Fischer W.B. Viral Membrane Proteins: Structure, Function, and Drug Design
Подождите немного. Документ загружается.

favorable (G 艐 11 kcal mol
1
) the 38CCLPFLVVAGAYLAKVDA55 (i-o)-TMD would
not spontaneously remain inserted at the membrane(G 艐2 kcal mol
1
). Exposure to
increasing membrane thickness during protein trafficking might affect 6K membrane topol-
ogy. In particular we proposed that the interfacial “aroma” region helix, whose main axis is
approximately parallel to the bilayer plane, followed by a single TMD, would be a more
favorable topology adopted by the secreted versions of 6K (Sanz et al., 2003). Indeed,
WW-2 suggests that a single 22LCIPLAAFIVLMRCCSCCLPFLVVAGAYLA51 (o-i)-TMD
would be energetically favored (G 艐 10 kcal mol
1
).
2.2. Inducible Synthesis of 6K in E. coli
The system developed by Studier’s group (Studier and Moffat, 1986; Rosemberg et al.,
1987; Studier et al., 1990) has been used successfully for the expression of toxic proteins in
E. coli. This system employs an E. coli strain, BL21(DE3), bearing a prophage (DE3) inte-
grated in its genome that includes the sequence codifying for T7 RNA polymerase under the
control of a lac promoter. The gene encoding the toxic protein to be induced is cloned into
pET plasmids under the control of a T7 RNA polymerase promoter. A variant of this system,
which is even more repressed, makes use of the plasmid pLys, which encodes lysozyme, an
inhibitor of T7 RNA polymerase. Besides, rifampicin selectively blocks transcription driven
by bacterial polymerase, but has no effect on the T7 RNA polymerase itself. Under these
conditions, only recombinant proteins are synthesized.
Inducible expression of the 6K gene is achieved in both systems, BL21(DE3) and
BL21(DE3)pLys E. coli cells. Significant synthesis of the highly toxic 6K protein is found
when 6K is cloned in pET11 and expressed in BL21(DE3)pLys cells in the presence of IPTG
and rifampicin. 6K synthesis cannot be detected in cultures of BL21(DE3) and plasmid
pET3-6K that are less repressed. The toxicity of 6K induces a positive selection of cells that
do not synthesize this protein. This selection results from the loss of the prophage. The E. coli
cells that have lost the prophage do not contain T7 RNA polymerase and consequently do not
express 6K. All of the colonies obtained after transfection of E. coli BL21(DE3) with
pET3-6K plasmid are unable to produce 6K. Even the expression of 6K under the more
repressed conditions may frequently give rise to a mixture of cells that are capable and inca-
pable of expressing 6K. This is an important point to consider if results are to be correctly
interpreted.
2.3. Synthesis of 6K in Mammalian Cells
The expression of 6K in mammalian cells has been achieved using Sindbis Virus (SV)
replicons derived from an infective SV cDNA clone (Hahn et al., 1992; Sanz et al., 2001).
These replicons retain the sequence for the nonstructural proteins and the noncodifying
sequences at the 3 and 5 ends of the genomic RNA (Figure 16.3). The genes encoding for
the structural proteins can be deleted without affecting its replication ability. Moreover, the
presence of the capsid sequence enhances the translation of the subgenomic mRNAs (Frolov
and Schlesinger, 1994, 1996). The SV sequences in the form of cDNA are located after
a T7 promoter. Therefore, they can be in-vitro transcribed from the corresponding plasmid
using the T7 phage RNA polymerase. These transcribed RNAs from SV cDNA are then
electroporated into BHK cells (Liljeström et al., 1991; Soumalainen and Garoff, 1994).
