Ellis A.M., Feher M., Wright T. Electronic and Photoelectron Spectroscopy: Fundamentals and Case Studies
Подождите немного. Документ загружается.

10 Lasers
85
Laser
output
Pump
laser
CL
BS1
BS2
OCODCBEE
DG
PDC ADC
q
Laser cavity
M
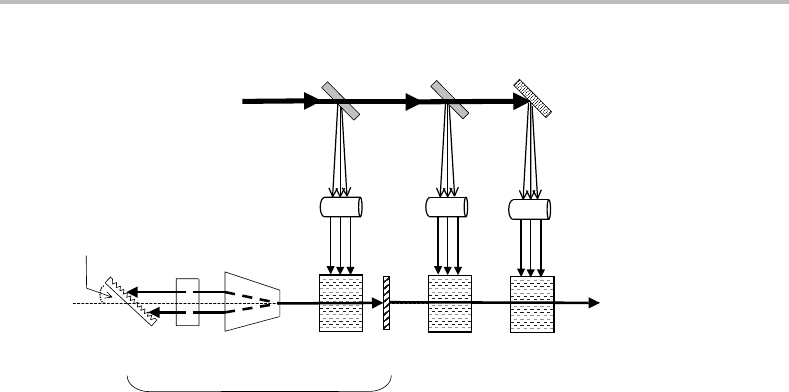
Figure 10.5 Optical arrangement of a tunable pulsed dye laser. The dye laser is pumped by pulsed
radiation from another laser. Abbreviations are as follows: BS, beamsplitter; M, mirror; CL, cylindrical
lens; OC, output coupler (end mirror); ODC, oscillator dye cell; PDC, preamplifier dye cell; ADC,
amplifier dye cell; BE, beam expander; E, intracavity etalon (optional); DG, diffraction grating. The
preamplifier and amplifier dye cells are used to increase the intensity of the dye laser beam produced
in the laser cavity. This amplification process can increase the intensity by more than two orders of
magnitude.
The output wavelength can be extended outside of the traditional dye operating ranges
using non-linear optical techniques. The most commonly used is frequency doubling, in
which the dye laser fundamental is passed through a suitable crystal to generate the second
harmonic (v
out
= 2v
in
). This crystal must possess the correct non-linear optical properties,
as well as being able to withstand very high laser intensities. -barium borate is one of the
best materials currently available, with KDP as a cheaper alternative for some wavelength
ranges. Efficient harmonic generation requires correct phase matching of the fundamental
and higher harmonic beams. Phase matching is the process by which the refractive indices
of the input and output beams are equalized, and this requires a specific orientation of
the crystal relative to the incoming laser beam. Frequency doubling allows coverage of
the whole of the near-ultraviolet (205–400 nm), and more advanced techniques can extend
the wavelength into the vacuum ultraviolet region (<200 nm). At the long wavelength end,
tunable radiation beyond 1 m can be generated using difference frequency generation [5].
10.7 Titanium:sapphire laser
The Ti:sapphire laser is a tunable solid state laser based on transitions of Ti
+
ions doped in
a sapphire host. The crystalline lattice broadens the electronic energy levels of Ti
+
to such
an extent that tunability far exceeding that of a single laser dye is achieved. However, the
Ti:sapphire laser is not really a competitor to the dye laser since their tunability ranges only
partially overlap. One of the strengths of the Ti:sapphire laser is that much of its tunability

86 Experimental techniques
range, 660–1180 nm, is in a difficult region for dye lasers. It also possesses better frequency
stability and a narrower linewidth than dye lasers. Output in the near-ultraviolet and blue
regions is possible by frequency doubling the fundamental output.
10.8 Optical parametric oscillators
These are tunable laser sources that offer the promise of eventually superseding dye lasers.
