurred in active-site residues of the enzyme. This makes sense, as it is those
residues which are involved in binding the inhibitors. In particular, there were changes to
Arg292 (to lysine), one of the three active-site arginines which interact with the natural sub-
strate carboxylate group (which is also present in 4-guanidino-DANA and GS4071), and to
Glu119 (to glycine) which lies in the pocket occupied by the 4-guanidino group of Relenza.
A structural study of the Arg292Lys N9 mutant bound to various inhibitors (Varghese et al.,
1998) revealed that the structural and binding effects of the mutation were most marked for
those inhibitors which were least like the natural ligand. For binding of GS4071 in neu-
raminidase, residue Glu276 rotates to form a salt link with Arg224, creating a hydrophobic
pocket. In the Arg292Lys mutant, Glu276 appears to be anchored by an ionic link to Lys292
not present in the wild-type enzyme, and cannot rotate to form the necessary salt link with
Arg224, thus reducing the binding of GS4071.
The active-site residues which have been found to mutate are also involved in catalysis,
and the mutant neuraminidases were found to be “crippled” in some way, making the mutant
virus less able to infect animals. These findings suggest that mutants resistant to Relenza and
Tamiflu might arise infrequently in the human population.
5. Conclusions
The four drugs described above represent the first example of antiviral drugs rationally
designed from knowledge of the X-ray crystal structure of the target protein of the virus.
These safe drugs, which are most effective if given within a day of symptoms appearing,
have had a rocky ride being accepted into the market place. For example, currently in Britain,
the normal time taken to see a General Practitioner is about two days. Because Relenza and
Tamiflu are only available on prescription, by the time they are administered it is too late for
efficacy. A further problem is that they are only effective against influenza viruses. This would
be remedied if a cheap, rapid, and accurate diagnostic test for flu were available.
However, in the event of an influenza pandemic, it is generally accepted that antiviral
drugs will provide the first line of defense against the new virus. Of these, the neuraminidase
inhibitors are the drugs of choice (WHO, 2003).
Acknowledgments
We thank Drs. Kim and Wu for their personal insights into the inhibitor discovery process,
and Stephen Lee, Martin Noble, Atlanta Cook, and James Murray for help with the figures.
References
Air, G.M. and Laver, W.G. (1986). The molecular basis of antigenic variation in influenza virus. Adv. Virus Res. 31,
53–102.
Atassi, M.Z. (1980). Molecular immune recognition of proteins: The precise determination of protein antigenic sites
has led to synthesis of antibody combining sites and other types of protein binding sites. In Laver, W.G. and
Air, G.M. (eds.), Structure and Variation in Influenza Virus, Elsevier North Holland, Inc, pp. 241–271.
Structure, Function, and Inhibition of Influenza Virus Neuraminidase 265
Babu, Y.S., Chand, P., Banta, S., Kotian, P., Dehghani, A., El-Katan, Y. et al. (2000). BCX-1812 (RWJ-27021).
Discovery of a novel, highly potent, orally active and selective influenza virus neuraminidase inhibitor through
structure-based drug design. J. Med. Chem. 43, 3482–3486.
Baker, A.T., Varghese, J.N. Laver, W.G. Air, G.M. and Colman, P.M. (1987). Three-dimensional structure of
neuraminidase of subtype N9 from an avian influenza virus. Proteins 2, 111–117.
Blow, D. (2002). Outline of Crystallography for Biologists. OUP, Oxford, United Kingdom. 0198510519
Burmeister, W.P., Ruigrok, R.W.H., and Cusack, S. (1992). The 2.2 Å resolution crystal structure of influenza B
neuraminidase and its complex with sialic acid. EMBO J. 11, 49–56.
Burnet, F.M. (1948). quoted in: Aust. J. Exp. Biol. Med. Sci. 26, 410.
Colman, P.M., Laver, W.G., Varghese, J.N., Baker, A.T., Tulloch, P.A., Air, G.M. et al. (1987). Three dimensional
structure of a complex of antibody with influenza virus neuraminidase. Nature 326, 358–363.
Colman, P.M., Varghese, J.N., and Laver, W.G. (1983). Structure of the catalytic and antigenic sites in influenza virus
NA. Nature 303, 41–44.
