Elder K. Human preimplantation embryo selection
Подождите немного. Документ загружается.


HUMAN PREIMPLANTATION EMBRYO SELECTION
stages.
38,64,70,71
Overall, amino acid depletion increases
from early cleavage stage up to the blastocyst stage.
In human embryos, the amino acid profile differed
between embryos that reached the blastocyst stage
compared with those that arrested.
39,72
Developing
embryos depleted amino acids from the culture
medium to a lesser extent than did those that arrested,
and the profile was also different, in that developing
embryos took up significantly more alanine,
glycine, and serine.
39
However, the amino acid pro-
file could not be related to embryo morphology.
Appearance of alanine in the culture medium dur-
ing early cleavage could be used to select embryos
that will arrest their development. Houghton et al
39
reported that a higher concentration of alanine in
the culture medium was observed for arrested
embryos, and suggested that alanine is used to
metabolize ammonium production by embryos. The
presence of high levels of alanine in the culture
medium can therefore reflect the level of amino
acid turnover and ammonium production. As
developing embryos have a low turnover level, the
amount of alanine in the culture medium can be
related to embryo viability and therefore may help
to select embryos before transfer.
72
Leucine is
another amino acid that could be useful to assay, as
leucine is the only amino acid that is significantly
depleted from the culture by early cleavage stage
human embryos.
39,72
OXYGEN CONSUMPTION
Oxygen is essential for the conversion of ADP to
ATP in oxidative phosphorylation through its role
as an electron acceptor in the mitochondrial elec-
tron transport chain. Compared with the extensive
analysis of the effects of carbohydrates or amino
acids on early embryo development, few data are
available for oxygen consumption. However, oxygen
consumption reflects embryo metabolism and via-
bility more closely. Indeed, oxidative phosphoryla-
tion requires both oxygen and mitochondrial
integrity. Oxygen uptake by mouse embryos is pres-
ent from the 1-cell stage, and increases drastically at
the blastocyst stage.
72
A similar pattern is observed
for bovine embryos, although the consumption of
oxygen during oocyte maturation is as high as that
during blastocyst formation.
OTHER MARKERS OF EMBRYO VIABILITY
HUMAN CHORIONIC GONADOTROPIN
The culture of embryos up to the blastocyst stage is
a means of improving embryo selection prior to
transfer, as it is considered that grossly abnormal
embryos will arrest their development before reach-
ing the blastocyst stage. Blastocyst embryos are also
more metabolically active. Dokras et al
73
therefore
tested the secretion of human chorionic gonado-
tropin (hCG) by individual postcompaction stage
human embryos. Unfortunately, although secretion
of hCG correlated well with blastocyst morphology,
it could not be detected until day 8, and therefore is
not detectable early enough to assist in the selection
of embryos for transfer.
73
CONCLUSIONS
Early embryo development is a complex process
involving many different metabolic reactions that
require the production of energy. Deficiencies in
ATP production can be responsible for alterations
in gene expression, chromosomal segregation, and
signal transduction cascades. Alterations in the meta-
bolic status of early embryos can therefore impair
embryo viability. Embryo viability is dependent on
both the oocyte from which it derives and in vitro
culture conditions. In this respect intrafollicular
and ooplasmic conditions may also have an
important influence on the chromosomal status or
cytoplasmic reserve necessary for the embryos to
undergo the first cellular division and to survive
in vitro conditions. Indeed, these conditions impose
a great deal of cellular stress on preimplantation
embryos. Although early cleavage stage embryos
possess a great plasticity, their adaptation to in vitro
conditions impairs their viability as reflected by
HPE_Chapter15.qxp 7/14/2007 5:54 PM Page 186
UPTAKE AND RELEASE OF METABOLITES IN HUMAN PREIMPLANTATION EMBRYOS
lower cleavage rates, cleavage arrest, poor morphology,
retarded genome activation, abnormal gene
expression, or energy production. The more an
embryo has to adapt, the less likely it is to be viable.
Similar to the rat,
74
in the mouse and human there
is little vasculature in the vicinity of the implanta-
tion site for several hours. Glycolysis will therefore
be the only available means of energy production
for the blastocyst during this period.
10
Analysis of
variations in metabolic activity during the different
stages of development could be of great help in the
evaluation of embryo viability and its potential to
form a neonate.
In summary, different metabolic studies show
that embryos are relatively metabolically quiescent
prior to compaction, and oxidative phosphoryla-
tion is the major pathway for energy production.
After activation of the embryonic genome, embryos
are more active and a shift to energy production
occurs with the suppression of phosphofructokinase
inhibition, the enzyme that converts fructose
6-phosphate to fructose 1,6-diphosphate, or at the
level of hexokinase, where it is converted into glu-
cose 6-phosphate (Figure 15.1). This adaptation is
thought to be necessary for embryos to survive in
hypoxic conditions at the time of implantation,
although oxidative phosphorylation is still active.
The formation of the blastocoel cavity and the
first two lineages are processes that require a large
amount of energy. They are accompanied by a
drastic increase in gene expression and protein syn-
thesis.
6,22,74
Pyruvate, glucose, and amino acid con-
sumption and lactate production are all related to
embryo viability. Oxygen, which is one of the most
important substrates in energy production, has
been relatively less studied. All experiments seem to
agree that embryos with a relatively low metabolic
activity, i.e. low level of lactate production and
amino acid turnover, are those with the highest
potential.Viable embryos that do not have to strug-
gle to develop in vitro are probably those with a
metabolic activity comparable with that of those
developed in vivo.
1,75
Indeed, transformation of
high amounts of glucose into lactate is associated
with a general suppression of respiration and
oxidative phosphorylation (Crabtree-like effect)
22
and modification of the redox status of the embryo.
