Elder K. Human preimplantation embryo selection
Подождите немного. Документ загружается.

HPE_Chapter25.qxp 7/14/2007 5:52 PM Page 336
26. The sperm centriole: its effect on the
developing embryo
Calvin R Simerly and Christopher S Navara
Centrosomes are classically defined as a pair of cen-
trioles, structures of nine triplet microtubules with-
out a central microtubule pair, surrounded by the
pericentrosomal matrix (PCM). The PCM is the
amorphous material that nucleates the microtubules
and defines their intrinsic polarity (i.e. minus ends
(slower turnover) anchored at each centrosome;
plus ends (faster turnover) radiating outward).
1,2
The past 25 years have seen an explosion in under-
standing basic centrosomal biology with regards to
molecular constituents and the minimal structure
required to promote microtubule nucleation from
this structure. Rapid advances in understanding
centrosomal inheritance, assembly, duplication, and
segregation in a variety of cell types provide crucial
clues for understanding how the centrosome medi-
ates intracellular motility, cytoplasmic organization,
and the many other cellular processes linked to cen-
trosomal activities.
3–5
These advances are beginning
to translate to modern molecular medicine, where
clinical challenges including infertility treatments
and contraception require a greater understanding
of this crucial cellular organelle.
Within the fields of reproductive and develop-
mental biology, the cellular and molecular events
during fertilization are intrinsically grounded in
understanding the role of the centrosome. Boveri
6
recognized more than a century ago that at fertiliza-
tion the sperm contributes the centrosome, the cell’s
major microtubule organizing center (MTOC) and
the structure that organizes the mitotic spindle poles
(cleavage centers). However, the process of centro-
some reduction and restoration during meiosis,
fertilization, and mitosis has remained perplexing
7
(Figure 26.1). In somatic cell cycles, the chromo-
somes, cytoplasm, and centrosome double during
interphase, with each daughter cell receiving one
diploid chromosome set, one centrosome, and half
of the cell volume following division (Figure 26.1).
During fertilization, however, there is ambiguity
about the inheritance and reconstitution of the func-
tional zygotic centrosome (Figure 26.1). Although
each gamete contributes a haploid genome at insem-
ination, the egg provides most of the cytoplasmic
contribution. Boveri first recognized that the egg
typically loses the centrosome during oogenesis and
that the sperm typically introduces this structure at
fertilization.Yet, understanding centrosome behavior
has remained a persistent problem in cell biology
and an especially enigmatic one during mammalian
development.
The foundations for the fields of cell, molecular,
developmental, and reproductive biology, provided
by the study of fertilization in lower animal species
(i.e. sea urchins, frogs) is being greatly extended by
enhanced methods for performing in vitro fertiliza-
tion (IVF) in many mammals, including humans.
A century after the discovery of the centrosome,
human IVF was achieved
8
– more than one million
IVF babies have now been born.
9
The otherwise dis-
carded gametes and embryos from IVF clinics are
providing a precious and unique research resource
for the modern-day centrosomal physician/scientist.
As a result, reproductive errors like polyspermy
(fertilization by more than one sperm) and partheno-
genesis (development beginning in an activated egg
without any sperm) have provided insights that
subtly challenge aspects of the unipaternal centro-
some inheritance theory. Promising investigations
in non-human primates appear to align closely to
humans, and avoid the many complexities in work-
ing with fertilized human oocytes for research.
10
Such advancements have direct implications in clin-
ical reproductive medicine, which in many ways has
pioneered amazing clinical achievements in assisted
reproductive technologies (ART) such as intracyto-
plasmic sperm injection (ICSI) that have produced
thousands of human babies.
11
Although fundamental
HPE_Chapter26.qxp 7/13/2007 5:32 PM Page 337
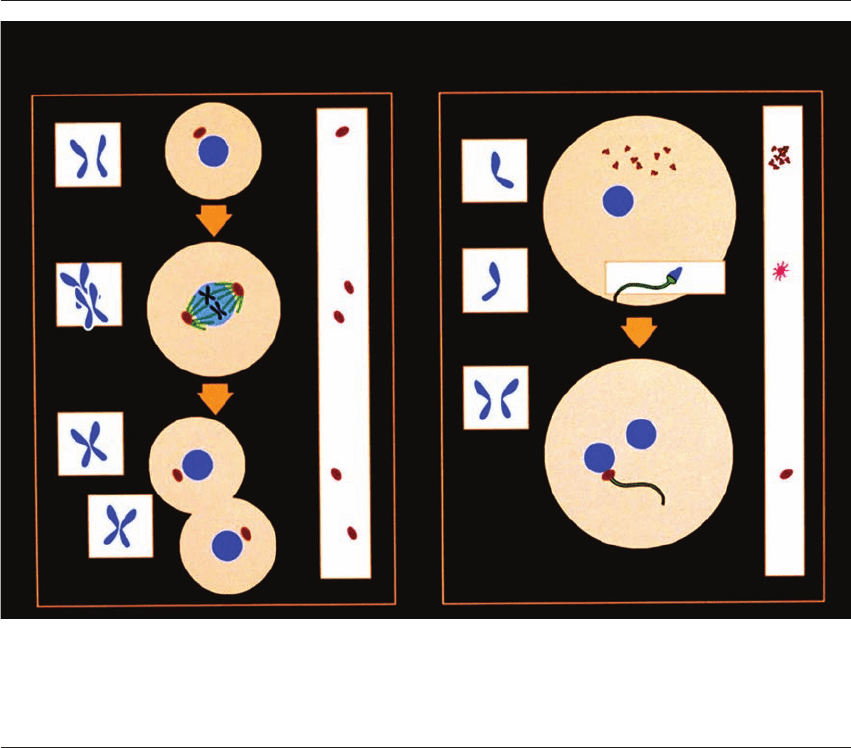
HUMAN PREIMPLANTATION EMBRYO SELECTION
research still lags in efforts to understand these
clinical accomplishments, biomedical researchers
are now poised to begin investigations on the
cellular and molecular events that underlie ART
advances.