236 M.A. Sanz et al.
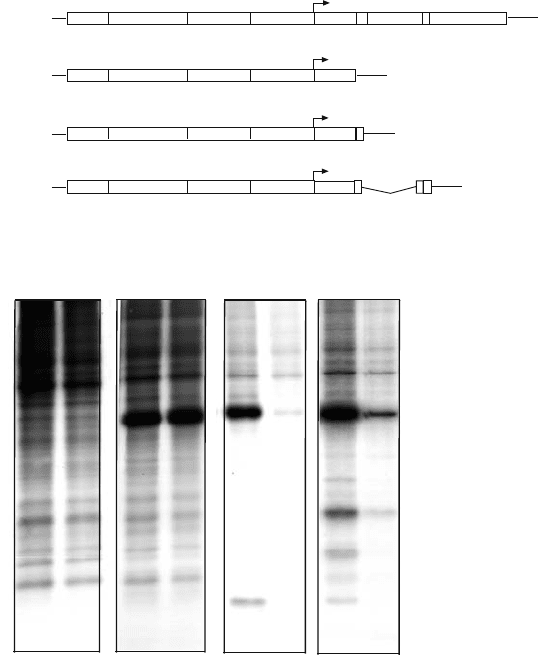
Systems to express the alphavirus 6K gene, based on SV replicons that synthesize the
6K protein in large amounts, have been developed. Two different constructs have been
employed (Figure 16.3). One of them uses a construct containing the 6K sequence adjacent
to the capsid gene. In this replicon, the 6K product released after C autoproteolysis contains
four additional amino acids in its N-terminal end, two from the E3 sequence and two from the
Nde I site introduced in the cloning process. Upon translation of the subgenomic mRNA,
the Capsid (C) protein is liberated by its autocatalytic activity and the rest of the protein is
made at equimolar levels as compared with C. As 6K is an integral membrane protein and
its insertion into the membrane is necessary to position the polyprotein precursor correctly it
is possible that this 6K may not acquire a genuine topology in the membrane. To circumvent
The Alphavirus 6K Protein 237
Poly A
Cap
Non-structural proteins SV
3' n.c. SVnsP1 nsP2 nsP3 nsP4
C6KE2E3 E1
Structural proteins SV
Cap
C
Cap
C
6K
Cap
C ∆E3 6K∆E2
SINDBIS
rep C
rep C+6K
rep C+ ∆(E3/E2) 6K
Poly A
Poly A
Poly A
A
_
+
HB
BHK rep C+6K
rep C+
∆(E3/E2) 6K
rep C
_
+
_
+
_
+
C
6K
B
Figure 16.3. Permeabilization of BHK cells to hygromycin B by the expression of SV 6K. (A) Schematic
representation of the SV genome, the replicon that only encodes C protein, and the two constructs that express SV
6K. (B) Permeabilization to hygromycin. BHK cells were transfected with the indicated RNAs. Protein synthesis was
estimated at 16 hr post-transfection, as indicated in Figure 16.1.
this problem, another construct was engineered, which retains the ER translocation sequence
from E3 and the end of the E2 sequence. The two sites of proteolytic cleavage, those between
C–E3 and E2–6K, have been maintained in this construct in order to synthesize a genuine 6K
protein with no extra amino acids (Figure 16.3). The cleavage between E2–6K which is made
by a signalase present at the ER demonstrates that 6K has reached this cellular compartment.
Large amounts of 6K are synthesized from both replicons and the permeabilizing ability of
6K in both cases is similar (Figure 16.3).
3. Synthesis of 6K During Virus Infection
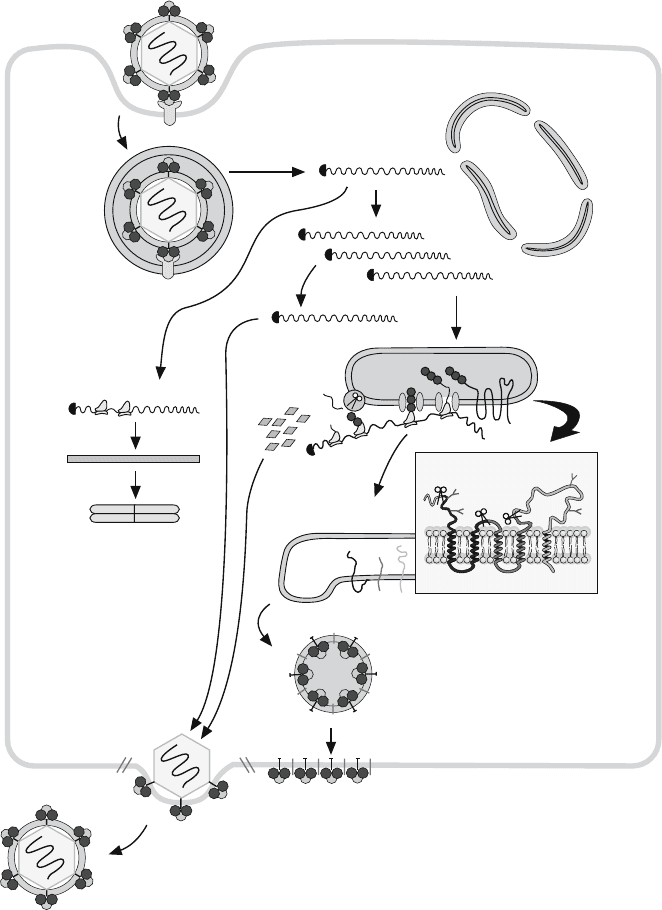
The schematic representation of the alphavirus lytic cycle is depicted in Figure 16.4.