Glucose phosphorylation into glucose 6-phosphate
by hexokinase depletes the stock of cellular and
mitochondrial ATP. An excessive production of
lactate decreases the level of NADH necessary for
oxidative phosphorylation. Both mechanisms impair
the function of the mitochondrial electron trans-
port chain reactions, which in turn decreases the
amount of energy available for biosynthetic
processes.
14,22
The ratio of ATP/ADP decreases,
raising phosphofructokinase inhibition and result-
ing in the activation of the glycolytic pathway. This
excessive production of lactate during the cleavage
stage could therefore impair embryo viability, by
altering mitochondrial energy production.
However, the lack of prediction shown by meta-
bolic measurements could be due to different factors.
First, those performed during early cleavage stages
represent oocyte competence more than that of the
future embryos. Although correct oocyte matura-
tion and preservation of the integrity of cellular
components and metabolic pathways are important
to sustain future development, it is probably not
sufficient to predict viability of later stages. Second,
carbohydrates and amino acids represent only a
fraction of embryo metabolism. Little is known
about lipid metabolism, which can be a valuable
source of energy substrate. Third, the majority of
nutrient uptake and production studies have been
performed in suboptimal culture media. Further
experiments should be performed in better adapted
culture media, for example a sequential salt solution
supplemented with human serum albumin, pyru-
vate, low level of glucose (0.5 mmol/l), so-called
‘non essential’ amino acids with glycine, taurine, and
leucine for cleavage stage embryos and all amino
acids with 1 mmol/l glucose for later embryo develop-
ment. Such culture media should probably contain
some growth factors, but this remains to be ana-
lyzed in detail. Fourth, human embryos display a
large range of values for pyruvate, lactate, glucose, or
amino acids, which renders it difficult to use a single
value to predict viability. Finally, all the methods
actually employed to measure metabolic activity –
even if simplified – are too time consuming for
routine use in an IVF program.
HPE_Chapter15.qxp 7/14/2007 5:54 PM Page 187
HUMAN PREIMPLANTATION EMBRYO SELECTION
ACKNOWLEDGMENTS
I thank Professor Kate Hardy for welcoming me into
her laboratory. With great patience and kindness,
she trained me to handle preimplantation embryos
in culture in vitro as well as in the different tech-
niques used in this work. She was most generous
with her time in teaching me indispensable analyti-
cal methods; her comments were always pertinent
and most helpful. I am grateful to Professor Yvon
Englert for enabling me to pursue my research suc-
cessfully and his constant encouragement and many
constructive discussions. The work was supported
by the Belgian Funds for National Research.
REFERENCES
1. Gardner DK, Sakkas D. Assessment of embryo viability: the ability to
select a single embryo for transfer – a review. Placenta 2003; 24: S5–12.
2. Hardy K. Development of human blastocysts in vitro. In: Bavister BD,
ed. Preimplantation Embryo Development. New York: Springer-Verlag,
1993: 184–99.
3. Khosla S, Dean W, Reik W et al. Culture of preimplantation emryos
and its long-term effects on gene expression and phenotype. Hum
Reprod Update 2001; 7: 419–27.
4. Pedersen ME, Ozdas OB, Farstad W et al. Effects of bovine oviduct
epithelial cells, fetal calf serum and bovine serum albumin on gene
expression in single bovine embryos produced in the synthetic oviduct
fluid culture system. Reprod Fertil Dev 2005; 17: 251–7.
5. Lane M, Gardner DK. Selection of viable mouse blastocysts prior to
transfer using a metabolic criterion. Hum Reprod 1996; 11: 1975–8.
6. Fleming TP, Kwong WY, Porter R et al. The embryo and its future. Biol
Reprod 2004; 71: 1046–54.
7. Epstein C, Smith SA. Amino acid uptake and protein synthesis in
preimplantation mouse embryos. Dev Biol 1973; 33: 171–84.
8. Houghton FD, Thompson JG, Kennedy CJ et al. Oxygen consumption
and energy metabolism of the early mouse embryo. Mol Reprod Dev
1996; 44: 476–85.
9. Thompson JG, Bell ACS, Pugh PA et al. Metabolism of pyruvate by
pre-elongation sheep embryos and effect of pyruvate and lactate con-
centrations during culture in vitro. Reprod Fertil Dev 1993; 5: 417–23.
10. Leese HJ. Metabolism of the preimplantation mammalian embryo. In:
Oxford Review of Reproductive Biology, Milligan S.R. (Ed.). Oxford:
Oxford University Press, 1991; 13: 35–72.
11. Goodall H, Johnson MH. The nature of intercellular coupling within
the preimplantation mouse embryo. J Embryo Exp Morphol 1984;
79: 53–76.
12. Tarkowsk AK, Wroblewska J. Development of blastomeres of mouse
eggs isolated at the 4- and 8-cell state. J Embryol Exp Morphol 1967;
18: 155–80.
13. Benos DJ, Balaban RS. Current topic: transport mechanisms in pre-
implantation mammalian embryos. Placenta 1990; 11: 373–80.
14. Leese HJ, Conaghan J, Martin KL et al. Early human embryo metabo-
lism. Bioassays 1993; 15: 259–64.
15. Dale B, Menezo Y, Cohen J et al. Intracellular pH regulation in the
human oocyte. Hum Reprod 1998; 13: 964–70.
16. Brinster RL. Carbon dioxide production from lactate and pyruvate by
the preimplantation embryo. Exp Cell Res 1967; 47: 634–7.
17. Brinster RL. Carbon dioxide production from glucose by the preim-
plantation embryo. Exp Cell Res 1967; 47: 271–7.
18. Wales RG, Whittingham DG. The metabolism of specifically labelled
lactate and pyruvate by two-cell mouse embryos. J Reprod Fertil 1973;
33: 207–22.