This review focuses on the role of the centro-
some during fertilization, with special attention to
human reproduction and development. We also
consider the centrosome in devastating disease dis-
orders that arise in human development resulting
from aberrant genomic imprints. These new research
frontiers represent unique challenges for the next
generation of centrosome biologists, owing in large
part to the ethical, moral, political, and religious hur-
dles that accompany working with human gametes.
Consequently, experimental results are obtained
and/or corroborated by studying non-human pri-
mate development. This is essential, ironically,
because fertilization in outbred mice, hamsters,
and rats – while vital for fundamental research
investigations – represent rare exceptions to Boveri’s
theory on centrosome inheritance.
7
Somatic cells cycles Gametes at fertilization
2N
1N
1N
1N + 1N
2(2N)
2N
2N
AB
100%
100%
zygote
100%
egg
>99%
sperm
>1%
?
?
?
200%
100%
Cytoplasm CytoplasmChromosome ChromosomeCentrosomes Centrosomes
Figure 26.1 The problem of centrosome inheritance. During each cycle in somatic cells (A), the chromosomes (2N1→2N2),
cytoplasm (100→200%), as well as the centrosomes (·→··), duplicate during interphase. During cell division, the chromosomes,
cytoplasm, and centrosomes all split in two. Each of the gametes during fertilization (B) contributes a haploid chromosome set
to the zygote (1N
1
⫹ 1N
1
→ 2N
1
), and while the egg contributes the vast bulk of the cytoplasm (⬎99%), the relative contributions
of the sperm and the egg to the zygotic centrosome are not yet understood (*). Reprinted with permission from Schatten.
7
HPE_Chapter26.qxp 7/13/2007 5:32 PM Page 338
THE SPERM CENTRIOLE: ITS EFFECT ON THE DEVELOPING EMBRYO
CENTROSOMES DURING HUMAN
FERTILIZATION
Centrosome activity in spermatogenic cells centers
around the generation of the sperm axoneme required
for sperm motility. With the exception of rodents,
where the paternal centrioles are completely disman-
tled, mammalian sperm reduce the pair of centrioles
to a single inactive structure during spermatogenesis,
the proximal centriole.
12
The vast majority of the
proteins of the pericentriolar material are shed in the
cytoplasmic droplet during sperm maturation during
migration through the epididymal tract. Conversely,
oocytes lose both centrioles during oogenesis as
investigated by electron microscopy, but retain a sig-
nificant pool of pericentriolar proteins as observed by
immunocytochemistry and Western blotting.
13
Centrosomal inheritance during human fertil-
ization
14
is depicted from the organization of micro-
tubules and DNA observed in discarded human
oocytes (Figure 26.2) and mirrors the inheritance
pathway found in most animals, except rodents.
7,15,16
Microtubules are only observed in the metaphase-
arrested second meiotic spindle in the unfertilized
human oocyte (Figure 26.2A), unlike the rodent
that retains multiple cytoplasmic microtubule asters
(‘cytasters’).Within 6 hours postinsemination, a small
microtubule aster emanates from the introduced
sperm proximal centriole (Figure 26.2B and C). The
activated oocyte extrudes the second polar body,
observed here attached to the developing female
pronucleus by the microtubule based midbody
structure (Figure 26.2B and C). The enlarged sperm
centrosome (the ‘zygote centrosome’) nucleates
microtubules that assemble the first microtubule-
based structure in the fertilized egg – the sperm
aster. The sperm aster in fertilized human oocytes is
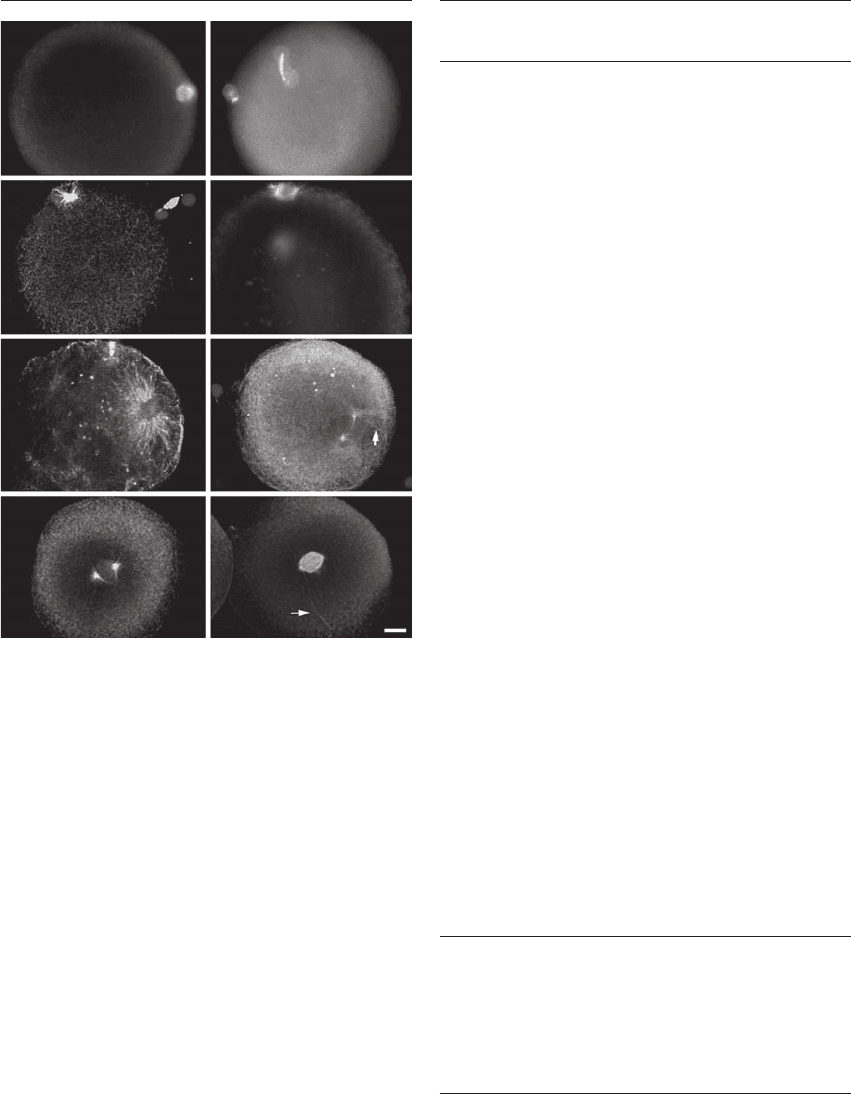
Figure 26.2 Microtubule and DNA organization in normal
inseminated human oocytes. The meiotic spindle in mature,
unfertilized human oocytes is anastral, oriented radially to the
cell surface, and asymmetric, with a focused pole abutting the
cortex and a broader pole facing the cytoplasm (A). No other
microtubules are detected in the cytoplasm of the unfertilized