Tunability in optical parametric oscillators (OPOs) is achieved by non-linear optical pro-
cessing of a single input (pump) beam. It is useful to think of this as the opposite of frequency
doubling in a non-linear crystal. In essence, a single high intensity laser beam is passed
through the non-linear crystal. The input beam can ‘split’ into two output beams, one known
as the signal and the other the idler, such that v
in
= v
signal
+ v
idler
. The exact reverse of fre-
quency doubling would correspond to equal idler and signal frequencies. However, any
combination of v
signal
and v
idler
is, in principle, achievable providing the sum equals v
in
, and
a particular combination can be amplified if the mixing process is carried out in a tunable
laser cavity. By combining the tunability of a diffraction grating in the laser cavity, and the
orientation of the crystal for optimum phase matching, efficient generation of tunable radi-
ation over a wide spectral range is possible. Commercial OPOs are available which operate
over the whole of the visible region and these can be extended into the near-ultraviolet by
frequency doubling.
References
1. Principles of Lasers,O.Svelto, New York, Plenum Publishing Corporation, 1998.
2. Lasers,A.E.Siegman, Mill Valley, California, University Science Books, 1986.
3. Laser Fundamentals,W.T.Silvast, Cambridge, Cambridge University Press, 1996.
4. R. H. Lipson, S. S. Dimov, P. Wang, Y. J. Shi, D. M. Maxo, X. K. Hu, and J. Vanstone,
Instrum. Sci. Technol. 28 (2000) 85.
5. A. S. Pine, J. Opt. Soc. Am. 70 (1980) 1568.

11
Optical spectroscopy
Consider a beam of light of intensity I
0
incident on some absorbing sample. Providing only
a small fraction of the light is absorbed,
1
and assuming that losses caused by light scattering
are negligible, the transmitted light intensity, I,isgoverned by the familiar Beer–Lambert
law,
A = log
10
I
0
I
= ε(ν)cl (11.1)
where A is known as the absorbance. The absorbance is dependent upon the concentration
of absorbing species, c, the optical path length, l (distance travelled by the light through
the sample), and the molar absorption coefficient, ε. The molar absorption coefficient is a
measure of the intrinsic absorbing power of the sample and is frequency dependent, which
is why it has been written as ε(ν). It is customary to give c in units of mol dm
−3
and l in
cm, and so ε is often quoted in the rather strange mixture of units dm
3
mol
−1
cm
−1
.Asone
might expect, ε is related to the Einstein B coefficient introduced in Chapter 7.
The absorbance is an important quantity because it is directly proportional to the con-
centration. If monochromatic radiation is passed through a material of known thickness
and known molar absorption coefficient, the concentration of the absorbing species can
be determined from a measurement of the absorbance. This is a widely used feature of
absorption spectroscopy.
11.1 Conventional absorption/emission spectroscopy
A schematic of an absorption spectrometer is shown in Figure 11.1. Ideally, the light source is
continuous over the wavelength region of interest and shows no major variations in intensity.
Resistively heated filaments are good sources of near-continuum light. One example is a
white-hot tungsten filament, which will cover the whole of the visible and parts of the
near-ultraviolet and near-infrared. A wavelength selector is central to the spectrometer and
is usually a monochromator built around a diffraction grating, thus allowing tunability.
In order to obtain a spectrum, light intensity transmitted through the monochromator is
1
If the fraction of light absorbed is large, then the light intensity varies strongly as the sample is traversed and the
Beer–Lambert law no longer holds.
87
88 Experimental techniques
P
M
T
Sample
Light
source
Entrance
slit
Exit
slit
Monochromator
l
2
l
1
PC
Lenses
Diffraction
grating
Figure 11.1 Schematic of a conventional grating-based absorption spectrometer. The monochromator
is of the Czerny–Turner type in which the entrance and exit slits are placed at the focal points of curved
mirrors.
Excitation
source
Sample
Mono-
chromator
Detector
+ PC
Figure 11.2 Block diagram of a standard emission spectrometer.
measured as a function of wavelength. The light intensity is measured by a photomultiplier
tube (PMT), a photodiode, or some other light-detecting device.
In an emission spectrometer, the sample must be driven up to excited quantum states in
order for emission to occur. This is normally achieved by an electrical discharge, although
broadband optical excitation is also possible. As indicated in Figure 11.2, the monochroma-
tor is now used to select a specific emission wavelength from the sample and the intensity at
this wavelength is measured by imaging the light onto a detector such as a PMT. An emission
spectrum is obtained by recording the PMT signal as a function of emission wavelength.
Monochromators such as that shown in Figure 11.1 have both entrance and exit slits.