After virus entry, the genomic single-stranded RNA is translated and subsequently transcribed
to generate the late subgenomic 26S mRNA. The alphavirus structural proteins are synthe-
sized from this subgenomic mRNA, which encodes a proteolytically processed polyprotein
(for reviews see Strauss and Strauss, 1994; Schlesinger and Schlesinger, 1996; Garoff et al.,
1998). C protein is first synthesized and detaches from the rest of the polyprotein by autocat-
alytic proteolysis at its carboxy terminus. Once C protein has been liberated to the cytoplasm,
polyprotein synthesis continues associated with ER membranes. After C cleavage, the
exposed amino terminus contains a signal sequence that interacts with ER membranes and
directs the glycoprotein precursor (E3-E2-6K-E1) into the lumen of the ER vesicles. This
precursor becomes associated with the ER membrane, spanning the lipid bilayer five times.
This precursor is then cleaved at both ends of the 6K protein by a cellular protease present in
the ER, generating the products PE2 (E3E2), 6K and E1. PE2 and E1 then associate with
each other to form dimers that travel with 6K through the vesicular system to the plasma
membrane. In addition, the PE2-E1 dimers oligomerize, with the result that the dimers adopt
a trimeric structure. As a final proteolytic step, PE2 is cleaved by a furin-like protease pres-
ent in a post-Golgi compartment, giving rise to glycoproteins E3 and E2. The glycoproteins
transported to the plasma membrane expose their amino-terminal ectodomains to the external
medium, while the carboxy domains remain facing the cytoplasm. The virus genomes repli-
cated in the cytoplasm interact with the C protein to form nucleocapsids. The assembled
nucleocapsids subsequently interact with the carboxy domain of E2. This interaction pro-
vokes the wrapping of the capsid by the lipid envelope, concomitant with the budding of virus
particles.
4. Cell Membrane Permeabilization by 6K
Alphavirus 6K is a very hydrophobic protein of about 60 amino acid residues that has
been classified as a viroporin (Carrasco, 1995). 6K is a heavily acylated integral membrane
protein (Gaedigk-Nitscho and Schlesinger, 1990; Lusa et al., 1991). There are a number of
methods for testing the permeabilization ability of a protein based on the entry or release of
compounds that usually do not cross the cellular plasma membrane (Lama and Carrasco,
1992; Sanz et al., 1994; Carrasco, 1995). The hygromycin B test has been widely employed
to analyze permeability changes produced by the isolated expression of alphavirus 6K in bac-
terial and mammalian cells. The entry of this aminoglycoside antibiotic into cells inhibits
translation that can be easily detected by a radioactive protein labeling assay.
238 M.A. Sanz et al.
The Alphavirus 6K Protein 239
Nucleus
Lumen
P E2
E1
Cytoplasm
Golgi
E2 E16K
ER
6K
P1234
nsP1 nsP3
nsP4nsP2
Figure 16.4. Schematic representation of the alphavirus lytic cycle. For details, see text.
Synthesis of 6K is very toxic for bacterial cells as judged by the rapid lysis observed
after its induction (Sanz et al., 1994). In addition to the entry of hygromycin B, the efflux of
3
H-coline from preloaded E. coli cells can also be detected after inducible expression of 6K.
However, enhanced passive diffusion of compounds with a greater MW, such as ␣-sarcin,
does not take place (Figure 16.1). The function of alphavirus 6K in cell membrane perme-
abilization is more probably related to its pore-formation ability. The simple insertion of 6K
variants into the bacterial membrane is not sufficient to promote permeabilization, since 6K
variants with mutations in the aromatic residues located at the N-terminal region outside the
TMD are defective for this function (Sanz et al., 2003).