19. Biggers JD, Stern S. Metabolism of the preimplantation mammalian
embryo. Adv Reprod Physiol 1973; 6: 1–59.
20. Leese HJ, Barton AM. Pyruvate and glucose uptake by mouse ova and
preimplantation embryos. J Reprod Fertil 1984; 72: 9–13.
21. Gardner DK, Leese HJ. Assessment of embryo metabolism and viability.
Handbook of In Vitro Fertilization.Boca Raton: CRC Press, 1993: 195–211.
22. Bavister BD. Culture of preimplantation embryos: facts and artifacts.
Hum Reprod Update 1995; 1: 91–148.
23. Rieger D, Guay P. Measurement of the metabolism of energy substrates
in individual bovine blastocysts. J Reprod Fertil 1988; 83: Z85–91.
24. Pinyopummintr T, Bavister BD. Energy substrate requirements for
in vitro development of early cleavage-stage bovine embryos. Mol Reprod
Dev 1996; 44: 193–9.
25. Fridhandler L, Wastila WB, Palmer WM. The role of glucose in meta-
bolism of the developing mammalian preimplantation conceptus.
Fertil Steril 1967; 18: 819–30.
26. Brinster RL. Radioactive carbon dioxide production from pyruvate
and lactate by preimplantation rabbit embryo. Exp Cell Research
1969; 54: 205–9.
27. Gardner DK, Lane M, Batt P. Uptake and metabolism of pyruvate and
glucose by sheep preattachment embryos developed in vivo. Mol
Reprod Dev 1993; 36: 313–9.
28. Brison DR, Leese HJ. Energy metabolism in the late preimplantation
rat embryo. J Reprod Fertil 1991; 93: 245–51.
29. Flood MR, Wiebold JL. Glucose metabolism by preimplantation pig
embryos. J Reprod Fertil 1988; 84: 7–12.
30. Wales RG, Whittingham DG, Hardy K et al. Metabolism of glucose by
human embryos. J Reprod F 1987; 79: 289–97.
31. Gardner DK, Leese HJ. The role of glucose and pyruvate transport in
regulating nutrient utilization by preimplantation mouse embryos.
Development 1988; 104: 423–9.
32. Hardy K, Handyside AH, Winston RML. The human blastocyst: cell
number, death and allocation during late preimplantation develop-
ment in vitro. Development 1989; 107: 597–604.
33. Gardner DK, Leese HJ. Non-invasive measurement of nutrient uptake
by single cultured pre-implantation mouse embryos. Hum Reprod
1986; 1: 25–7.
34. Lowry OH, Passoneau JV. A flexible system of enzymatic analysis. New
York: Academic Press, 1972.
35. Hardy K, Hooper MAK, Handyside AH et al. Non-invasive measure-
ment of glucose and pyruvate uptake by individual human oocytes
and preimplantation embryos. Hum Reprod 1989; 4: 188–91.
36. Leese HJ, Hooper MAK, Edwards RG et al. Uptake of pyruvate by early
human embryos determined by a non-invasive technique. Hum Reprod
1986; 1: 181–2.
37. Martin KL, Hardy K, Winston RML et al. Activity of enzymes of
energy metabolism in single human preimplantation embryos.
J Reprod Fertil 1993; 99: 259–66.
38. Orsi NM, Leese HJ. Ammonium exposure and pyruvate affect the
amino acid metabolism of bovine blastocysts in vitro. Reproduction
2004; 127: 131–40.
39. Houghton FD, Hawkhead JA, Humpherson PG et al. Non-invasive
amino acid turnover predicts human embryo developmental capacity.
Hum Reprod 2002; 17: 999–1005.
HPE_Chapter15.qxp 7/14/2007 5:54 PM Page 188
UPTAKE AND RELEASE OF METABOLITES IN HUMAN PREIMPLANTATION EMBRYOS
40. Sturmey RG, Leese HJ. Energy metabolism in pig oocytes and early
embryos. Reproduction 2003; 126: 197–204.
41. Pantaleon M,Kaye PL. Glucose transporters in preimplantation develop-
ment. Rev Reprod 1998; 3: 77–81.
42. Rieger D, Loskutoff NM, Betteridge KJ. Developmentally related
changes in the uptake and metabolism of glucose, glutamine and
pyruvate by cattle embryos produced in vitro. Reprod Fertil Dev 1992;
4: 547–57.
43. McKiernan SH, Bavister BD, Tasca RJ. Energy substrate requirements
for in-vitro development of hamster 1- and 2-cell embryos to the
blastocyst stage. Hum Reprod 1991; 6: 64–75.
44. Cross PC, Brinster RL. The sensitivity of one-cell mouse embryos to
pyruvate and lactate. Exp Cell Res 1973; 77: 57–62.
45. Biggers JD, Whittingham DG, Donahue RP. The pattern of energy
metabolism in the mouse oocyte and zygote. Proc Natl Acad Sci USA
1967; 58: 560–7.
46. Conaghan J, Handyside AH, Winston RML et al. Effects of pyruvate
and glucose on the development of human preimplantation embryos
in vitro. J Reprod Fertil 1993; 99: 87–95.
47. Devreker F, Hardy K, Van den Bergh M et al. Non-invasive assessment
of glucose and pyruvate uptake by human embryos after ICSI and
during the formation of pronuclei. Fertil Steril 2000; 73: 947–54.
48. Roberts R, Franks S, Hardy K. Culture environment modulates matura-
tion and metabolism of human oocytes. Hum Reprod 2002; 17: 2950–6.
49. Conaghan J, Hardy K, Handyside AH et al. Selection criteria for
human embryo transfer: a comparison of pyruvate uptake and mor-
phology. J Assist Reprod Genet 1993; 10: 21–30.