These are crucial to the wavelength selection process. Narrowing the entrance and exit slits
can improve the spectral resolution, but it does so at the expense of sensitivity because of
the reduced light throughput. Improvements can be made that make more efficient use of the
available light. For example, the exit slits in an emission spectrometer can be dispensed with
if a multichannel detector is available. Examples are photodiode arrays and charge-coupled
devices (CCDs). These measure the light intensity as a function of position on the detector
surface and so are able to record a large portion of the spectrum simultaneously. Another
alternative is Fourier transform spectroscopy, which does away with both the entrance and
exits slits as well as the diffraction grating. Fourier transform spectroscopy is described
later in this chapter.
11 Optical spectroscopy
89
Photomultiplier
tube
Collection lens
Dye laser beam
Filter
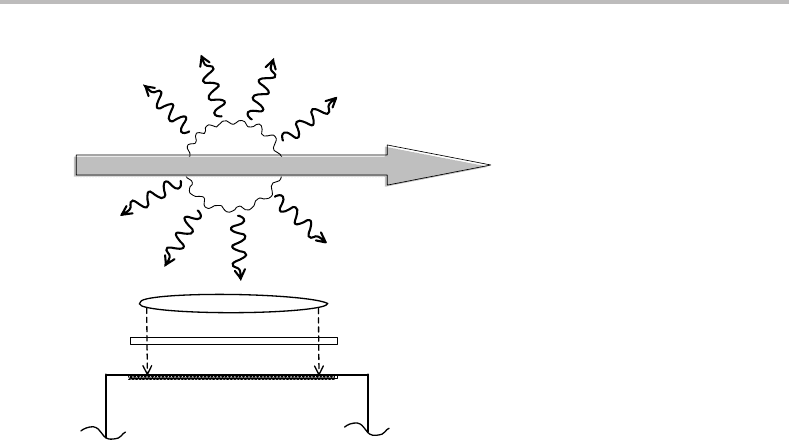
Figure 11.3 Experimental arrangement for laser-induced fluorescence spectroscopy. Fluorescence
radiates in all directions, a portion of which is collected by the lens and transmitted to the detector, a
photomultiplier tube. The filter, which is used to reduce the amount of scattered laser light reaching
the detector, is optional. The arrangement shown is for laser excitation spectroscopy. For dispersed
fluorescence spectroscopy the filter is replaced with a scanning monochromator.
11.2 Laser-induced fluorescence (LIF) spectroscopy
This is one of the principal techniques for studying electronic transitions of both neutral
molecules and molecular ions at high sensitivity and at high resolution. In LIF spectroscopy
an electronic transition of the molecule is excited using a tunable laser and any fluorescence
generated is monitored. There are two complementary methods that parallel, respectively,
conventional absorption and emission spectroscopy.
Suppose the wavelength of a tunable laser is scanned through the electronic absorption
band of a molecule. Absorption will occur at resonant wavelengths and could be monitored
by measuring the intensity of the transmitted laser beam. The high intensity of a laser
can greatly increase the probability of absorption compared with low intensity non-laser
light sources and thus it might be thought that laser absorption spectroscopy would be
very sensitive. Unfortunately, this is not the case because the fractional absorption by a
sample will still normally be very low. Thus a small change in intensity is superimposed
on a large background signal. When fluctuations in intensity of the laser beam and noise
from the light detector are factored in, this approach turns out to have a very limited
sensitivity.
However, instead of measuring absorption directly it can be monitored indirectly by
detecting fluorescence from the excited electronic state. The experimental arrangement is
remarkably simple, and is outlined in Figure 11.3.Atunable laser is passed through the
sample and any fluorescence produced is collected off-axis, usually at right angles to the
laser beam, by a collection lens. The light is then detected by a photosensitive device, most

90 Experimental techniques
usually a PMT. PMTs have phenomenal sensitivities and are even capable of detecting single
photons in some cases. When the laser is off-resonance, no fluorescence will be produced,
and therefore the PMT registers no signal. However, at resonant wavelengths fluorescence
is possible and so absorption can be registered by detecting emission from the excited state.
This is the basic idea of laser excitation spectroscopy,inwhich a spectrum is obtained by
measuring the fluorescence intensity as a function of laser wavelength.