Davies, D.R., Sheriff, S., Padlan, E.A., Silverton, E.W., Cohen, G.H., and Smith-Gill, S.J. (1989). Three dimensional
structures of two Fab complexes with lysozyme. In S. Smith-Gill and E. Sercarz (eds.), The Immune Response
to Structurally Defined Protein: The Lysozyme Model. Adenine Press, New York, ISBN 0-940030-27-6,
pp. 125–132.
Downie, J.C. Hinshaw, V., Laver, W.G. (1977). The ecology of influenza. Isolation of type “À” influenza viruses from
Australian pelagic birds. Aust. J. Exp. Biol. Med. Sci. 55, 635–643.
Els, M.C., Air, G.M., Murti, K.G., Webster, R.G., and Laver, W.G. (1985). An 18-amino acid deletion in an influenza
neuraminidase. Virology 142, 241–247.
Fujii, Y., Goto, H., Watanabe, T., Yoshida, T., and Kawaoka, Y. (2003). Selective incorporation of influenza virus
RNA segments into virions. PNAS 100, 2002–2007.
Garman, E., E. Rudino-Pinera, P. Tunnah, S.C. Crennell, R.G. Webster, and W.G. Laver (1995). Unpublished
structure of N6 neuraminidase.
Garman, E.F. and Laver, W.G. (2004). Controlling influenza by inhibiting the virus’s neuraminidase. Curr. Drug
Targets 5, 119–136.
Garman, E.F. and T.R. Schneider, (1997). Macromolecular cryocrystallography. J. Appl. Cryst. 30, 211–237.
Goodford, P. (1996). Multivariate characterisation of molecules for QSAR analysis. J. Chemometrics 10, 107–117.
Gottschalk, A. (1957). The specific enzyme of influenza virus and Vibrio cholerae. Biochem. Biophys. Acta 23,
645–646.
Hirst, G.K. (1941). The agglutination of red cells by allantoic fluid of chick embryos infected with influenza virus.
Science 94, 22–23.
Kati, W.M., D. Montgomery, C. Maring, V.S. Stoll, V. Giranda, X., Chen et al. (2001). Novel - and -amino acid
inhibitors of influenza virus neuraminidase. Antimicrob. Agents Chemother. 45, 2563–2570.
Kim, C.U., Williams, M.A., Lui, H., Zhang, L., Swaminathan, S., Bischofberger, N. et al. (1997). Influenza neu-
raminidase inhibitors possessing a novel hydrophobic interaction in the enzyme active site: Design, synthesis
and structural analysis of carbocyclic sialic acid analogues with potent anti-influenza activity. J. Am. Chem. Soc.
119, 681–690.
Laver, W.G. (1964). Structural studies on the protein subunits from three strains of influenza virus. J. Mol. Biol. 9,
109–124.
Laver, W.G. (1978). Crystallization and peptide maps of neuraminidase “heads” from H2N2 and H3N2 influenza
virus strains. Virology 86, 78–87.
Laver, W.G. and Valentine, R.C. (1969). Morphology of the isolated hemagglutinin and neuraminidase subunits of
influenza virus. Virology 38, 105–119.
Laver, W.G., Air, G.M., Webster, R.G., and Smith, G.S. (1990). Epitopes on protein antigens: Misconceptions and
realities. Cell 61, 553–556.
Laver, W.G., Colman, P.M., Webster, R.G., Hinshaw, V.S. and Air, G.M. (1984). Influenza virus neuraminidase with
hemagglutinin activity. Virology 137, 314–323.
Laver, W.G., Webster, R.G., and Colman, P.M. (1987). Crystals of antibodies complexed with influenza virus
neuraminidase show isosteric binding of antibody to wild type and variant antigens. Virology 156, 181–184.
Mayron, L.W., Robert, B., Winzler, R.J. and Rafelson, M.E. (1961). Studies on the neuraminidase of influenza virus 1.
Separation and some properties of the enzyme from Asian and PR8 strains. Arch. Biochem. Biophys. 92, 475–483.
Mitnaul, L.J., Castrucci, M.R., Murti, K.G., and Kawaoka, Y. (1996). The cytoplasmic tail of influenza A virus neu-
ramindase (NA) affects NA incorporation into virions, virion morphology, and virulence in mice but is not
essential for virus replication. J. Virol. 70, 873–879.
266 Elspeth Garman and Graeme Laver