50. Hardy K, Robinson FM, Parascos T et al. Normal development and
metabolic activity of preimplantation embryos in vitro from patients
with polycystic ovaries. Hum Reprod 1995; 10: 2125–35.
51. Devreker F, Winston RML, Hardy K. Glutamine improves human
preimplantation development in vitro. Fertil Steril 1998; 69: 293–9.
52. Turner K, Martin KL, Woodward BJ et al. Comparison of pyruvate
uptake by embryos derived from conception and non-conception
cycles. Hum Reprod 1994; 9: 2362–6.
53. Payne D,Flaherty SP, Barry MF et al. Preliminary observations on polar
body extrusion and pronuclear formation in human oocytes using
time-lapse video cinematography. Hum Reprod 1997; 12: Z32–41.
54. Gardner DK, Lane M, Stevens J et al. Noninvasive assessment of
human embryo nutrient consumption as a measure of developmental
potential. Fertil Steril 2001; 76: 1175–80.
55. Tiffin GJ, Rieger D, Betteridge KJ et al. Glucose and glutamine meta-
bolism in pre-attachment cattle embryos in relation to sex and stage of
development. J Reprod Fertil 1991; 93: 125–32.
56. Gardner DK, Lane M. Culture and selection of viable blastocyst: a
feasible proposition for human IVF? Hum Reprod Update 1997; 3:
367–82.
57. Gardner DK, Leese HJ. Concentrations of nutrients in mouse oviduct
fluid and their effects on embryo development and metabolism
in vitro. J Reprod Fertil 1990; 88: 361–8.
58. Van den Bergh M, Devreker F, Emiliani S et al. Glycolytic activity: a pos-
sible tool for human blastocyst selection. Reproduction Biomedicine
Online 2001; 3(Suppl1): 8.
59. Biggers JD. Reflections on the culture of the preimplantation embryo.
Int J Dev Biol 1998; 42: 879–84.
60. Van Winkle LJ. Amino acid transport regulation and early embryo
development. Biol Reprod 2001; 64: 1–12.
61. Miller JGO, Schultz GA. Amino acid content of preimplantation rab-
bit embryos and fluids of the reproductive tract. Biol Reprod 1987; 36:
125–9.
62. Leese HJ, Aldridge S, Jeffries KS. The movement of amino acids into
rabbit fluid. J Reprod Fertil 1979; 56: 623–6.
63. Lane M, Gardner DK. Differential regulation of mouse embryo develop-
ment and viability by amino acids. J Reprod Fertil Dev 1997; 109:
153–64.
64. Partridge RJ, Leese HJ. Consumption of amino acids by bovine preim-
plantation embryos. Reprod Fertil Dev 1996; 8: 945–50.
65. Gardner DK, Lane M, Spitzer A et al. Enhanced rates of cleavage and
development for sheep zygotes cultured to the blastocyst stage in vitro
in the absence of serum and somatic cells: amino acids, vitamins, and
culturing embryos in groups stimulate development. Biol Reprod
1994; 50: 390–400.
66. Kane MT, Foote RH. Culture of two- and four-cell rabbit embryos to
the expanding blastocyst stage in synthetic media. Proc Soc Exp Biol
Med 1970; 133: 921–5.
67. Kishi J, Noda Y, Narimoto K et al. Block to development in cultured rat
one-cell embryos is overcome using medium HECM-1. Hum Reprod
1991; 6: 1445–8.
68. Devreker F, Van den Bergh M, Biramane J et al. Effects of taurine on
human embryo development in vitro. Hum Reprod 1999; 14: 2350–6.
69. Devreker F, Hardy K, Van den Bergh M et al. Amino acids decrease cell
death in human embryos cultured in vitro. Hum Reprod 2001; 16:
749–56.
70. Donnay I, Partridge RJ, Leese HJ. Can embryo metabolism be used for
selecting bovine embryos before transfer? Reprod Nutr Dev 1999; 39:
523–33.
71. Lamb VK, Leese HJ. Uptake of a mixture of amino acids by mouse
blastocysts. J Reprod Fertil 1994; 102: 169–75.
72. Houghton FD, Leese HJ. Metabolism and developmental competence
of the preimplantation embryo. Eur J Obstet Gynecol 2004; 115S:
S92–6.
73. Dokras A, Sargentt IL, Ross C et al. The human blastocyst: morpho-
logy and human chorionic gonadotropin secretion in vitro. Hum
Reprod 1991; 6: 1143–51.
74. Rogers PAW, Murphy CR, Rogers AW et al. Capillary patency and per-
meability in the endometrium surrounding the implanting rat blasto-
cyst. Int J Microcirc Clin Exp 1983; 2: 241–9.
75. Harvey AJ, Kind KL, Thompson JG. REDOX regulation of early
embryo development. Reproduction 2002; 123: 479–86.
HPE_Chapter15.qxp 7/14/2007 5:54 PM Page 189
HPE_Chapter15.qxp 7/14/2007 5:54 PM Page 190
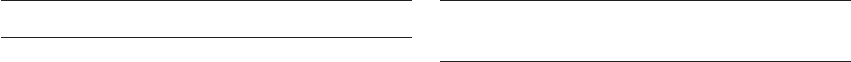
INTRODUCTION
The goal of in vitro fertilization (IVF) and embryo
culture is to provide high quality embryos capable
of continued development and implantation, which
will result in the birth of healthy babies. Considerable
progress has been made in culturing preimplantation
embryos since the initial studies were undertaken. We
began to design and define new, more complex culture
media in the early 1970s, which were based on the
composition of genital tract secretions.
1
During the
initial stages of zygote formation and early cleavage
divisions, the cells carry out only a minimal level of
transcription, since early preimplantation develop-
ment, i.e. up to the stage of maternal to zygotic tran-
sition (MZT), is maternally driven. A mature oocyte
must contain a storage pool of proteins and/or
mRNA transcripts in order to maintain its viability
during these early stages: all of the enzymes required
for metabolic pathways must be present and in har-
mony with the components of the culture medium.