There are several important points to note about laser excitation spectroscopy. First, while
there is a clear similarity between laser excitation spectroscopy and absorption spectroscopy,
there is also an important difference. The intensity of peaks in a laser excitation spectrum
depends on both the absorbance of the sample and the fluorescence quantum yield of the
excited state. The fluorescence quantum yield is defined as
f
=
rate of photon emission by excited state
rate of photon absorption
(11.2)
A fluorescence quantum yield of unity implies that all molecules excited to the upper
electronic state relax via photon emission. However, competition from other decay routes
(see below) may not only lower
f
,but may also cause it to change from one excited state
level to another. As a result absorption and fluorescence excitation spectra may look very
different.
The high sensitivity of LIF spectroscopy arises from the low background signal received
by the PMT at off-resonance laser wavelengths. Even though any fluorescence produced
may be very small, it is easily detected by the PMT and therefore if the off-resonance
signal is much smaller still then an extremely high signal-to-noise ratio can be achieved. In
practice the off-resonance signal is never zero. The principal cause is scattered light from
the laser. This can be minimized by keeping potential scattering sites out of the path of the
laser. Furthermore, scattered laser light can be virtually eliminated if at least a portion of
the fluorescence is at longer wavelengths than the laser. If this condition is satisfied, and
it often is for many molecules, then an optical filter, which will only transmit wavelengths
longer than that of the laser, can be inserted in front of the PMT.
In laser excitation spectroscopy the fluorescence serves only as a means of detecting the
absorption process. However, the fluorescence itself clearly contains spectroscopic infor-
mation since it arises from emission to lower energy levels. If the emission is dispersed in
a monochromator, the spectrum obtained will be the emission spectrum originating from
a specific (laser-excited) upper state. This type of spectroscopy goes by several names,
including dispersed fluorescence spectroscopy, laser-excited emission spectroscopy, and
single vibronic level fluorescence spectroscopy;wewill use the first of these throughout
this text.
In laser excitationspectroscopythe resolution is often limited by the linewidth of the laser.
For pulsed dye lasers, linewidths of ∼0.03 cm
−1
can be obtained relatively straightforwardly.
If narrower linewidth lasers are used, such as CW dye lasers or specialized pulsed dye lasers,
other factors may begin to limit the resolution, such as Doppler broadening. If steps are taken
to minimize Doppler broadening, a resolution of better than 0.001 cm
−1
can be attained.
With such a high resolution, rotationally resolved electronic spectra of quite large molecules
can be tackled.
11 Optical spectroscopy
91
AB
A + B
A + B*
AB*
Repulsive
state
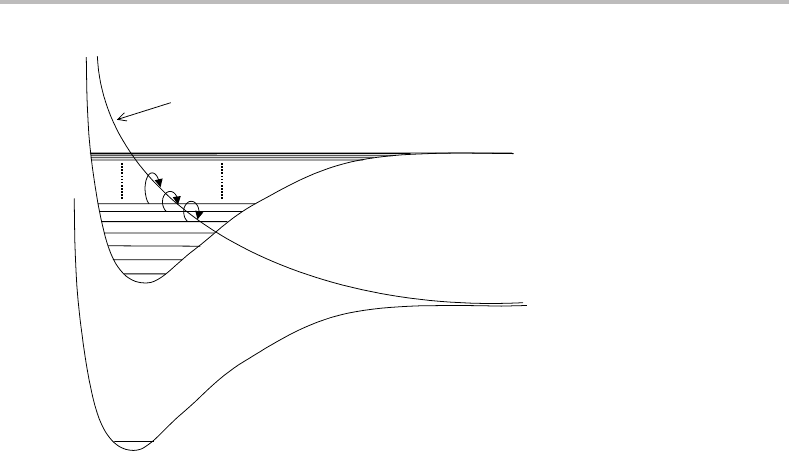
Figure 11.4 Predissociation caused by the crossing of two potential energy curves. Notice that only
those energy levels above the crossing point can undergo predissociation.
In dispersed fluorescence spectroscopy, the use of a scanning monochromator is normally
the principal factor limiting the resolution. Even a large monochromator may only have a
resolution of about 1 cm
−1
. Thus dispersed fluorescence spectroscopy is normally concerned
with vibrationally resolved emission spectra.