human oocyte. Shortly after sperm incorporation (3–6.5 hours
postinsemination), sperm astral microtubules assemble around
the base of the sperm head, as the inseminated oocytes complete
second meiosis and extrude the second polar body (B)–(E). M,
male pronucleus; F, female midbody. The close association of
the meiotic midbody identifies the female pronucleus (B)–(E).
Short, sparse, disarrayed cytoplasmic microtubules can also be
observed in the cytoplasm following confocal microscopic
observations of these early-activated oocytes (C). As the male
pronucleus continues to decondense in the cytoplasm, the
microtubules of the sperm aster enlarge, circumscribing the
male pronucleus (D) and (E). By 15 hours postinsemination,
the centrosome splits and organizes a bipolar microtubule array
that emanates from the tightly apposed pronuclei (F). The
sperm tail is associated with an aster (arrow) (F). At first mitotic
prophase (16.5 hours postinsemination), the male and female
AB
M
M
M
M
F
F
F
F
CD
EF
GH
chromosomes condense separately as a bipolar array of micro-
tubules marks the developing first mitotic spindle poles (G). By
prometaphase, when the chromosomes begin to align on the
metaphase equator, a barrel-shaped, anastral spindle forms in
the cytoplasm (H). The sperm axoneme remains associated with
one small aster found at one of the spindle poles (arrow) (H).
Bar ⫽ 10 m. Reprinted with permission from Simerly et al.
14
HPE_Chapter26.qxp 7/13/2007 5:32 PM Page 339
HUMAN PREIMPLANTATION EMBRYO SELECTION
the typical radially arrayed monaster juxtaposed to
the sperm nucleus (Figure 26.2D and E) (which is
called the ‘male pronucleus’) after the sperm chro-
matin has decondensed within the egg cytoplasm.
These microtubules elongate throughout the cyto-
plasm during early development, some contacting
the female pronucleus and initiating pronuclear
migration (Figure 26.2E and F). As in most animal
eggs, the sperm tail enters the egg (Figure 26.2F).
Often, one or two punctate foci are found at the cen-
ter of the sperm aster exactly at the junction between
the sperm axoneme and the male pronuclear surface
which correspond to the sperm centriole(s).
15
The
reconstituted zygotic centrosome duplicates and splits
during late interphase, presumably under the control
of cell cyle regulatory machinery
17,18
(Figure 26.2F).
Mitotic prophase commences with the separate
chromosomal condensation of the male and female
pronuclei, as the zygotic centrosomes nucleate the
microtubules of the bipolar mitotic spindle apparatus
(Figure 26.2G). By late prometaphase, the condensing
parental chromosomes align along the equator of
the bipolar, anastral mitotic spindle (Figure 26.2H),
completing the fertilization process in humans.
These data confirm that humans inherit their
centrosomes from their fathers.
Evidence supporting the sperm centriolar complex
as the foundation for zygotic centrosome formation in
humans comes from studies on polyspermic fertiliza-
tion and parthenogenesis.
16,19
As shown in Figure 26.3,
when two sperm enter the oocyte, each paternal cen-
triolar complex organizes a sperm aster at the base of
the sperm head (Figure 26.3A and B). Conversely,
parthenogenetic (artificial) activation of oocytes, in
which no contribution of the paternal centrosome
is provided, causes random, disarrayed interphase
microtubule patterns (Figure 26.3C and D). Collec-
tively, these data reinforce the observation that the
centrosome is paternally inherited in humans.
7,14
CENTROSOME DYSFUNCTION AND HUMAN
INFERTILITY
Human gametes discarded from infertility clinics
performing ART and donated for research provide
important insights into the diagnosis of novel forms
of human infertility, especially male factor infertil-
ity.
14,20,21
Centrosome dysfunction after the sperm
enters the oocyte is being recognized as a new cause
of male factor infertility during human reproduc-
tion (Figure 26.4).