As described above, a system has been designed, based on SV replicons that synthesize
large amounts of 6K protein in BHK cells. HB readily entered into BHK cells transfected with
full-length RNA from wt SV, leading to a profound inhibition of viral translation. The expres-
sion of wt 6K enhanced membrane permeability to HB, whereas this effect was not as strong
with the 6K variants in the interface region located at the amino terminus (Sanz et al., 2003).
These findings are consistent with the hypothesis that the integrity of the interfacial sequence
of 6K is crucial for the correct activity of this protein for enhancing membrane permeability.
Notably, a 6K expressed just after the C sequence exhibited the same permeabilization abil-
ity as the genuine 6K containing the E3 signal sequence (Figure 16.3). These results suggest
that the 6K is able by itself to interact with and permeabilize the plasma membrane.
The 6K proteins of Ross River virus and Barmah Forest virus exhibit the ability to form
cation-selective ion channels in planar lipid bilayers (Melton et al., 2002). Bacterially
expressed 6K proteins were purified and inserted into lipid bilayers with a defined orienta-
tion that is, N-terminal cis, C-terminal trans. Channel conductances varied from 40–800 ps,
suggesting that the protein is able to form channels with different oligomerization states.
5. Function of 6K During the Alphavirus Life Cycle
Despite the relative structural simplicity of 6K, this protein plays several roles during
the virus life cycle, including those we shall now mention. The sequences of 6K located at the
amino and carboxy termini provide cleavage sites during polyprotein processing (Welch and
Sefton, 1980). The second hydrophobic region of 6K directs the translocation of glycoprotein
E1 into the lumen of the ER (Liljeström and Garoff, 1991). After synthesis and proteolytic
processing, 6K may interact with glycoproteins E1 and pE2 to regulate their trafficking to the
plasma membrane (Yao et al., 1996). Another well-documented activity of 6K is its mem-
brane permeabilization capacity (Sanz et al., 1994, 2003; Melton et al., 2002). Finally, the
major function of 6K is to participate in efficient virus budding from infected cells (Gaedigk-
Nitschko et al., 1990; Gaedigk-Nitschko and Schlesinger, 1991; Liljeström et al., 1991;
Schlesinger et al., 1993; Ivanova et al., 1995; Yao et al., 1996; Loewy et al., 1995; Sanz and
Carrasco, 2001; Sanz et al., 2003). Despite the association of the 6K protein with the plasma
membrane and its interaction with E1-E2, very little 6K is incorporated into mature virus
particles (Gaedigk-Nitschko et al., 1990; Lusa et al., 1991).
The acquisition and analysis of a number of variants of alphavirus, defective in 6K
function, have helped to elucidate its role during the virus life cycle. These studies have
yielded evidence indicating that 6K may be considered an accessory protein, since defective
6K virions can be obtained, albeit in small amounts (Liljeström et al., 1991; Loewy et al.,
1995; Sanz et al., unpublished results). Although 6K protein provides the cleavage sites in the
240 M.A. Sanz et al.
glycoprotein precursor for signalase activity, a Semliki Forest virus (SFV) variant lacking the
entire 6K is processed between E2 and E1 (Liljeström et al., 1991; Loewy et al., 1995). This
mutant virus is not defective in synthesis and transport of glycoproteins or in nucleocapsid
formation; its major defects concern the budding process. Notably, E1 is properly translocated
in the 6K-deleted SFV mutant. Similarly, SV variants with single or multiple amino acid sub-
stitutions in the 6K have defects in virion release, leading to the formation of multinucleated
virus particles (Gaedigk-Nitschko et al., 1990; Gaedigk-Nitschko and Schlesinger, 1991;
Ivanova et al., 1995). Proper proteolytic processing of the virus glycoproteins is hampered in
an SV variant bearing an insertion of 15 amino acids in the 6K protein. This variant exhibits
a transdominant phenotype, but virus particles display a similar morphology to that observed
with wt virus (Schlesinger et al., 1993). An SV variant with deleted 6K 22 amino acids shows
defects in glycoprotein proteolytic processing (Sanz et al., 2001). A revertant of this mutant
that had corrected these defects was subsequently isolated, but virus release was still
impaired. Further, the expression in trans of a genuine 6K from an extra subgenomic pro-
moter placed in the same genome did not produce appreciable reversion. In addition, the func-
tions of the 6K protein cannot be rescued by the corresponding counterparts from related
virus species. Thus, the substitution of the SV 6K gene by the 6K counterpart from Ross River
virus leads to the small plaque phenotype and reduced formation of infectious virus (Yao
et al., 1996). This SV variant with the 6K gene from Ross River virus was able to cleave the
glycoprotein precursors and to transport them into the plasma membrane, although the bud-
ding process was impaired. More recently, a clear defect has been observed with three SV 6K
variants in the interface region located at the amino terminus (Sanz et al., 2003). In all three
cases, virus particles accumulated at the plasma membrane. Although their morphology
showed no anomaly, these particles were unable to detach efficiently from cells. Together,
these observations suggest a function for 6K in the release of virions from infected cells.