The principal disadvantage of LIF is the need for a fluorescent excited state. Fast non-
radiative decay routes may reduce the fluorescence quantum yield to zero and in these cases
LIF cannot be used. An example of a non-radiative decay process is predissociation, which is
illustrated in Figure 11.4. Predissociation results from a crossing of potential energy surfaces
of two excited electronic states, one of which is repulsive (dissociative). If the molecule
is excited to the bound potential energy curve, it may hop over onto the repulsive curve at
the crossing point and will then undergo dissociation. If the probability of predissociation
is not too high, there may still be sufficient fluorescence for LIF detection. In such cases,
the occurrence of predissociation manifests itself by a broadening of spectral lines, since
the effect of predissociation is to decrease the lifetime of the level and hence increase the
lifetime broadening.
Depopulation mechanisms such as predissociation are particularly troublesome for large
molecules because of their high density of rovibrational energy levels. Usually the coupling
mechanism, the process which actually brings about the interaction between the electronic
states, will be restricted by symmetry in the same way that symmetry restricts electric dipole
transitions. However, the importance of symmetry restrictions decreases as the overall point
group symmetry of a molecule is lowered, and large molecules tend to have low symmetry.
It is for these reasons that LIF is a particularly powerful technique for investigating small
molecules, but is more limited in scope for large molecules.

92 Experimental techniques
11.3 Cavity ringdown (CRD) laser absorption spectroscopy
Direct laser absorption electronic spectroscopy is appealing for several reasons. First, the
narrow linewidths of lasers can be exploited. Second, it does not rely on the occurrence
of a secondary process for detection, as in LIF spectroscopy. Third, the absorbance can
be directly related to the concentration of the absorbing species, thus allowing absolute
concentration measurements to be made.
2
However, as discussed in the previous section,
when done in the conventional manner laser absorption spectroscopy is a low-sensitivity
technique.
Cavity ringdown spectroscopy is a form of laser absorption spectroscopy in which the
absorbance is determined but in a rather ingenious manner. It is a relatively new technique,
first appearing in 1988, but is based on a simple idea.
Suppose a gas is placed between two highly reflecting mirrors which act as an optical
cavity. If a pulse of laser light is injected into the cavity, as shown in Figure 11.5, then
laser light will reflect backwards and forwards and, if the spacing between the mirrors is
relatively small, interference will occur as a consequence of the coherence of the laser
beam. However, the coherence of a laser beam is restricted to a finite distance known as
the coherence length.
3
The finite coherence length is brought about by uncertainty in the
frequency of the light, which in turn is a result of the non-zero linewidth. The coherence
length, l
coh
,isgivenby
l
coh
=
c
ν
(11.3)
where ν is the linewidth (FWHM) of the laser. For typical pulsed dye lasers without
intracavity etalons, the linewidth is 0.2 cm
−1
and so equation (11.3) yields l
coh
= 5 cm.
Consequently, if the mirror separation is significantly larger than 5 cm, interference is not
an issue; this is the starting point for cavity ringdown spectroscopy.
If the mirrors are able to transmit a small proportion of the incident light, then each time
the laser light pulse impinges on a mirror some is lost from the cavity. Gradually, at a rate
determined by the mirror reflectivities, the intensity of the light trapped within the cavity
will decay to zero. In fact the decay is exponential, and the time taken for the intensity
to decay to 1/eofits initial value is known as the ringdown time.Itcan be measured by
placing a sensitive light detector, usually a photomultiplier tube, behind one of the mirrors,
as shown in Figure 11.5.
Now suppose that an absorbing sample is placed inside the cavity. Absorption of the
laser light by the sample will accelerate the ringdown process, resulting in a faster ringdown
time. The larger the absorbance, the shorter the ringdown time. Hence it is possible to record
something akin to an absorption spectrum by measuring the change in ringdown time as
a function of laser wavelength. In fact there is a simple and exact relationship linking
2
It is difficult to deduce absolute concentrations of an absorbing species from LIF spectroscopy, although changes
in relative concentrations can easily be measured.