14,21–26
For instance, when the
assembled sperm centrosome after incorporation is
dysfunctional in microtubule assembly or organiza-
tion, the sperm aster may fail to form or be so
poorly organized as to be inconsistent with the abil-
ity to conduct pronuclear apposition. Sperm that
fail to activate the oocyte and initiate exit from
metaphase arrest often have underdeveloped micro-
tubule asters adjacent to incorporated sperm heads
(Figure 26.4A and B). Other sperm may nucleate
multiple asters (Figure 26.4C and D), or can fail to
correctly organize the sperm astral microtubules
Mt
M
M
M
F
F
DNA
BA
DC
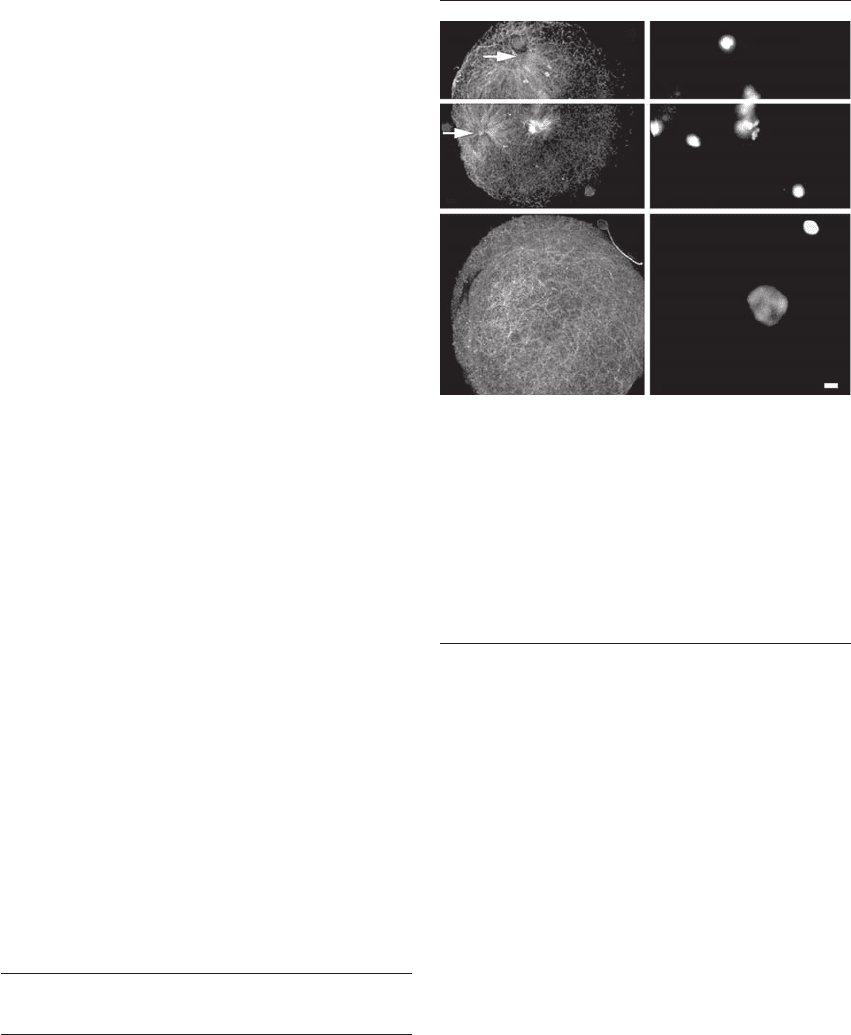
Figure 26.3 Microtubules (Mt) and DNA organization during
human polyspermic fertilization and parthenogenesis. (A) and
(B) During dispermic fertilization, a microtubule-based sperm
aster assembles at the base of each incorporated sperm head
(M) (B) from the site of the paternal proximal centriole
(arrows) (A). (C) and (D) Activation of the human oocyte
without sperm penetration (parthenogenesis) results in
random, disarrayed cortical microtubule assembly during
interphase. F, female pronucleus. Bar ⫽ 10 m. Reprinted
images with permission from Simerly et al.
14
The white bar
dividing A and B indicates the two focal planes.
HPE_Chapter26.qxp 7/13/2007 5:32 PM Page 340
THE SPERM CENTRIOLE: ITS EFFECT ON THE DEVELOPING EMBRYO
after incorporation resulting in oocytes that fail the
fertilization process and arrest in early development
(Figure 26.4E and F). About 25% of the human
oocytes classified as ‘failed to fertilize’ demonstrated
successful sperm incorporation, but with the oocytes
failing to properly assemble sperm astral micro-
tubules and/or undergo pronuclear formation.
14,25
These data show that the formation and functioning
of the sperm aster is essential during human
fertilization, and that naturally occurring defects are
causes of fertilization failures.
A study on the phenotypic expression of bull
centrosomes further illustrates the clinical, agricul-
tural, and fundamental importance of the centro-
some during fertilization.