6. A Model of 6K Function in Virion Budding
A major gap in our understanding of 6K function concerns the link between pore
activity and the stimulation of virus budding. Several models have been put forward to explain
this connection. One such model proposes that the function of 6K is merely mechanical. The
interaction of 6K with membranes would lead to phospholipid bilayer bending (Gaedigk-
Nitschko et al., 1990; Loewy et al., 1995). This deformation of the membrane promotes virus
budding. The most significant aspect of this model is the actual interaction of 6K with the
membrane, but it does not take into consideration the ion-channel activity of 6K.
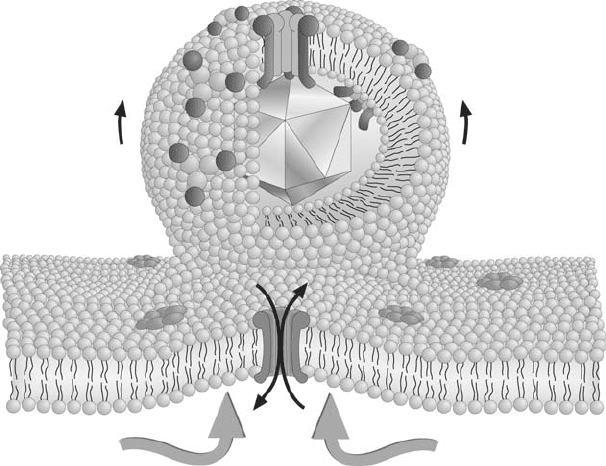
Another possibility, which we have advanced, is that pore formation by 6K serves to
dissipate ion gradients, thereby providing the energy to push the viral particles out of the cell
(Sanz et al., 2001, 2003). In addition, the interaction of 6K with membranes may also partic-
ipate in the bending of the phospholipid bilayer. The initial synthesis of 6K-E2-E1 trimers
is followed by its oligomerization into trimers of PE2-6K-E1 trimers. These oligomers
then travel to the plasma membrane, concentrating in some selected regions at the cell sur-
face. The interaction of the nucleocapsid with the cytoplasmic tail of E2, may expel 6K from
these hetero-oligomers, giving rise to 6K homo-oligomerization and consequent pore forma-
tion. Thus, these pores would be generated surrounding the budding virions (Figure 16.5).
This leads to the dissipation of membrane potential in the immediate vicinity of these
particles. The dissipation energy pushes the virus particles out of the cells. In fact, ionic
The Alphavirus 6K Protein 241
changes in the culture medium that modifies membrane potential hamper the release of viri-
ons into the medium (Waite and Pfefferkorn, 1970; Li and Stollar, 1995).
Acknowledgments
We acknowledge the financial support of the CAM (project number 07B/0010/2002)
and DGICYT (project numbers PM99-0002 [MAS, VM, and LC] and BIO2000-0929 [JLN]).
Further support to JLN was obtained from the Basque Government (PI-1998-32) and the
University of the Basque Country (UPV 042.310-G03/98). CBM was awarded an institutional
grant by the Fundación Ramón Areces, Spain.
References
Carrasco, L. (1978). Membrane leakiness after viral infection and a new approach to the development of antiviral
agents. Nature 272, 694–699.
Carrasco, L. (1981). Modification of membrane permeability induced by animal viruses early in infection. Virology
113, 623–629.