3
The coherence length is a measure of the distance over which the phase relationships between the constituent waves
in a light source are maintained. For optical path differences exceeding this difference, the phase relationships are
lost and so interference effects become negligible.
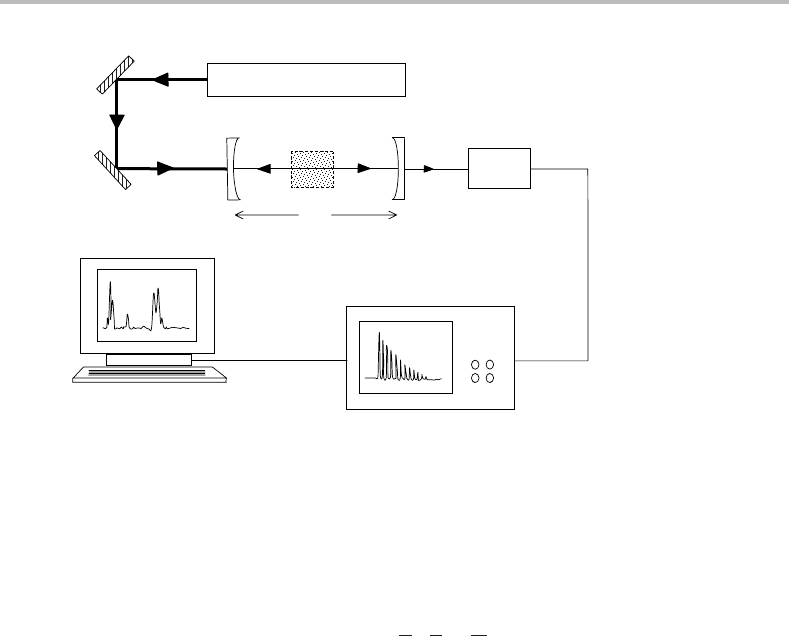
11 Optical spectroscopy
93
PMT
Pulsed laser
L
Sample
Oscilloscope
PC
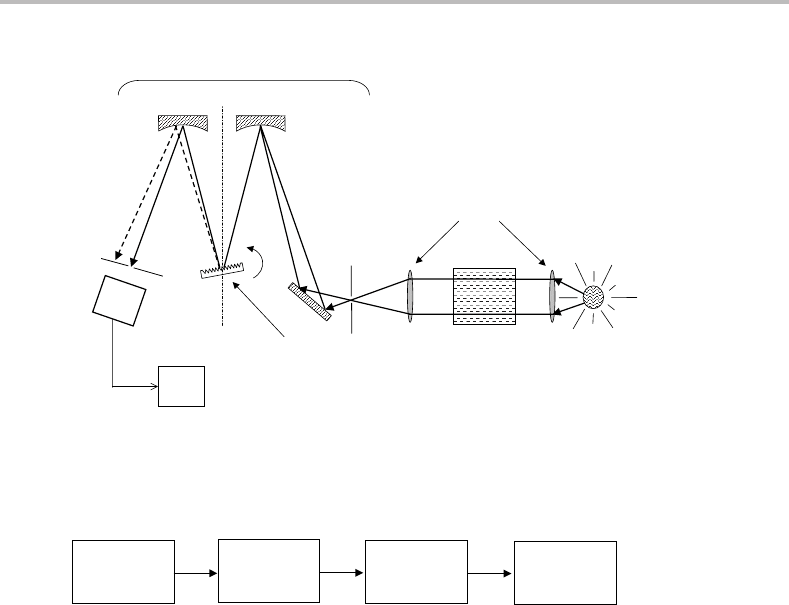
Figure 11.5 Experimental arrangement for pulsed cavity ringdown laser absorption spectroscopy.
The cavity is defined by the two plano-concave mirrors. Concave mirrors are preferred over plane
mirrors because the former can produce a so-called stable optical cavity, making it easier to ‘trap’ the
radiation within the cavity.
ringdown time to the sample absorbance, A,which takes the form
A =
L
c
1
τ
−
1
τ
0
(11.4)
where L is the cavity length, c is the speed of light, and τ
0
and τ are the ringdown times in
the absence and presence of an absorber, respectively. Thus the cavity ringdown spectrum
can easily be converted into a conventional absorption spectrum.