During and after the cycle of MZT, the longest cycle
of preimplantation development, transcription of
the new zygote genome then results in an increase
in mRNA levels. The requirements of the embryo
before and after MZT differ, and the environment,
i.e. culture conditions, will have a direct impact on
transcription and translation. Moreover, epigenetic
reprogramming throughout early preimplantation
development is also important, and this has generated
concerns regarding the role of culture conditions in
assisted reproductive technologies.
2–6
In this chap-
ter we describe the basis of embryo metabolism, and
the impact of culture media composition on embryo
quality and viability.
16. Preimplantation embryo metabolism
and embryo interaction with the
in vitro environment
Yves J R Ménézo and Pierre Guérin
IN VITRO CULTURE CONDITIONS AND
ENVIRONMENTAL FACTORS
The physical conditions used during in vitro culture
differ significantly from conditions in vivo, in terms
of light, variations in pH, pCO
2
/O
2
,temperature,
static medium, etc. Physiological pH is regulated
by a HCO
3
/CO
2
buffer according to the equation
pH = pKa ⫹ log (HCO
3
)/(CO
2
). This Henderson-
Hasselbach equation allows pH to be calculated under
a 5% CO
2
atmosphere, in relation to the concentra-
tion of bicarbonate. The embryo has an alkaline pH
(7.4), and is not able to deal with an acidic pH before
the stage of MZT. The gas phase may be from 5% to
6.5% CO
2
in air, or 5% CO
2
,5% O
2
and 90% N
2
.
Based on the genital tract environment and an
increased potential to decrease free radical forma-
tion, a reduced O
2
atmosphere might appear to be
more physiological. However, no clear-cut data
support the superiority of reduced oxygen tension in
human embryo culture in terms of ongoing pregnancy
rates, particularly if the medium is well protected by
the addition of agents that counteract the reactive
oxygen species (ROS). Gas phase contaminants
such as NO and CO are deleterious, as are volatile
organic compounds (VOCs). The effect of ammonia
is discussed in the paragraph describing amino acid
metabolism.
An osmolarity between 280 and 300 mosmol
allows fertilization and early embryonic development.
Redox potential is difficult to evaluate, and is there-
fore rarely considered. Based on oviduct and uterine
secretions, redox potential should be calibrated to
⫺0.1 mV. In culture conditions, antioxidants can
turn into pro-oxidants, this is the case for vitamin C,
HPE_Chapter16.qxp 7/13/2007 5:26 PM Page 191
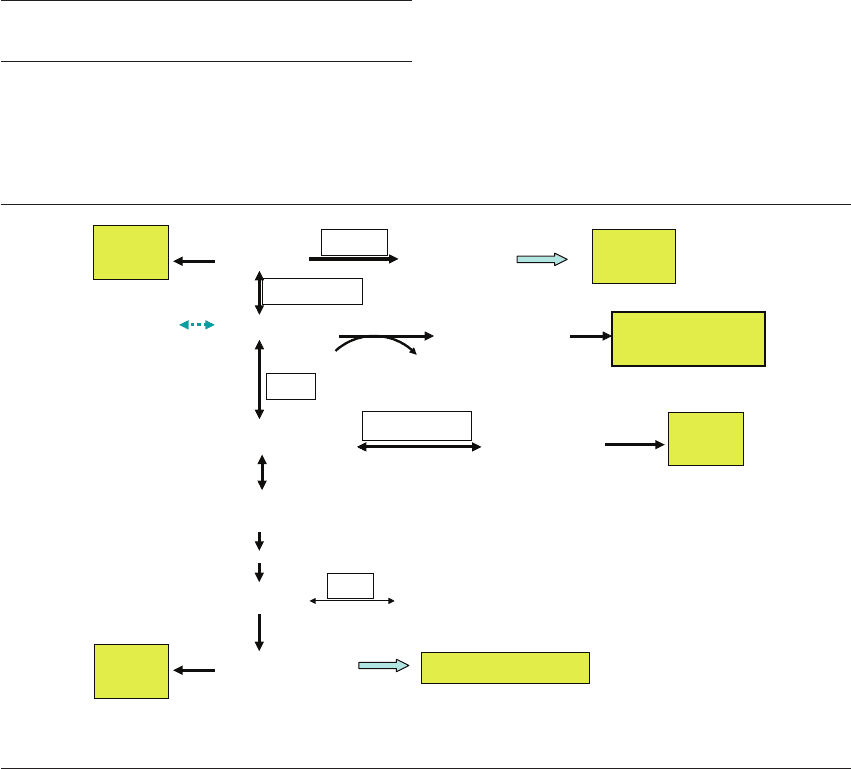
HUMAN PREIMPLANTATION EMBRYO SELECTION
especially in the presence of ferric ions. On the other
hand, an excessively reducing redox potential may
have harmful effects on protein tertiary structure.
Glutathione is sometimes added to culture media
as a reducing agent, but it should be noted that this
cannot enter the embryo. The addition of serum
destabilizes the culture media, by contributing
enzymes as well as unknown metabolites and catabo-
lites. Serum does not make a useful contribution, and
may even be deleterious by potentially increasing
pathologies, in particular those linked to imprint-
ing.
7,8
GLUCOSE AND ITS METABOLITES
(FIGURE 16.1)
High levels of glucose have been considered to be a
major factor in precipitating embryonic develop-
mental arrest during in vitro culture. The present
trend towards removing glucose and phosphate from
mammalian embryo culture media is not physiolog-
ical, and merely replaces one artifact with another.