Carrasco, L. (1995). Modifications of membrane permeability by animal viruses. Adv. Virus Res. 45, 61–112.
Fernández-Puentes, C. and Carrasco, L. (1980). Viral infection permeabilizes mammalian cells to protein toxins. Cell
20, 769–775.
242 M.A. Sanz et al.
Figure 16.5. Connection between viroporin activity and alphavirus budding. Pore formation by the alphavirus 6K
around the budding area leads to dissipation of membrane potential providing the energy to push the viral particle
out of the cell.
Frolov, I. and Schlesinger, S. (1994). Translation of Sindbis virus mRNA: Effects of sequences downstream of the
initiating codon. J. Virol. 68, 8111–8117.
Frolov, I. and Schlesinger, S. (1996). Translation of Sindbis virus mRNA: Analysis of sequences downstream of the
initiating AUG codon that enhance translation. J. Virol. 70, 1182–1190.
Gaedigk-Nitschko, K. and Schlesinger, M.J. (1990). The Sindbis virus 6K protein can be detected in virions and is
acylated with fatty acids. Virology 175, 274–281.
Gaedigk-Nitschko, K. and Schlesinger, M.J. (1991). Site-directed mutations in Sindbis virus E2 glycoproteins cyto-
plasmic domain and the 6K protein lead to similar defects in virus assembly and budding. Virology 183, 206–214.
Gaedigk-Nitschko, K., Ding, M.X., Levy, M.A., and Schlesinger, M.J. (1990). Site-directed mutations in the Sindbis
virus 6K protein reveal sites for fatty acylation and the underacylated protein affects virus release and virion
structure. Virology 175, 282–291.
Garoff, H., Hewson, R., and Opstelfen, D.-J.E. (1998). Virus maturation by budding. Microbiol. Mol. Biol. Rev. 62,
1171–1190.
Garry, R.F., and Dashb, S. (2003). Proteomics computational analyses suggest that hepatitis C virus E1 and pestivirus
E2 envelope glycoproteins are truncated class II fusion proteins. Virology 307, 255–265.
Garry, R.F., Bishop, J.M., Parker, J., Westbrook, K., Lewis, G., and White, M.R.F. (1979). Na
and K
concentra-
tions and the regulation of protein synthesis in Sindbis virus-infected chick cells. Virology 96, 108–120.
Hahn, C.S., Hahn, Y.S., Braciale, T.J., and Rice, C.M. (1992). Infectious Sindbis virus transient expression vectors
for studying antigen processing and presentation. Proc. Natl. Acad. Sci. USA 89, 2679–2683.
Ivanova, L., Le, L. and Schlesinger, M.J. (1995). Characterization of revertants of a Sindbis virus 6K gene mutant
that affects proteolytic processing and virus assembly. Virus Res. 39, 165–179.
Jeetendra, E., Robison, C.S., Albritton, L.M., and Whitt, M.A. (2002). The membrane-proximal domain of vesicular
stomatitis virus G protein functions as a membrane fusion potentiator and can induce hemifusion. J. Virol. 76,
12300–12311.
Lama, J. and Carrasco, L. (1992). Expression of poliovirus nonstructural proteins in Escherichia coli cells.
Modification of membrane permeability induced by 2B and 3A. J. Biol. Chem. 267, 15932–15937.
Li, M.C. and Stollar, V. (1995). A mutant of Sindbis virus which is released efficiently from cells maintained in low
ionic strength medium. Virology 210, 237–243.
Liljeström, P. and Garoff, H. (1991). Internally located cleavable signal sequences direct the formation of Semliki
Forest virus membrane proteins from a polyprotein precursor. J. Virol. 65, 147–154.
Liljeström, P., Lusa, S., Huylebroeck, D., and Garoff, H. (1991). In vitro mutagenesis of a full-length cDNA clone
of Semliki Forest virus: The small 6.000-molecular-weight membrane protein modulates virus release. J. Virol.
65, 4107–4113.
Loewy, A., Smyth, J., Von, C.-H., Bonsdorff, Liljeström, P., and Schlesinger, M.J. (1995). The 6-kilodalton mem-
brane protein of Semliki Forest virus is involved in the budding process. J. Virol. 69, 469–475.