27
Sperm from bulls were
selected and classified as superb, average, or subfer-
tile based on estrous non-return rates of 2500 cows
who underwent artificial insemination in field
studies, as well as blastocyst production rates
Bull A
0
20
40
60
80
100
Sperm aster size (µm)
Sperm aster quality
120
A
B
B
a
2
1
0
n = 100
n =91
n =81
Bull B Bull C
b
c
G
A
C
EF
M
M
M
D
B
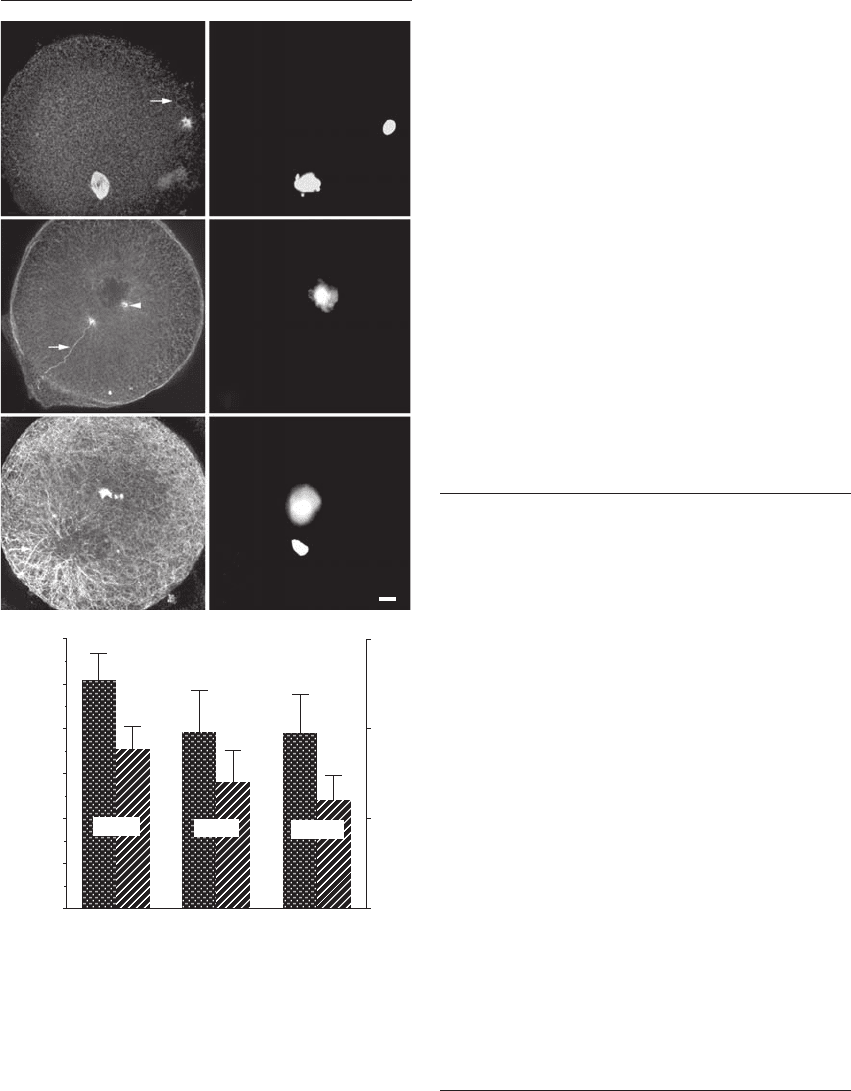
Figure 26.4 Microtubule (A), (C) and (E) and DNA (B), (D)
and (F) organization in ‘fertilization failures’ and phenotypic varia-
tions among paternal centrosomes. Common examples of the
stages at which human fertilization arrests after sperm incorpo-
ration include incomplete sperm aster assembly (A) and (B);
inappropriate detachment of the sperm aster and tail from the
male pronucleus (M), as well as androgenesis (C) and (D); and
disarrayed sperm astral organization (E) and (F). (A) and (B) A
truncated aster at the base of the sperm head has formed, but
not enlarged, by 24 hours postinsemination and the oocyte
remains arrested at a late meiotic phase. The incorporated
sperm tail is observed (arrow) (A). (C) and (D) The insemi-
nated oocyte displays a single pronucleus separated from the
nearby sperm tail (arrow) (C), indicating the presence of a male,
but not female, pronucleus. This example of androgenesis may
have occurred after the loss of the maternal chromosomes dur-
ing first or second polar body extrusion. Two small asters are
visible; one in association with the incorporated sperm tail
(arrow), while the other is detected at the male pronucleus
(arrowhead) (C). (E) and (F) A defect in microtubule aster
organization around the decondensing male pronucleus (E).
(G) Comparison of sperm aster size and quality in three bulls of
known fertility. Diameter of the sperm aster at its largest plane
was measured using the confocal microscope. A quality score
was also given to each aster. The bull with the highest field fertil-
ity and in vitro fertility (bull A) also had the largest and best-
organized sperm asters with averages of 101.4 m and 1.8,
respectively. Bull B had an average sperm aster diameter of
78.2 m and an aster quality score of 1.4. The bull with the
worst in vitro fertility (bull C) had the smallest (77.9 m) and
most poorly organized sperm asters (1.2). These data represent
five repetitions: comparisons were made using the protected
means of the least squares method. Different letters indicate
significant differences (p ⫽ 0.025); bars indicate standard error.
Bar ⫽ 10 m. Images (A)–(F) reprinted with permission from
Simerly et al.
14
Graph reprinted from Navara et al.
27
HPE_Chapter26.qxp 7/13/2007 5:32 PM Page 341

HUMAN PREIMPLANTATION EMBRYO SELECTION
following IVF. Using randomized bovine oocytes,
each bull sperm set was used for IVF and zygotes
fixed at a selected timepoint. As shown in Figure
26.4G, the organization and size of the sperm aster
varies according to the bull sperm, suggesting that
the quality or quantity of the sperm centrosome
directly affects the success and speed of fertilization,
and is correlated with the frequency of live births.
Perhaps variations in centrosomal vigor occur as
is found in other inherited components, a view
strengthened by recent observation of impaired cat
embryonic development in vitro correlated with
poor centrosomal function of cat testicular sperma-
tozoa following ICSI.
28
Collectively, these investigations have the
potential to be developed into novel screens for
male fertility.
7,19,22
Most sperm assays examine
parameters – motility, morphology, and counts –
that are factors more geared to successful fusion of
the sperm and egg plasma membranes. Yet, fertiliza-
tion is not successfully concluded until the sperm
and egg genomes align at metaphase of first mitosis,
and this requires the proper formation and func-
tioning of the zygotic centrosome and sperm aster.
29
DIAGNOSING MALE INFERTILITY BY
CENTROSOME FUNCTION ASSAYS
Zygotic centrosome formation as an early critical
step towards the accurate completion of the fertil-
ization process is a multistep pathway occurring
between the end of second meiosis and the transi-
tion into interphase of the first cell cycle. Central to
this process is assembling microtubules in the proper
organization to form the sperm aster that can
quickly direct proper pronuclear migration. Later,
the zygotic centrosome, duplicated under cell cycle
control, will define the site of first bipolar mitotic
spindle assembly within the activated cytoplasm
and participates in spindle organization by serving
as a dominant MTOC at the spindle poles.