The mouse model should be interpreted with care
as glucose metabolism in rodents may be impaired
due to a metabolic cul de sac that gives glucose poor
entry into the TCA cycle, with a block at the level of
glucose 6-phosphate isomerase that leads to a useless
accumulation of glycogen. However, there is a species
specific difference between rodents and humans,
and the human zygote has a completely different fea-
ture in its enzyme activity: hexokinase activity is high,
and glycogen synthase is low.
9
The activity of the
pentose phosphate pathway is equally high in human
and in rodents. High levels of glucose also have a
deleterious effect through an increase in free radical
formation. This aspect is particularly obvious in dia-
betic mammals, and is more than probably the case
in humans. Glucose itself is not toxic per se, and its
metabolism is necessary for the synthesis of ATP.
Hexokinase
G6Pi
Nucleic
acids
CO
2
+ H
2
O + ATP
G6PDH
Glycoproteins,
glycolipids…
Amino
acids
Tricarboxylic
cycle
Amino
acids
Fructose 6-phosphate
Fructose 1,6-diphosphate
Pyruvate
LDH
Glycogen
Amino
acids
Lactate
Hexokinase
Fructose
Glucosamine-6-
phosphate
Ribose
Deoxyribose
Glucose
Glu
Glucose 6-phosphate
Gln
Figure 16.1 Glucose and fructose metabolism in the embryo. Glucose metabolism leads to the synthesis of nucleic acids and amino acids,
and produces energy. G6Pi, glucose 6-phosphoisomerase; G6PDH, glucose 6-phosphate dehydrogenase; LDH, lactate dehydrogenase.
HPE_Chapter16.qxp 7/13/2007 5:26 PM Page 192
EMBRYO METABOLISM AND INTERACTION WITH IN VITRO ENVIRONMENT
The problem arises when too much glucose is present
in a medium that has an inadequate balance of sub-
strates. This is the case for several metabolic pathways,
including purine salvage in bovine (Figure 16.2),
where excess of glucose also induces shifts in the sex
ratio towards female
10
as the female embryo has a
more effective antiapoptotic mechanism. A relation-
ship between glucose-related apoptosis and monozy-
gotic twinning in humans has been proposed.
11
Pyruvate, on the other hand, is an interesting com-
pound, in that it acts not only as an energy source,
but can also detoxify ammonia in the embryo, through
transamination and export of alanine formed as a
result (Figure 16.3). It also plays a role in protection
against oxidative stress by preventing peroxide
induced injury. Lactate is an end product of metab-
olism, and therefore adding it to culture media is of
questionable value. In order to be re-introduced into
metabolism, lactate must be converted (oxidized) to
pyruvate, with generation of NADH. These short
chain carboxylic acids require monocarboxylate trans-
porters (MCTs) and their chaperone protein basigin
to allow their entry into metabolic pathways; both of
these are present in human oocytes and embryos.
12
These C2/3 carbon chains are also used for the syn-
thesis of lipids. Other glucose-derived metabolites
(such as acetate, citrate, malate, etc.) are sometimes
added to media, although no real impact on devel-
opment has been established; they will be used for
the synthesis of amino acids.
LIPID METABOLISM
The embryo needs to synthesize ‘sophisticated’
lipids at a very early stage. For this purpose, it is able
to ‘pick up’ saturated and unsaturated fatty acids,
free or bound to proteins, from its environment. The
ratio of individual fatty acids is much more impor-
tant than the presence of any single one. Phospholipid
synthesis can be carried out with exogenous choline
and glucose (metabolized toward fatty acid synthesis).
In the mouse oocyte, cholesterol levels increase
(three fold) from the 1-cell to the blastocyst stage.
13
Cholesterol can be taken up from the environment,
and it can be synthesized from the precursors meval-
onate and lanosterol, as well as from acetate that is
present as a result of pyruvate (and probably lactate)
decarboxylation. Blocking the metabolic pathway
for cholesterol synthesis with specific inhibitors
such as compactine or diosgenine leads to develop-
mental arrest before the blastocyst stage (Figure 16.4).
Cholesterol and fatty acids bound to albumin can also
be directly incorporated into the embryo.
AMINO ACIDS
Amino acids are necessary very early after fertiliza-
tion, and even for short-term embryo handling.
Active amino acid synthesis is grafted onto the tricar-
boxylic acid (TCA) cycle. Amino acids are used for
protein synthesis after translation of the mRNA stored
during maturation, and again for messages that are
translated after MZT. Moreover, there is an accelerated
protein turnover under in vitro conditions. Micro-
array technology has demonstrated that expression
of 114 genes is affected in culture without amino
acids, versus 29 genes with affected expression in cul-
ture with amino acids.
8
Glycine is present at a higher
HK
HK
HPRT
HPRT
G6PDH
G6PDH
O
2
–
.
Glucose
Pentose
phosphate
pathway
Purines
Hypoxanthine
Xanthine
11
2233
Glucose 6-phospate
Figure 16.2 Glucose and purine metabolism. Excess glucose
will inhibit the purine salvage pathway and thus increase
reactive oxygen species (ROS) formation. (1) Glucose inhibits
hypoxanthine phosphoribosyl transferase (HPRT). (2) Purine
catabolism. (3) Purine salvage. HK, hexokinase.
HPE_Chapter16.qxp 7/13/2007 5:26 PM Page 193
HUMAN PREIMPLANTATION EMBRYO SELECTION
concentration than any other amino acid in the
female genital tract.It can reach 5 mmol,10–50 times
higher than the other amino acids that are present
during the embryo’s transition through the tract.