Lusa, S., Garoff, H., and Liljeström, P. (1991). Fate of the 6K membrane protein of Semliki Forest virus during virus
assembly. Virology 185, 843–846.
Melton, J.V., Ewart, G.D., Weir, R.C., Board, P.G., Lee, E., and Gage, P.W. (2002). Alphavirus 6K proteins form ion
channels. J. Biol. Chem. 277, 46923–46931.
Muñoz, A., Castrillo, J.L., and Carrasco, L. (1985). Modification of membrane permeability during Semliki Forest
virus infection. Virology 146, 203–212.
Otero, M.J. and Carrasco, L. (1987). Proteins are cointernalized with virion particles during early infection. Virology
160, 75–80.
Rosemberg, A.H., Lade, B.N., Chui, D., Lin, S., Dunn, J.J., and Studier, F.W. (1987). Vectors for selective expres-
sion of cloned DNAs by T7 RNA polymerase. Gene 56, 125–35.
Sanz, M.A. and Carrasco, L. (2001). Sindbis virus variant with a deletion in the 6K gene shows defects in glycopro-
tein processing and trafficking: Lack of complementation by a wild-type 6K gene in trans. J. Virol. 75, 7778–7784.
Sanz, M.A., Madan, V., Carrasco, L., and Nieva, J.L. (2003). Interfacial domains in Sindbis virus 6K protein.
Detection and functional characterization. J. Biol. Chem. 278, 2051–2057.
Sanz, M.A., Pérez, L. and Carrasco, L. (1994). Semliki Forest virus 6K protein modifies membrane permeability
after inducible expression in Escherichia coli cells. J. Biol. Chem. 269, 12106–1211.
Schlesinger, S. and Schlesinger, M.J. (1996). In Fields, B.N. (ed.), Virology, Lippincott-Raven, Philadelphia,
pp. 825–841.
Schlesinger, M.J., London, S.D. and Ryan, C. (1993). An in-frame insertion into the Sindbis virus 6K gene leads to
defective proteolytic processing of the virus glycoproteins, a trans-dominant negative inhibition of normal virus
formation, and interference in virus shut off of host-cell protein synthesis. Virology 193, 424–432.
The Alphavirus 6K Protein 243
Suomalainen, M. and Garoff, H. (1994). Incorporation of homologous and heterologous proteins into the envelope
of Moloney murine leukemia virus. J. Virol. 68, 4879–4889.
Strauss, J.H. and Strauss, E.G. (1994). The alphaviruses: Gene expression, replication, and evolution. Microbiol. Rev.
58, 491–562.
Studier, F.W. and Moffatt, B.A. (1986). Use of bacteriophage T7 RNA polymerase to direct selective high level
expression of cloned genes. J. Mol. Biol. 198, 113–130.
Studier, F.W., A.H. Rosenberg, and Dunn, J.J. (1990). Use of T7 RNA polymerase to direct expression of cloned
genes. Methods in Enzymology 185, 60–89.
Suárez, T., Gallaher, W.R., Agirre, A., Goñi, F.M., and Nieva, J.L. (2000). Membrane interface-interacting sequences
within the ectodomain of the HIV-1 envelope glycoprotein: Putative role during viral fusion. J. Virol. 74,
8038–8047.
Waite, M.R. and Pfefferkorn, E.R. (1970). Inhibition of Sindbis virus production by media of low ionic strength:
Intracellular events and requirements for reversal. J. Virol. 5, 60–71.
Welch, W.J. and Sefton, B.M. (1980). Characterization of a small, nonstructural viral polypeptide present late
during of BHK cells by Semliki Forest virus. J. Virol. 33, 230–237.
White, S.H., Ladokhin, A.S., Jayasinghe, S., and Hristova, K. (2001). How membranes shape protein structure. J.
Biol. Chem. 276, 32395–32398.
Wimley, W. and White, S.H. (1996). Experimentally determined hydrophobicity scale for proteins at membrane inter-
faces. Nature Struct. Biol. 3, 842–848.
Yao, J.S., Strauss, E.G., and Strauss, J.H. (1996). Interactions between PE2, E1, and 6K required for assembly of
alphaviruses studied with chimeric viruses. J. Virol. 70, 7910–7920.
244 M.A. Sanz et al.
Part IV
Membrane-Spanning/Membrane
Associated