7
Understanding centrosome reconstitution during
fertilization is inherently important for exploring
the molecular components necessary for determin-
ing centrosome parental origin and function.
7,19
Cell-free cytoplasmic extracts obtained from cyto-
static factor (CSF)-arrested Xenopus laevis oocytes
have effectively explored centriole formation and
microtubule assembly in vitro.
30–32
These pioneering
studies demonstrated that the crucial constituents
necessary for centrosome construction and reproduc-
tion reside in the oocyte. With regards to the relative
parental contributions to the zygote’s centrosome, the
Xenopus studies demonstrate that the sperm centro-
some contains conserved centrosomal constituent
proteins like centrin (a ubiquitous Ca
2⫹
-binding
protein of centrosomes) and pericentrin (a 220 kDa
component of the centrosomal matrix),
30,33
but
undetectable amounts of ␥-tubulin, a rare, invariant
constituent of the centrosome required for micro-
tubule nucleation and for defining the intrinsic
polarity of assembled microtubules from the cen-
trosome.
5
After exposure to Xenopus egg extracts,
both ␥-tubulin and phosphorylated epitopes are
detected on the sperm centrosome. Careful experi-
mental analysis of sperm in egg extracts suggests that
the Xenopus sperm contributes a structure capable
of binding maternal ␥-tubulin, does not require
assembled microtubules or microfilaments, but
does require egg extract and is ATP-dependent.
30–32
The sperm centrosome thus becomes competent for
nucleating microtubule growth into sperm asters in
vitro.
Analysis of human and bovine sperm in X. laevis
CSF-arrested extracts provides a basis for studying
the assembly of a zygotic centrosome capable of
nucleating and organizing microtubules in vitro.
19,34
Mammalian sperm exposed to increased calcium
levels, plasma membrane destabilization, and disul-
fide bond reduction unveils paternal ␥-tubulin and
other centrosomal protein binding sites, concomi-
tant with the onset of pronuclear decondensation.
This ‘procentrosome’ structure is thus primed to
attract and bind maternal ␥-tubulin from the egg’s
cytoplasmic pool. Conversely, other paternal cen-
trosomal proteins predicted to be critical for the
reorganization of the sperm centrosomal complex
following insemination (i.e. centrin) are modified
following exposure to the egg’s cytoplasm.
33,35
Exposure to an elevated kinase activity within the
meiotic cytoplasm then shifts the microtubule
HPE_Chapter26.qxp 7/13/2007 5:32 PM Page 342

THE SPERM CENTRIOLE: ITS EFFECT ON THE DEVELOPING EMBRYO
useful prospective centrosomal assay system.
22
This test permits observation of pronuclear apposi-
tion mediated by a functional human sperm centro-
some within a living egg, as opposed to just
microtubule assembly in egg extracts. Research has
shown that the recipient oocyte must be from a
species other than rodents, i.e. species that follow
the paternal inheritance of the centrosome.
36
The
zona-free hamster oocyte sperm penetration test,
for assaying human male infertility, is a uniquely
inappropriate model for the investigation and diag-
nosis of impaired sperm centrosome function of
human sperm, since hamster oocytes retain their
maternal centrosomes from oogenesis.
23
Instead,
oocytes from animals like the rabbit or cow, which
support paternal centrosomal functioning, are
more relevant models to investigate human centro-
some reconstitution, sperm aster formation, and
sperm-mediated pronuclear apposition.
Centrosome microinjection therapy could theo-
retically overcome defective centrosomes responsi-
ble for fertilization arrest in certain types of male
infertility.
7,19
However, research suggests that only
centrosomes introduced from intact sperm will
complete the entire fertilization process and cor-
rectly segregate their chromosomes at first cell
division,
37
indicating that centrosome numbers and
positioning at the pronuclear surface is a critical
parameter for completion of fertilization. While still
speculative, if successful as a therapeutic treatment,
the resulting embryo would have two fathers: a
genomic father and a centrosomal one.
POLYSPERMY AND THE ‘DISPERMY
HYPOTHESIS’ FOR THE ORIGINS
OF GENOMIC IMPRINTED
DISORDERS IN HUMANS
Polyspermy is invaluable for testing the relative
parental contributions of the centrosome, since the
paternal contribution is multiplied. In most animals,
dispermic insemination introduces two centrosomes
that duplicate and separate at mitosis to form
tetrapolar spindles. The resultant embryos, however,
are aneuploid because the triploid chromosome
dynamics to a state conducive to nucleation and
polymerization.