To achieve high absorption sensitivity, high mirror reflectivities are required since these
lengthen the ringdown times and therefore make it easier to observe small changes. Mirrors
with reflectivities better than 99.995% are available in the visible and near-ultraviolet. Of
course, one problem with such high mirror reflectivities is that only a tiny proportion of the
light from the laser is injected into the cavity in the first place since the laser beam enters
through one of the end mirrors! However, with sensitive detectors such as PMTs this does
not cause a significant problem.
The discussion so far has focussed on cavity ringdown using pulsed lasers. However, it is
also possible to record CRD spectra with continuous lasers (CW-CRD). Typically, a narrow
linewidth tunable diode laser is employed as the light source. It is still necessary to inject a
pulse of light into the cavity. One way this can be achieved is to scan the cavity length by
mounting one of the end mirrors on a piezoelectric transducer. If the cavity length does not
match one of the longitudinal modes of the cavity, no significant light can be injected. This
restriction is normally unimportant in pulsed laser CRD because the relatively broad laser
linewidths mean that there is always some radiation that matches longitudinal modes of the
cavity. As the mirror is moved in CW-CRD, at some stage the standing wave condition will
be met and light will be injected into the cavity. An electro-optical switch, known as an

94 Experimental techniques
acousto-optical modulator, is then used to block the laser beam so that a pulse of laser light
remains in the cavity. A ringdown profile is then measured in the normal manner.
CW-CRD is growing in importance. One reason for this is that it is capable of much
higher spectral resolution than pulsed laser CRD. Much higher pulse repetition rates can
also be employed giving improved detection sensitivity [1].
11.4 Resonance-enhanced multiphoton ionization (REMPI) spectroscopy
Highly excited electronic states can be studied by vacuum ultraviolet (VUV) absorption
spectroscopy. One of the problems in working with VUV light sources is the low resolution
achieved in this region. Although tunable laser radiation can be obtained in parts of the
VUV, this is not as routine to generate, nor is it as cheap, as visible and near-ultraviolet
laser sources. Fortunately, many VUV transitions can be accessed by multiphoton transitions
using visible or near-ultraviolet laser light. Resonance-enhanced multiphoton ionization
(REMPI) spectroscopy is a particularly powerful and widely used example of a multiphoton
spectroscopic technique.
REMPI is a two-stage process. In the first step, molecules are promoted to an excited
electronic state by the absorption of one or more photons. It may at first sight seem strange
to suggest that more than one photon can be absorbed in a spectroscopic transition, since
we normally regard them as single-photon resonant processes. However, there is nothing
intrinsically impossible in using two or more photons of lower energy to achieve the same
task, providing (i) their combined energy satisfies the resonance condition, e.g. for two
photons having the same frequency, E
2
− E
1
= 2hv, and (ii) all selection rules are satisfied
(see later).
The principal reason why multiphoton transitions are not normally considered is that such
processes are extremely improbable at normal light intensities. The photons must arrive at
the molecule at virtually the same instant in time in order to be simultaneously absorbed.
With ordinary light sources, such as lamps or low intensity lasers, this hardly ever happens.
However, if extremely high light intensities are employed, as is the case with powerful
pulsed lasers, then multiphoton transition probabilities need no longer be negligible. Even
so, it is easy to appreciate that the probability will rapidly decrease as the number of photons
to be absorbed increases.
Once the molecule has reached the excited electronic state by absorption of one or more
photons, it may absorb one or more further photons to climb abovethe ionization limit. This is
a REMPI process. Compare this with direct (non-resonant) multiphoton ionization. Clearly
REMPI and direct (non-resonant) multiphoton ionization have the same overall photon
order, i.e. the same total number of photons is absorbed. However, in REMPI the ionization
is achieved by two steps of lower photon order, each with a much higher probability (many
orders of magnitude) than the non-resonant multiphoton ionization process. In other words,
the ionization probability is dramatically increased by breaking the ionization process down
into two separate, sequential steps.
This suggests a means of detecting electronic transitions. If the laser is tuned to a wave-
length that is not resonant with an energy level in the excited electronic state manifold
of the neutral molecule, then ionization is only possible by the non-resonant route, and