Glycine, glutamine, alanine, and taurine may act as
organic osmolytes, allowing endogenous osmolarity
and volume regulation, and preventing an eventual
‘salting out’ effect. Amino acid uptake takes place
through active transport, and the overall scheme is
complex. Different amino acids will compete for the
same class of transporters, and individual affinities
will allow a higher or lower incorporation. Moreover,
some transporters are subject to a kinetic catabolism
before genomic activation, while others appear after
MZT. It is impossible to have a clear idea of the quan-
titative exchanges for each amino acid in the develop-
ing embryo, as the efficiency of uptake may differ
widely, as is the case for glycine and methionine
(Met). Glycine transport in the embryo is severely
reduced in the presence of Met (Figure 16.5). It
should also be noted that the Met concentration in
vivo is much lower than the concentration of glycine.
The concentrations of the different amino acids
2 Acetyl CoA
Acetoacetyl CoA
β HMG-CoA
MEVALONATE
β HMG-CoA reductase
NADPH
2
NADP
+
CoA
CHOLESTEROL
NADPH
2
CO
2
Fatty acid
synthesis
Diosgenine
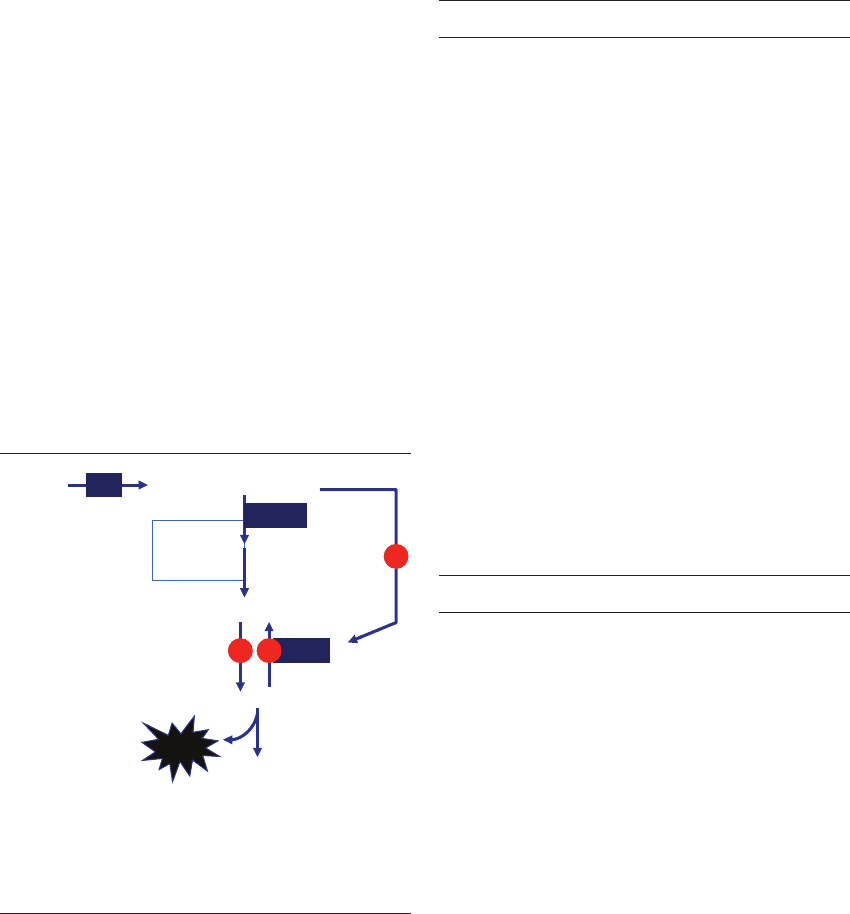
Figure 16.4 Cholesterol synthesis and its inhibition in the
mouse embryo. Inhibition of this pathway will cause the embryo
to arrest before blastocyst stage. HMG, hydroxymethyl-glutaryl.
Glucose
Glycine
Glycine
Glyoxylate
GPT
GPT
GOT
GOT
pyruvatepyruvate
Pyruvate
glutamine
Glutamine
TCA
Cycle
TCA
cycle
Alanine
Lactate
Pyruvate Glutamate
Oxoglutarate
Aspartate
Acetyl CoA
Oxaloacetate
Oxaloacetate
Alanine
Malate
NH
3
Figure 16.3 Transamination reactions in the embryo. GPT, glutamate-pyruvate transaminase; GOT, glutamate-oxaloacetate
transaminase.
HPE_Chapter16.qxp 7/13/2007 5:26 PM Page 194
EMBRYO METABOLISM AND INTERACTION WITH IN VITRO ENVIRONMENT
found in vivo are probably an important considera-
tion for the composition of culture media. Several
points requiring clarification are discussed below.
ESSENTIAL AMINO ACIDS AND
SULFUR AMINO ACIDS
It has been suggested that ‘essential amino acids’
may be toxic during preimplantation development
before the stage of MZT.
14
However, the distinction
between essential and non-essential amino acids is
somewhat unclear, as some amino acids can be pro-
duced from others. Phenylalanine, valine, trypto-
phan, threonine, isoleucine, methionine, histidine,
arginine, lysine, leucine, cysteine, and tyrosine were
described by Waymouth in a particular context
(diploid cell culture).
15
Leucine uptake is consid-
ered to be a good marker of embryo quality before
genomic activation (MZT), indicating that this ‘essen-
tial amino acid’ may be important for human
embryo construction.