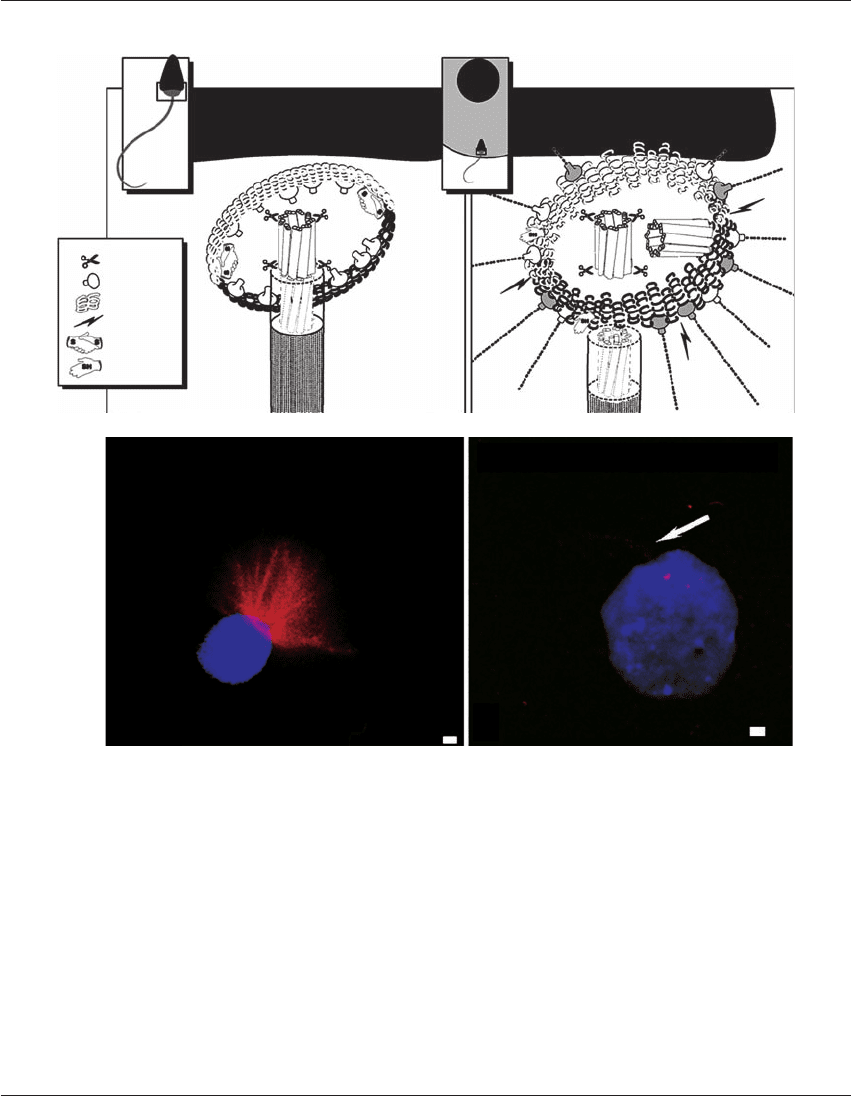
A model for mammalian sperm reconstitution is
presented in Figure 26.5.
7
In the mature human
sperm, centrin is found on the proximal centriole
(Figure 26.5A), with the doublet microtubules of
the sperm tail anchored to the triplet microtubules
of the centriole(s). The centrosome is probably
not phosphorylated, but extensively crosslinked by
disulfide bonds that mask the presence of paternal
␥-tubulin. After sperm incorporation, a functional
zygotic centrosome is quickly assembled (Figure
26.5B). Paternal centrin is lost, perhaps caused by
the binding of calcium ions released during oocyte
activation, and this triggers a calcium-induced trans-
formation of the proximal centriole, with its axial
microtubules, into a structure resembling a func-
tioning centrosome that can participate in the
organization of radial arrays of microtubules
post-insemination. The sperm centriolar complex is
predicted to be a coiled-coil structure,
3,7
and the lat-
tice structure is unravelled by the reduction of
disulfide bonds through the reducing environment
of the oocyte’s cytoplasm. This exposes minute
amounts of paternal ␥-tubulin and probably addi-
tional binding sites onto which maternal ␥-tubulin
complexes can attach. These events, along with
centrosome phosphorylation, lead to the nucleation
of the microtubules that can assemble the sperm
aster.
Clinical assays for the initial molecular charac-
terization of the human sperm centrosome have
been proposed (Figure 26.5).
7
Examining micro-
tubule assembly in vitro from the disulfide-reduced
(i.e. ‘primed’) sperm centrosome can be assayed
using Xenopus egg extracts in combination with
polymerization-competent rhodamine-tagged tubu-
lin protein.
19
This prospective assay may measure
the ability of a population of a patient’s sperm to
nucleate microtubules after sperm incorporation,
identifying men who are poor candidates for ART
procedures, prior to couples’ undergoing arduous
procedures of ART such as ICSI.
Examining microtubule assembly and centrosome
function after microinjecting human sperm into
mature bovine or rabbit oocytes is also a potentially
HPE_Chapter26.qxp 7/13/2007 5:32 PM Page 343
HUMAN PREIMPLANTATION EMBRYO SELECTION
Sperm centrosome
Centrin
γ-Tubulin
Pericentrin, etc.
Phosphorylation
Disulfide bonds
Sulthydryl groups
AB
Zygote centrosome
Human
+ Frog extract
No extractHuman
C
D
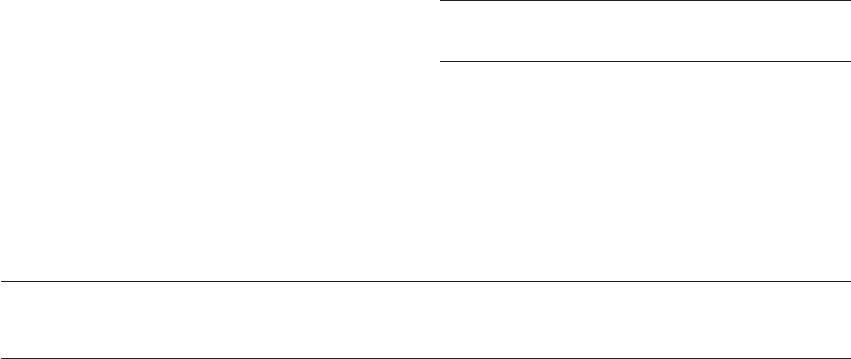
Figure 26.5 Molecular dissection of the human sperm centrosome and its reconstruction in the zygote leading to assembly of sperm
astral microtubules. (A) The human sperm centrosome has centrin concentrated in one or two focal sites, corresponding to the
centrioles. ␥-Tubulin is not apparent in mature human sperm, but becomes detectable after ‘centrosomal priming’ of the sperm with
disulfide reducing agents; this is a novel type of cytoplasmic capacitation. ␥-Tubulin is also detectable on Western blots with intact or
sonicated human sperm. The centrosome is not phosphorylated and the sperm tail microtubules extend from a centriole. The coiled-
coil infrastructure of the centrosome probably anchors the centrosome to the sperm nucleus and regulates the exposure of, and bind-
ing sites for, ␥-tubulin. In the zygote centrosome (B), after permeabilization and incubation in extracts from Xenopus oocytes, the
human sperm becomes phosphorylated and heavily immunoreactive with antibodies to ␥-tubulin. The ␥-tubulin found in the
human sperm is probably a combination of some paternal (light) and largely maternal protein (dark). The binding of calcium ions,
released during the transient increase during egg activation, to centrin is predicted to result later in a centrin-induced severing of the
doublet sperm tail microtubules from the triplet microtubules of the centriole. Perhaps the severing of the tail microtubules from the
basal body frees the basal body complex so that it can bind additional ␥-tubulin and undergo transformation into a centriole. The
coiled-coil domains of the centrosome are drawn as unraveling, expanding, and averting in the zygote; this exposes paternal ␥-tubulin and
also exposes binding sites for maternal ␥-tubulin. The halo of ␥-tubulin nucleates the microtubules, which assemble into the sperm
aster. (C) and (D). Human sperm exposed to 5 mol/l ionomycin and primed with 5 mol/l dithiothreitol, (DTT) demonstrate
assembly of microtubules in vitro from the centrosomal region after 40–60 minutes of incubation in CSF-arrested Xenopus
HPE_Chapter26.qxp 7/13/2007 5:32 PM Page 344
THE SPERM CENTRIOLE: ITS EFFECT ON THE DEVELOPING EMBRYO
complement is abnormally divided into the result-
ant four blastomeres at the end of cell division. Such
experimental evidence clearly supports the hypoth-
esis that only one centrosome – duplicated under
cell cycle control – is required for correctly forming
a bipolar spindle that can accurately segregate the
chromosomes.
2,17,18
In mammals, however, polyspermy experiments
argue against this central dogma on the universal
contribution of the sperm centrosome. Rodents vio-
late the notion of the paternal centrosome contribu-
tion at fertilization, as both the distal and proximal
sperm centrioles degenerate during spermatogene-
sis
12
and no sperm aster assembles at the base of the
sperm head in the cytoplasm following incorpora-
tion. Furthermore, di- or trispermic mouse zygotes
divide from one to two,
38
indicating no dominant
sperm centrosomal contribution during mouse
fertilization. In most other mammals, including
marsupials, cows, sheep, pigs, rabbits, monkeys, and
humans, supernumerary sperm asters form after
polyspermy.
7,39–42
However, as shown in human
fertilization, these dispermic zygotes can divide
from one-cell into two-, three-, or even four-cell
embryos.
42,43
As shown in Figure 26.6,
19
dispermic
human zygotes at mitosis assemble bipolar metaphase
spindles in the presence of supernumerary centro-
somes. At prophase, a disorganized multipolar
spindle with four foci of ␥-tubulin assembles first
(Figure 26.6A–C), which surprisingly resolves into
a bipolar spindle with aligned chromosomes at
mitotic metaphase (Figure 26.6D–F) and divides
one to two cells. These observations in humans may
reflect requirements of non-centrosomal components
(i.e. molecular motors and spindle matrix proteins)
necessary for bipolar spindle assembly in somatic
cells.
1
Analysis of parthenogenetic development in
primate oocytes supports this view (discussed
below).
Dispermic fertilizations in humans are observed
frequently.
42,43
Diandric triploidy is one of the con-
sequences, although other mis-segregations leading
to chromosome mosaicism can be found. 2N/3N
mixoploidies can give rise to live births but with
a variety of developmental disorders.
44,45
Diploid
sperm, the result of male meiotic error, can also
produce triploidy.
46
Studies of postzygotic diploidization in triploid
embryos
47
have suggested the origins of disorders
arising from genomic imprinting errors. Mammals
have the paternal and maternal chromosomes specif-
ically modified by DNA-methylation of ‘imprinted’
genes, so that certain maternal genes are silenced, as
are complementary paternal genes. Uniparental
disomy disorders (UPD) like Angelman (AS) and
Prader-Willi syndromes (PWS)
47,48
result from the
loss of either two maternally (AS) or two paternally
(PWS) expressed genes. Since centrosomes in
human zygotes form bipolar spindles even after
dispermic insemination, the possibility exists that
daughter blastomeres after cell division inherit two
or more paternal or maternal chromosome sets.
While the exact origins for uniparental disomies
remain elusive, understanding the molecular conse-
quences of these imprinting errors in humans
would be enormously useful in resolving develop-
mental, neurological, and behavioral consequences
of these devastating diseases.
MATERNAL CENTROSOME ANOMALIES AND
BIRTH DEFECTS
A primary cause of reproductive failure in older
women may reflect aging of crucial maternal factors
relevant to genetic fidelity and/or assembling a func-
tional zygotic centrosome.
49
Aneuploidy rates as high
as 52–61% have been reported in preimplantation
cytoplasmic extract and subsequent exposure to rhodamine-conjugated bovine brain tubulin (C). Primed sperm not exposed to
CSF extract do not nucleate microtubules after incubation in rhodamine-conjugated bovine brain tubulin (D). Bars ⫽ 1 m.
(A)–(C) Reprinted with permission from Schatten.
7
(C) and (D) reprinted with permission from Simerly et al.
19
HPE_Chapter26.qxp 7/13/2007 5:32 PM Page 345