16
Methionine is classified as an essential amino
acid and is thus omitted in the majority of first phase
sequential media. This is a questionable concept,
because in mouse embryos, silent paternal alleles of
H19, Igf2, Grb10, and Grb7 are aberrantly expressed
and hypomethylated in simple media, but not in
media with amino acids. In reality, the balance
between Met and the other amino acids is of major
importance. Met has such a high affinity for the
transporter molecules that if it is present in too high
a concentration, it may prevent the uptake of other
amino acids, thus disrupting the equilibrium so that
the endogenous pool becomes unbalanced. Met is
required for the initiation of all protein synthesis
through Met-tRNA (Figure 16.6), and is incorpo-
rated by mouse, bovine, and human embryos. Equally
important, Met is the fuel of methylation through the
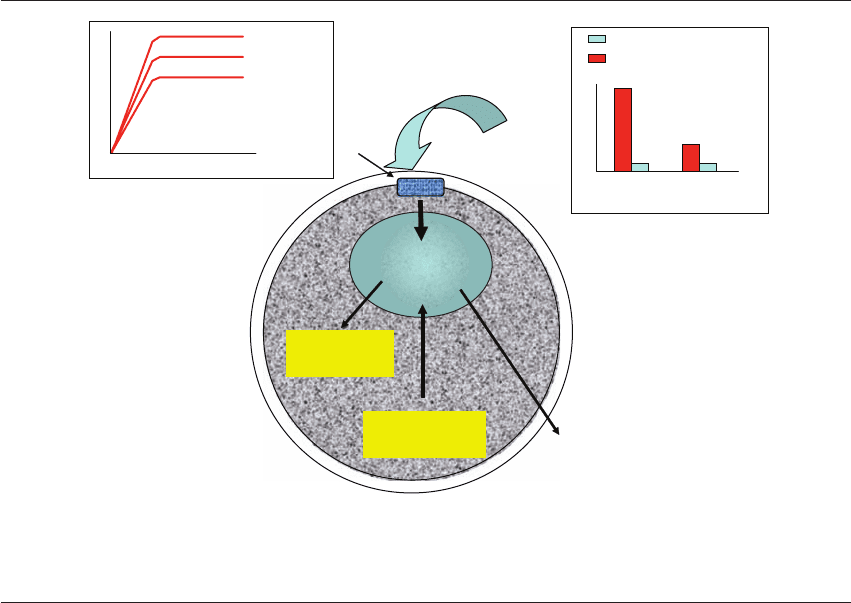
Glycine
free
Glycine
in proteins
Glycine uptake
With methionine
Without methionine
Export (ala)
(2) AA competition
(4) Protein
catabolism
Carrier
Glycine
(1) AA external
concentration
(3) Protein
synthesis
Methionine
100 µmol/l
250 µmol/l
AA uptake
Time
50 µmol/l
Amino acid
pool
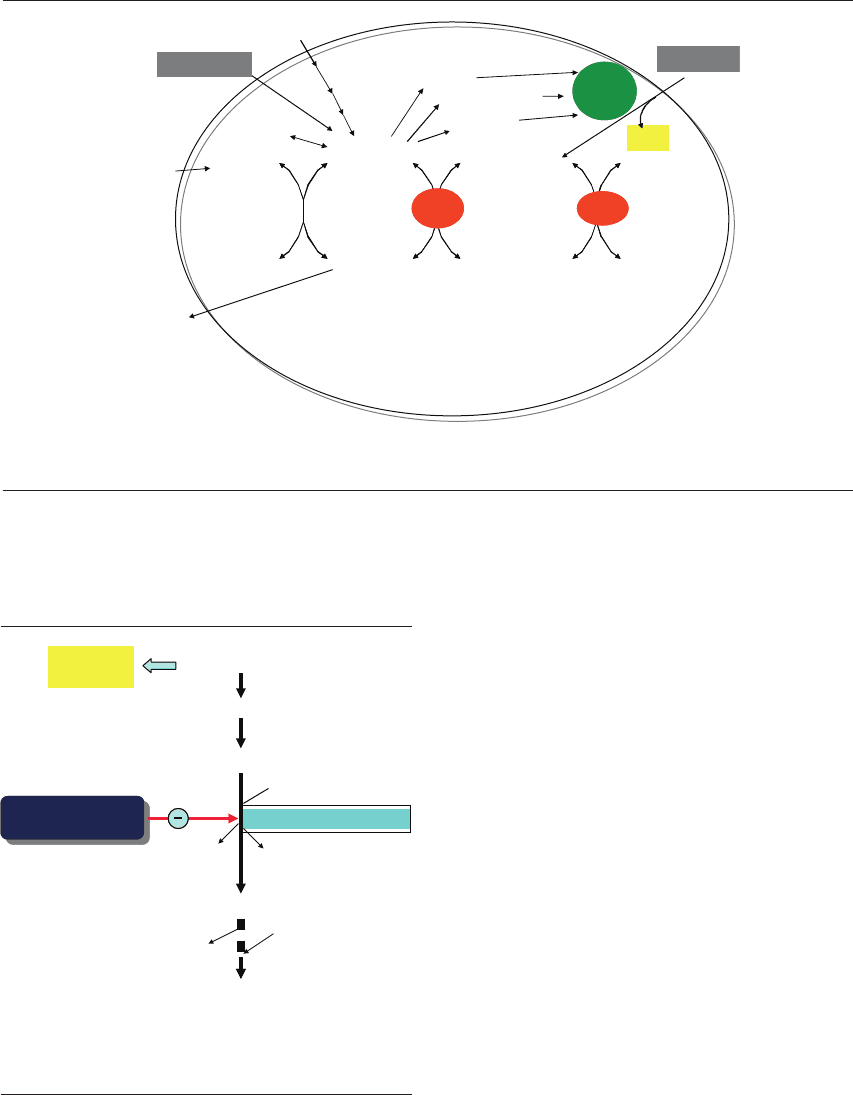
Figure 16.5 Factors influencing the endogenous pool of amino acids (AA) in the embryo. (1) The external concentration of each
amino acid affects its entry into the embryo. (2) Competition between amino acids for the same carriers will facilitate the entry of
some and reduce uptake of others. (3) Protein synthesis will decrease the endogenous pool. (4) Protein turnover will partly increase
the endogenous pool.
HPE_Chapter16.qxp 7/13/2007 5:26 PM Page 195
